ABSTRACT
Background: Hyperuricemia is a risk factor for the development of hypertension and is comorbid in many hypertensive patients. According to Japanese hypertension management guidelines published in 2019, a target serum uric acid level of ≤6.0 mg/dL is recommended in hypertensive patients with gout or asymptomatic hyperuricemia. Dotinurad is a novel uric acid-lowering drug classified as a selective urate reabsorption inhibitor. A pooled analysis was performed on the uric acid-lowering effect of dotinurad in 222 hypertensive patients with gout or asymptomatic hyperuricemia in four clinical trials (NCT02344862, NCT02416167, NCT03100318, NCT03372200). Moreover, we analyzed the long-term uric acid-lowering effect of dotinurad in 154 hypertensive patients with gout or asymptomatic hyperuricemia (NCT03006445).
Results: In the pooled analysis, the percent change in the decrease of serum uric acid with the use of dotinurad was 42.17 ± 12.42% at a dose of 2 mg and 60.42 ± 8.03% at a dose of 4 mg; the percentage of patients who achieved a serum uric acid level of ≤6.0 mg/dL was 82.8% and 100.0%. The long-term uric acid-lowering effect of dotinurad showed almost the same results. In this study, the concomitant use of diuretics or angiotensin II receptor blockers affected the uric acid-lowering effect of dotinurad at only a dose of 2 mg in the pooled analysis.
Conclusions: In the pooled analysis, dotinurad lowered serum uric acid levels. Dotinurad has an achievement rate of over 80% for serum uric acid level of ≤6.0 mg/dL in both analyses, and will be clinically useful for the management of hyperuricemic states in hypertensive patients.
Introduction
Hyperuricemia is frequently associated with hypertension and is considered a factor for the development of hypertension (Citation1). An enhanced renin-angiotensin system (RAS) has been proposed as a mechanism by which serum uric acid levels are increased. An increase in the number of renin-positive cells is observed in a rat model of hyperuricemia (Citation2), and a significant correlation between serum uric acid levels and plasma renin activity has been observed in humans (Citation3). Additionally, the generation of reactive oxygen species in the process of uric acid production causes a decrease in the levels of nitric oxide in vascular endothelial cells, which leads to increases in RAS activity, oxidative stress, and vascular smooth muscle cell proliferation, which can lead to hypertension (Citation4).
The frequency of hyperuricemia in hypertensive patients attending the outpatient clinic of a hypertension specialist was 40.6% for men and 8.6% for women in Japan (Citation5). If the serum uric acid level is ≥8.0 mg/dL in hypertensive patients, the initiation of uric acid-lowering drugs should be considered as stated in the Japanese Society of Hypertension Guidelines for the Managements of Hypertension 2019 (JSH2019) (Citation6). The target serum uric acid level is ≤6.0 mg/dL in the JSH2019 (Citation6).
Dotinurad, a novel selective urate reabsorption inhibitor (SURI), selectively inhibits urate transporter 1 (URAT1) in the kidney and does not inhibit ATP binding cassette sub-family G member 2 (ABCG2), which is responsible for uric acid secretion in the intestine and kidney (Citation7,Citation8).
We performed a pooled analysis of the serum uric acid-lowering effects of dotinurad in 222 hypertensive patients with gout or asymptomatic hyperuricemia enrolled in two Phase II (Citation9,Citation10) and two Phase III trials (Citation11,Citation12). Furthermore, we evaluated the long-term uric acid-lowering effect of dotinurad in a 34- or 58-week study of 154 hypertensive patients with gout or asymptomatic hyperuricemia (Citation13) to support the results obtained from the pooled analysis. To date, no stratification analysis for relationship between dotinurad and hypertension has been conducted. This is the first study to analyze the relationship. Clarifying the effect of dotinurad in hypertensive patients and its effect on blood pressure can optimize the treatment of gout and hyperuricemia in those patients.
Materials and methods
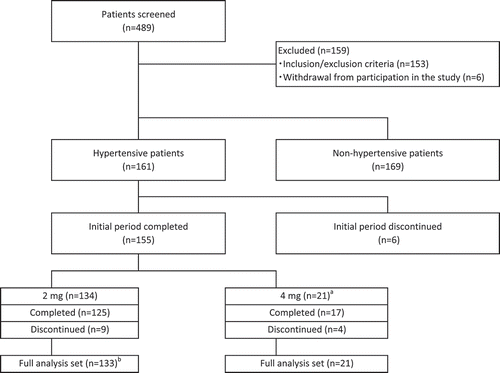
Hyperuricemic patients with hypertension in four 8 to 14-week Phase II and III trials of dotinurad, NCT02344862 (Citation9), NCT02416167 (Citation10), NCT03100318 (Citation11), and NCT03372200 (Citation12), were pooled and analyzed (hereafter referred to as the pooled analysis). A similar analysis was performed for a long-term study of 34 or 58 weeks, NCT03006445 (Citation13) (). The each full analysis set was used in these analyses. The evaluation time points for each trial used in the pooled analysis were different and the details of them were shown in Appendix 1. The long-term study was analyzed independently in this study. The reasons for this were as follows: first, the treatment periods of the four 8 to 14-week trials in the pooled analysis and the long-term study were up to 14 and 58 weeks, respectively, and the treatment periods were very different; second, patients were assigned evenly to each dose of dotinurad (0.5, 1, 2, and 4 mg) in the four 8 to 14-week trials, whereas in the long-term study, all patients were assigned to a dose of 2 mg and only those with insufficient response (who did not achieve a serum uric acid level of ≤6.0 mg/dL) at week 14 were increased to 4 mg, therefore each study has a very different study treatment. Flowcharts of the selection of patients in both analyses were shown in . In the pooled analysis, 9 patients in the dotinurad group discontinued the study treatment, and the reasons for the discontinuation were development of adverse drug reactions or adverse events in 4 patients and withdrawal of participation in clinical trials in 5 patients. In the long-term study, 13 patients in the dotinurad group discontinued the study treatment, and the reasons for the discontinuation were development of adverse drug reactions or adverse events in 6 patients, withdrawal of participation in clinical trials in 5 patients, and others in 2 patients.
Table 1. Clinical trials of dotinurad
Figure 1. Flowchart of selection of patients [pooled analysis (four studies)]
![Figure 1. Flowchart of selection of patients [pooled analysis (four studies)]](/cms/asset/8d03dc9d-da91-4c2b-81ef-20cd7c5a245c/iceh_a_1950752_f0001_b.gif)
Figure 2. Flowchart of selection of patients (Long-term study)
The Phase II and III trials and the long-term study were conducted with the approval of the institutional review board (IRB) of each participating medical site and in compliance with Japanese Good Clinical Practice (GCP). Patients with gout were also included in the analysis. All of the patients provided written informed consent prior to enrollment in each trial. The analysis was performed using patients classified as hypertensive according to the preferred term in the Medical Dictionary for Regulatory Activities (MedDRA). Pre-treatment serum uric acid levels, post-treatment serum uric acid levels, percent change in serum uric acid levels, change in serum uric acid levels, percentage of patients who achieved a serum uric acid level of ≤6.0 mg/dL, presence of concomitant drugs, systolic blood pressure, diastolic blood pressure, and pulse rate were evaluated as analysis items.
The main inclusion criteria in each trial were as follows: serum uric acid level during the run-in period of ≥7.0 mg/dL in patients with a history of gouty arthritis or gouty tophus, ≥8.0 mg/dL in hyperuricemic patients (on treatment or follow-up for hypertension, diabetes mellitus, and/or metabolic syndrome), or ≥9.0 mg/dL in hyperuricemic patients (without treatment or follow-up for any of the aforementioned complications).
The main exclusion criteria were as follows: gouty arthritis that had not become asymptomatic, complications involving renal calculus or clinical manifestations indicative of a urinary calculus (e.g., hematuria, back pain), and hyperuricemia classified as overproduction type or indeterminate, used drugs that might have affected serum uric acid levels, aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) levels ≥100 U/L, estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, systolic blood pressure ≥180 mmHg, and/or diastolic blood pressure ≥110 mmHg.
The classification of hyperuricemia was based on the criteria specified in Japanese guideline for the management of hyperuricemia and gout: second edition (Citation14) as follows: overproduction type, urinary uric acid excretion (EUA) >0.51 mg/kg/hr and uric acid clearance (CUA) ≥7.3 mL/min/1.73 m2; underexcretion type, EUA <0.48 mg/kg/hr or CUA <7.3 mL/min/1.73 m2; indeterminate type, EUA >0.51 mg/kg/hr and CUA <7.3 mL/min/1.73 m2; normal type, 0.48≤ EUA ≤0.51 mg/kg/hr and CUA ≥7.3 mL/min/1.73 m2. EUA and CUA were calculated as follows: EUA (mg/kg/hr) = [urine uric acid level (mg/dL) × urine output per hour (mL/hr)]/[weight (kg) × 100]; CUA (mL/min/1.73 m2) = [urine uric acid level (mg/dL) × urine output per hour (mL/hr)]/[serum uric acid level (mg/dL) × 60] × [1.73/body surface area (m2)].
An overview of the clinical trials used in the pooled analysis is shown in . Statistical analysis was performed using JMP® 10 (SAS Institute Inc., Cary, NC, USA). Patients were divided into two groups, with or without concomitant drugs, including thiazide diuretics and angiotensin receptor blockers (ARBs), and summary statistics were calculated using Wilcoxon’s rank-sum test. Statistical significance was defined as a two-tailed p-value of <0.05. The reason why we used the Wilcoxon’s rank-sum test was there were cases in which the number of patients in the groups with or without concomitant drugs differed significantly, so it was not appropriate to assume normality and homoscedasticity. Last observation carried forward method was used as an imputation for missing values.
Results
Patient characteristics
In the pooled analysis, the number of patients was 24 in the placebo group, 20 in the 0.5-mg group, 34 in the 1-mg group, 134 in the 2-mg group, and 34 in the 4-mg group. In the long-term study, the number of patients in the dotinurad 2- and 4-mg groups was 133 and 21, respectively. Patient characteristics are shown in . Office blood pressure was measured during the run-in period (-4 to -28 days before treatment).
Table 2. Baseline characteristics of patients analyzed
Efficacy of dotinurad
Pooled analysis
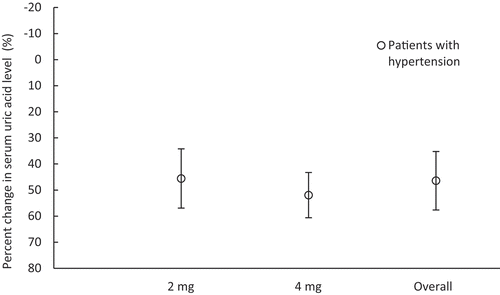
The percent change in serum uric acid levels at the end of treatment in the pooled analysis was 42.17 ± 12.42% at a dose of 2 mg and 60.42 ± 8.03% at a dose of 4 mg; the percentage of patients who achieved a serum uric acid level of ≤6.0 mg/dL at the end of treatment was 82.8% and 100.0%, respectively (, ).
Table 3. Uric acid-lowering effect of dotinurad in patients with hypertension [pooled analysis (four studies)]
Long-term study
The percent change in serum uric acid levels at the end of treatment in the long-term study was 45.55 ± 11.35% at a dose of 2 mg and 51.92 ± 8.67% at a dose of 4 mg; the percentage of patients who achieved a serum uric acid level of ≤6.0 mg/dL at the end of treatment was 88.7% and 90.5%, respectively (, ). In addition, the percent change in serum uric acid levels at week 14, which was the same as the longest treatment period in the pooled analysis, in the long-term study was 46.89 ± 9.08% at a dose of 2 mg and 32.15 ± 6.21% at a dose of 4 mg (Appendix 2).
Table 4. Uric acid-lowering effect of dotinurad in patients with hypertension (Long-term study)
Concomitant drugs
In the pooled analysis, the concomitant use of dotinurad with thiazide diuretics or ARBs did not affect the percent change in serum uric acid levels (). However, when the effects of concomitant drugs were evaluated at each dose, significant differences were observed in the 2-mg dose group for both concomitant drugs (both p < 0.05) ().
Table 5. Effects of concomitant drugs on percent change in serum uric acid level [pooled analysis (four studies)]
In the long-term study, the concomitant use of dotinurad with thiazide diuretics or ARBs did not affect the percent change in serum uric acid levels (). In the analysis performed in the 2-mg and 4-mg dose groups, no concomitant drugs affected the serum uric acid levels in either group ().
Table 6. Effects of concomitant drugs on percent change in serum uric acid level (Long-term study)
Effects on blood pressure and pulse rate
Pooled analysis
The office blood pressure and pulse rates at each dose in the pooled analysis are shown in . There is not much change in diastolic or systolic blood pressure or pulse rate before and after dotinurad administration.
Table 7. Blood pressure and pulse rate [pooled analysis (four studies)]
Long-term study
The office blood pressure and pulse rates at each dose in the long-term study are shown in . There is not much change in diastolic or systolic blood pressure or pulse rate before and after dotinurad administration, as in the pooled analysis.
Table 8. Blood pressure and pulse rate (Long-term study)
Discussion
In this pooled analysis of 222 hypertensive patients with gout or asymptomatic hyperuricemia, dotinurad lowered serum uric acid levels and the percentage of patients who achieved a serum uric acid level of ≤6.0 mg/dL was greater than 80% in 2- and 4-mg groups. The long-term uric acid-lowering effect of dotinurad in 154 hypertensive patients was similar. In the long-term study, the percent change in serum uric acid levels at week 14, which was the same as the longest treatment period in the pooled analysis, was 46.89 ± 9.08% at a dose of 2 mg and 32.15 ± 6.21% at a dose of 4 mg, and the percent change at 4 mg was lower than that at 2 mg (Appendix 2). The reason for this was that the study treatment was to increase the dose to 4 mg only in the patients who did not achieve a serum uric acid level of ≤6.0 mg/dL at week 14. The percent change in serum uric acid levels at week 58 of the end of treatment was 51.92 ± 8.67% at a dose of 4 mg after increasing the dose, and it was greater than that at 2 mg (). The percent change of the serum uric acid-lowering effects of dotinurad was 47.17 ± 11.18% (at week 58) at a dose of 2 mg and 57.35 ± 8.73% (at week 58) at a dose of 4 mg in the previous long-term study with pooled analysis of hypertensive and non-hypertensive patients (Citation13), and was similar to those of dotinurad in this study. No stratification analysis for relationship between dotinurad and hypertension was conducted in the previous studies. This is the first report to show the serum uric acid-lowering effect of dotinurad, a new SURI, in hypertensive patients with gout or asymptomatic hyperuricemia.
The number of hyperuricemia patients has increased in recent years, and persistent hyperuricemia causes gout (Citation15). Recent reports have shown that hyperuricemia is frequently associated with hypertension (Citation1). Furthermore, increased serum uric acid levels play a pivotal role in the progression of hypertension (Citation16,Citation17). JSH2019 recommends that the serum uric acid level should be reduced to ≤6.0 mg/dL with drug treatment (Citation6). These pooled and long-term analyses showed that this criterion was achieved at 4 mg of dotinurad. These results suggest that dotinurad exerts sufficient efficacy in hypertensive patients with gout or asymptomatic hyperuricemia.
Some drugs used to treat hypertension affect serum uric acid levels. Losartan, an angiotensin II type 1 antagonist, has URAT1 inhibitory effects (Citation18) and decreases uric acid levels (Citation19). It has been reported that thiazide diuretics increase serum uric acid levels (Citation20), the cause of which is controversial and may be due to a decrease in GFR (Citation21) or inhibitory effects on renal sodium phosphate transporter 4 (Citation22) by thiazide diuretics. Salicylic acid and salicylic acid derivatives decrease serum uric acid levels at high doses (Citation23). In this study, no significant differences were observed with the concomitant use of drugs, such as thiazide diuretics and ARBs, which have been reported to increase serum uric acid levels (). However, when analyzed by dosage, the uric acid reduction effects of dotinurad combined with thiazide diuretics or ARBs were attenuated only in the 2-mg dose group in the pooled analysis. No significant differences were observed between the groups at doses other than 2 mg. Although it is difficult to draw a conclusion based on the results of the analysis alone owing to the difference in the number of patients in each treatment group, it is necessary to accumulate data from patients of concomitant use of thiazide diuretics or ARBs in the future to verify clinical significance of the concomitant use because thiazide diuretics or ARBs may increase serum uric acid levels.
A close relationship between hyperuricemia and blood pressure has been reported in many studies; however, the effect of uric acid-lowering drugs on blood pressure is not apparent. Increased serum uric acid levels increase blood pressure through the enhancement of the RAS. Hypertensive patients tend to have higher uric acid levels than non-hypertensive patients and both systolic and diastolic blood pressure tend to increase with increased uric acid levels (Citation1). In a trial of Japanese patients, no changes in blood pressure were observed when febuxostat (40 mg) or benzbromarone (50 mg) was administered to hyperuricemic patients with hypertension (Citation24). In a Phase II trial of febuxostat in North America, a subgroup analysis showed a decrease in blood pressure in patients with normal renal function but no differences were found in the blood pressure in the whole patient population (Citation25). Hyperuricemia and hypertension seemed to be closely related and the uric acid level change caused by the administration of dotinurad could have affected blood pressure. However, no changes in office blood pressure or pulse rate were observed at any dose of dotinurad in both the pooled and long-term analyses. Therefore, the present results suggest that dotinurad does not affect office blood pressure and pulse rate in hypertensive patients.
As this was a pooled analysis of multiple studies, the study has the following limitations. First, there was a patient selection bias because this was a pooled analysis of hypertensive patients. Second, there was heterogeneity bias because the number of patients at each dose level varied. Third, the effects of dotinurad on office blood pressure may not have been accurately assessed because the hypertensive patients were selected at the discretion of the investigator based on their diagnosis, and not on the office blood pressure values. Fourth, the drug interactions between concomitant drugs and dotinurad may not have been accurately assessed because antihypertensive drugs other than losartan and thiazide diuretics were allowed to change their dosage during the treatment period, and the dose or duration of concomitant drugs used for hypertension could not be strictly controlled. Fifth, the vital sign data used in the analysis of this study were collected as a safety assessment of individual studies. Therefore, prospective clinical intervention studies in hypertensive patients for exact efficacy and blood pressure assessment are desirable in the future. Another limitation of the analysis in this study is that only hypertensive patients with hyperuricemia of underexcretion and normal types were analyzed, and the effect of dotinurad on hypertensive patients with hyperuricemia of overproduction and indeterminate types could not be evaluated.
e conducted this study to progress our knowledge of the optimal use of dotinurad in clinical practice by evaluating the effect of dotinurad on serum uric acid levels. To this effect, we pooled individual participant data of hypertensive patients with gout or asymptomatic hyperuricemia from previously published clinical studies. A high achievement rate of ≤6.0 mg/dL with dotinurad will be clinically useful for the management of hyperuricemic states in hypertensive patients. Large prospective studies on dotinurad will be needed to evaluate its serum uric acid-lowering effect in hypertensive patients with gout or asymptomatic hyperuricemia in the future.
Acknowledgments
We would like to express our gratitude to the physicians who cooperated in the present survey and provided valuable data.
Disclosure statement
Of the authors, Toshinari Takahashi is an employee of Mochida Pharmaceutical Co., Ltd., Takanobu Beppu is an employee of Fuji Yakuhin Co., Ltd., and Dr. Yuji Hidaka and Dr. Tatsuo Hosoya are medical advisors for this study. No potential competing interest was reported by the authors.
References
- Kuwabara M, Niwa K, Nishi Y, Mizuno A, Asano T, Masuda K, Komatsu I, Yamazoe M, Takahashi O, Hisatome I. Relationship between serum uric acid levels and hypertension among Japanese individuals not treated for hyperuricemia and hypertension. Hypertens Res. 2014;37(8):785–89. doi:https://doi.org/10.1038/hr.2014.75. Cited in: PMID: 24671018.
- Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–06. doi:https://doi.org/10.1161/hy1101.092839. Cited in: PMID: 11711505.
- Tani S, Nagao K, Hirayama A. Effect of febuxostat, a xanthine oxidase inhibitor, on cardiovascular risk in hyperuricemic patients with hypertension: a prospective, open-label, pilot study. Clin Drug Investig. 2015;35(12):823–31. doi:https://doi.org/10.1007/s40261-015-0349-8. Cited in: PMID: 26482071.
- Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB, Lozada LGS, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the national kidney foundation. Am J Kidney Dis. 2018;71(6):851–65. doi:https://doi.org/10.1053/j.ajkd.2017.12.009. Cited in: PMID: 29496260.
- Sakaki M, Tsuchihashi T. Current status of uric acid level in treated hypertensive patients. Gout Nucleic Acid Metabolism. 2013;37(2):103–09. doi:https://doi.org/10.6032/gnam.37.103.
- Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH2019). Hypertens Res. 2019;42(9):1235–481. doi:https://doi.org/10.1038/s41440-019-0284-9. Cited in: PMID: 31375757.
- Taniguchi T, Ashizawa N, Matsumoto K, Saito R, Motoki K, Sakai M, Chikamatsu N, Hagihara C, Hashiba M, Iwanaga T. Pharmacological evaluation of dotinurad, a selective urate reabsorption inhibitor. J Pharmacol Exp Ther. 2019;371(1):162–70. doi:https://doi.org/10.1124/jpet.119.259341. Cited in: PMID: 31371478.
- Ishikawa T, Takahashi T, Taniguchi T, Hosoya T. Dotinurad: a novel selective urate reabsorption inhibitor for the treatment of hyperuricemia and gout. Expert Opin Pharmacother. 2021;1–10. doi:https://doi.org/10.1080/14656566.2021.1918102. Cited in: PMID: 33926357.
- Hosoya T, Sano T, Sasaki T, Fushimi M, Ohashi T. Clinical efficacy and safety of dotinurad, a novel selective urate reabsorption inhibitor, in Japanese hyperuricemic patients with or without gout: an exploratory, randomized, multicenter, double-blind, placebo-controlled, parallel-group early phase 2 study. Clin Exp Nephrol. 2020;24(Suppl 1):S44–S52. doi:https://doi.org/10.1007/s10157-019-01802-w. Cited in: PMID: 31754882.
- Hosoya T, Sano T, Sasaki T, Fushimi M, Ohashi T. Clinical efficacy and safety of dotinurad, a novel selective urate reabsorption inhibitor, in Japanese hyperuricemic patients with or without gout: randomized, multicenter, double-blind, placebo-controlled, parallel-group, confirmatory phase 2 study. Clin Exp Nephrol. 2020;24(Suppl 1):S53–S61. doi:https://doi.org/10.1007/s10157-019-01818-2. Cited in: PMID: 31792640.
- Hosoya T, Sano T, Sasaki T, Fushimi M, Ohashi T. Dotinurad versus benzbromarone in Japanese hyperuricemic patient with or without gout: a randomized, double-blind, parallel-group, phase 3 study. Clin Exp Nephrol. 2020;24(Suppl 1):S62–S70. doi:https://doi.org/10.1007/s10157-020-01849-0. Cited in: PMID: 31980978.
- Hosoya T, Furuno K, Kanda S. A non-inferiority study of the novel selective urate reabsorption inhibitor dotinurad versus febuxostat in hyperuricemic patients with or without gout. Clin Exp Nephrol. 2020;24(Suppl 1):S71–S79. doi:https://doi.org/10.1007/s10157-020-01851-6. Cited in: PMID: 31970593.
- Hosoya T, Fushimi M, Okui D, Sasaki T, Ohashi T. Open-label study of long-term administration of dotinurad in Japanese hyperuricemic patients with or without gout. Clin Exp Nephrol. 2020;24(Suppl 1):S80–S91. doi:https://doi.org/10.1007/s10157-019-01831-5. Cited in: PMID: 31875931.
- Yamanaka H. Japanese society of gout and nucleic acid metabolism. Japanese guideline for the management of hyperuricemia and gout: second edition. Nucleosides Nucleotides Nucleic Acids. 2011;30(12):1018–29. doi:https://doi.org/10.1080/15257770.2011.596496. Cited in: PMID: 22132951.
- Hisatome I, Ichida K, Mineo I, Ohtahara A, Ogino K, Kuwabara M, Ishizaka N, Uchida S, Kurajoh M, Kohagura K, et al. Japanese Society of Gout and Uric & Nucleic Acids 2019 Guidelines for Management of Hyperuricemia and Gout 3rd edition. Gout and Uric Nucleic Acids. 2020;44(Suppl).
- Goicoechea M, Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, Arroyo D, Luño J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388–93. doi:https://doi.org/10.2215/CJN.01580210. Cited in: PMID: 20538833.
- Kuriyama S, Maruyama Y, Nishio S, Takahashi Y, Kidoguchi S, Kobayashi C, Takahashi D, Sugano N, Hosoya T, Yokoo T. Serum uric acid and the incidence of CKD and hypertension. Clin Exp Nephrol. 2015;19(6):1127–34. doi:https://doi.org/10.1007/s10157-015-1120-4. Cited in: PMID: 25967597.
- Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417(6887):447–52. doi:https://doi.org/10.1038/nature742. Cited in: PMID: 12024214.
- Nishida Y, Takahashi Y, Susa N, Kanou N, Nakayama T, Asai S. Comparative effect of angiotensin II type I receptor blockers on serum uric acid in hypertensive patients with type 2 diabetes mellitus: a retrospective observational study. Cardiovasc Diabetol. 2013;12:159. doi:https://doi.org/10.1186/1475-2840-12-159. Cited in: PMID: 24180232.
- Salem CB, Slim R, Fathallah N, Hmouda H. Drug-induced hyperuricaemia and gout. Rheumatology (Oxford). 2017;56(5):679–88. doi:https://doi.org/10.1093/rheumatology/kew293. Cited in: PMID: 27498351.
- Okusa MD, Persson AE, Wright FS. Chlorothiazide effect on feedback-mediated control of glomerular filtration rate. Am J Physiol. 1989;257(1 Pt 2):F137–F144. doi:https://doi.org/10.1152/ajprenal.1989.257.1.F137. Cited in: PMID: 2750918.
- Jutabha P, Anzai N, Kitamura K, Taniguchi A, Kaneko S, Yan K, Yamada H, Shimada H, Kimura T, Katada T, et al. Human sodium phosphate transporter 4 (hNPT4/SLC17A3) as a common renal secretory pathway for drugs and urate. J Biol Chem. 2010;285(45):35123–32. doi:https://doi.org/10.1074/jbc.M110.121301. Cited in: PMID: 20810651.
- Caspi D, Lubart E, Graff E, Habot B, Yaron M, Segal R. The effect of mini-dose aspirin on renal function and uric acid handling in elderly patients. Arthritis Rheum. 2000;43(1):103–08. doi:https://doi.org/10.1002/1529-0131(200001)43:1<103::AID-ANR13>3.0.CO;2-C. Cited in: PMID: 10643705.
- Ohta Y, Ishizuka A, Arima H, Hayashi S, Iwashima Y, Kishida M, Yoshihara F, Nakamura S, Kawano Y. Effective uric acid-lowering treatment for hypertensive patients with hyperuricemia. Hypertens Res. 2017;40(3):259–63. doi:https://doi.org/10.1038/hr.2016.139. Cited in: PMID: 27760998.
- Gunawardhana L, McLean L, Punzi HA, Hunt B, Palmer RN, Whelton A, Feig DI. Effect of febuxostat on ambulatory blood pressure in subjects with hyperuricemia and hypertension: a phase 2 randomized placebo-controlled study. J Am Heart Assoc. 2017;6(11):e006683. doi:https://doi.org/10.1161/JAHA.117.006683. Cited in: PMID: 29102979.
Appendix 1. Evaluation time points for each trial used in the pooled analysis
Appendix 2. Uric acid-lowering effect of dotinurad in patients with hypertension (Long-term study, at week 14)

![Figure 3. Percent change in serum uric acid level in patients with hypertension [pooled analysis (four studies)]](/cms/asset/f2e65cf8-55d1-4d28-ba95-8ddc4a8b2171/iceh_a_1950752_f0003_b.gif)