Abstract
The Minamata Convention on Mercury (Hg) calls for global efforts to reduce the release and risk of Hg. A better understanding of the global Hg budget, transformation and transport as well as toxicity of Hg in the environment, and the Hg emission controlling technologies, is important to minimize Hg pollution and exposure risks. Here, we summarized recent findings regarding the Hg cycle, transport, transformation, and controlling technologies, based on publications in Critical Review in Environmental Science and Technology (CREST) during 2017–2021. In terms of CREST publications we first focused on the biogeochemical cycle and impacts of Hg in the sensitive environment of the Tibet Plateau, and technologies being used to control Hg0 emission from thermal power plants. Second, we discussed the roles of forest in the global cycle of Hg. Third, we reviewed the transport of Hg at artisanal and small-scale gold mining sites, and the mobility and transformation of Hg species in the environment. This special issue covers the recent studies on the cycle, transport, and transformation of Hg, enhances our abilities to develop better strategies to minimize its risks. There are emerging concerns with climatic change and natural and human perturbations on the biogeochemical cycle of Hg in the environment and futures studies are warranted in these areas as well as the global Hg cycle, transport, and remediation.
Graphical abstract
1. Introduction and background
Mercury is a naturally-occurring element on our planet, and it is a global pollutant because its gaseous form (Hg0) underlies a long-range transport in the atmosphere with a residence time of 0.5-1 year (Beckers & Rinklebe, Citation2017). Mercury is extremely toxic, especially its organic form methylHg (MeHg), which can cross the blood-brain barrier to damage the human nervous system. A review by Dietz et al (Citation2022) recently reported on a risk assessment of Hg exposure to mammals in the Arctic. MeHg is critical due to its bioaccumulation in organism and biomagnification in the food chain. Also, the bioaccumulation of MeHg in rice grain can cause a significant exposure of MeHg to humans via rice consumption (Feng et al., Citation2008). Aiming to protect the human health and the environment from the adverse effects of Hg, a global treaty titled Minamata Convention on Hg entered into the force in 2017 (UNEP, Citation2013). To support Hg abatement and reach the Convention’s aims, scientific research is needed to address Hg0 emissions, transfer, remediation, and disposal.
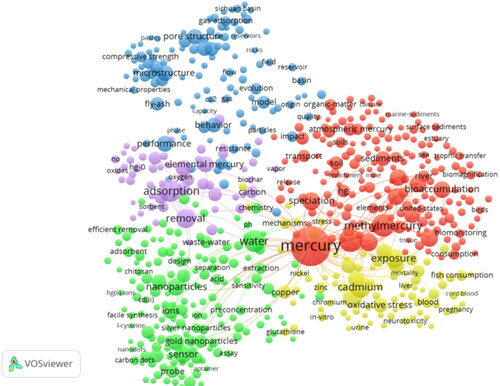
presents 19,305 peer-reviewed publications, where Hg research is involved, which can be grouped into five topical clusters. Cluster 1 (red) includes research on Hg speciation transformation, bioaccumulation, and atmospheric Hg0 deposition; Cluster 2 (green) includes research mainly on Hg removal from aqueous solution; Cluster 3 (purple) includes research on elemental Hg removal by oxidization and adsorption; Cluster 4 (yellow) includes research on Hg toxicity; and Cluster 5 (blue) includes research on Hg application in other fields. It is obvious that the scientific community has focused on the Hg cycle, remediation, and toxicity during the last 5 years.
Figure 1. Scientometric visualization of the top 600 keywords of all peer-reviewed publications from 2018. A total of 19,305 publications were retrieved from Web of Science with “mercury”as the searching keyword (all fields), and the database was selected as the “Web of Science Core Collection.” Collected data were analyzed using the built-in function of co-occurrence of all keywords, being plotted in “network visualization” using VOSviewer. Each circle represents a keyword, while its size represents the number of times that a pair of keywords has co-occurred in publications.
CREST published 12 reviews regarding Hg pollution issues in the period of 2017–2021. The topics covered Hg0 emission reduction technologies, forest Hg budget, biogeochemical transformation of Hg, and Hg remediation. Here, we collected those papers and aimed to provide a state-of-the-art editorial review on Hg0 emission reduction technologies, Hg cycle in Tibet Plateau and forest ecosystems, Hg methylation in anaerobic and aerobic environment, HgS formation and dissolution, Hg transport in artisanal and small-scale gold mining (ASGM)-impacted tropical rivers, and Hg remediation based on Hg-Se antagonism mechanisms.
2. Mercury research at CREST during 2017–2021
CREST manuscripts comprehensively reviewed the cycle of Hg in the environment, Hg biogeochemistry in the Tibetan Plateau, the Hg cycle in forest ecosystems, Hg0 emission reduction at coal-based thermal power plants, Hg speciation transformation in the environment, Hg transport in ASGM-impacted waters, and Hg remediation.
Beckers and Rinklebe (Citation2017) presented a general review on the biogeochemical transformation, transportation, and risk of Hg to better understand the Hg behavior in the environment. Mercury is a global pollutant, and its long-range transport in the atmosphere can cause contamination via deposition, leading to contamination of the environment far away from anthropogenic sources. The Tibetan Plateau is the “Roof of the World”. Sun et al. (Citation2021) reported that the Indian Summer Monsoon brought atmospheric Hg to the inland of Tibetan Plateau, and atmospheric Hg deposition to glacier, vegetation, and the soil was also largely affected by the “cold trapping effect”. Significant bioaccumulation of MeHg (>100 ng/g) was found in the Tibetan aquatic food chain. This clearly demonstrates that atmospheric Hg deposition can cause Hg contamination at sites even without obvious contamination sources. Therefore, reducing the Hg0 emission can be conclusive to reduce the global Hg risks.
Coal-based thermal power plants (CTPPs) are a significant anthropogenic Hg0 emission source, with about 477 tons of Hg being released annually. The development of technologies to control Hg0 emission at a CTPP is a hot research topic. The basic principle of Hg0 removal from the flue gas of the CTPP is the oxidation of Hg0 and subsequent removal of Hg2+. Balasundaram and Sharma (Citation2019) summarized the technologies/adsorbent for Hg0 removal from CTPP flue gas, with the adsorbents including activated carbon, metal oxides, spinels, fly ash, and natural mineral, Hg removal via photo-catalytic oxidation (PCO), fenton reagent, and Hg PCO-TiO2 mechanism. Although some progress has been achieved, Hg0 removal technologies still need further improvement due to the influence of flue gas components (e.g., SOx, Cl, HCl, and NOx) and operating conditions, and Hg behavior in flue gas.
Assessing the fate of atmospheric Hg is essential to assess the effectiveness of technologies in reducing Hg0 emission. Forest covers 31% of the global land area, and it strongly affect the global Hg budget and cycle (Wang et al., Citation2021). Foliar uptake of Hg0 mainly accounts for atmospheric Hg0 deposition, and knowledge regarding Hg behavior at leaf-air interface is crucial for a more precise assess of the contribution of forest in the global Hg budget. Liu et al. (Citation2021) reported that Hg fixed by foliage is a consequence of assimilation, re-volatilization, and washed off via throughfall; however, the accurate quantification of Hg fluxes during foliar Hg accumulation needs a combination of Hg flux measurement, Hg speciation analysis, isotope tracer techniques, element imaging, biological technologies, and ecologically microcosmic methods (Liu et al., Citation2021).
China is the largest atmospheric Hg0 emitter in the world. Therefore, knowledge about Hg in Chinese forests is important to calculate the Hg budget, thereby providing evidences for the effectiveness of the Minamata Convention on Hg reduction. Zhou et al. (Citation2020) reviewed the Hg fluxes, budgets, and pools in forest ecosystems of China. They reported that the annual THg retentions are 26.1-60.4 μg m−2 at subtropical forests and 12.4-26.2 μg m−2 at temperate forests in China, and the estimated THg retention by Chinese forest is 69 ton year−1, which is higher than predicted by global scale models (59 ton year−1). This greater Hg retention is due to the high input of Hg through atmospheric deposition and litterfall biomass (Zhou et al., Citation2020). Later, Wang et al. (Citation2021) reported the Hg budgets in global forests by synthesis of datasets of Hg flux measurements and Hg isotopic compositions. They concluded that forests are the largest atmospheric Hg sink in the terrestrial ecosystem, with total atmospheric Hg deposition of 2200–3400 Mg yr−1 and 500–1100 Gg stored in the surface soil and vegetation. Also, they reported that several hundred tons of Hg are re-released into the atmosphere through climate and land cover changes, deforestation and wildfire, and this Hg0 emission increases the risks for humans and the environment.
In addition to forest, Hg-contaminated sites around the world significantly affect the Hg budget by releasing ∼137–260 metric tons of Hg to the atmosphere and the hydrosphere annually (Kocman et al., Citation2013). Artisanal and small-scale gold mining (ASGM) is a significant Hg contributor to the environment, particularly due to the export of Hg via water. Moreno-Brush et al. (Citation2020) reviewed the fate of Hg from ASGM in tropical water using biogeochemical and hydrological information, and they explained that the mechanisms for the dispersion and transport of Hg in tropical rivers are different in rivers from midlatitudes and boreal areas. They reported that clay minerals and Al/Fe oxyhydroxide in suspended particulate matter played an important role controlling the accumulation and mobilization of Hg in river water.
Elucidating the speciation and transformation processes of Hg at contaminated sites, particularly the formation of the toxic MeHg and stable HgS, is critical for assessing risks of Hg contamination and may enhance the ability to manage those sites. Ma et al. (Citation2019) focused on the anaerobic microorganism-mediated methylation, and they reported that microorganisms possessing hgcAB genes are capable of Hg methylation such as sulfate-reducing bacteria, iron-reducing bacteria, and methanogens. Also, they found that Hg(II) crossed the plasma membrane of microorganisms through Mer-based transporters/passive diffusion/facilitated diffusion/active transporters for intracellular methylation. They proposed that the acetyl-coenzyme A pathway, methionine biosynthesis and biological degradation of simethylsulphoniopropionate are the main biochemical processes for Hg methylation. Wang et al. (Citation2021) emphasized in their review about the Hg methylation pathways in oxic marine waters, that this Hg methylation occurs via methyl donors such as organic compounds and organometallic complexes via abiotic aerobic microbes, and anaerobic microbes in anoxic microenvironments within oxic seawater. MeHg concentration in the environment is determined by both methylation (km) and demethylation rates (kd). Due to differences in experimental methods, the reported km and kd varied largely. Helmrich et al. (Citation2021) reviewed mercury methylation and MeHg demethylation rate constants in aquatic sediments for biogeochemical modeling. They provided important information concerning the use of km and kd to characterize MeHg formation.
HgS is a major sink of Hg in the environment, so its formation can remove Hg from the environmental cycling, while its dissolution can enhance Hg chemical and biological activities. Chen et al. (Citation2017) reported that HgS can be produced via chemical reaction between Hg2+, dissolved organic matter and S2–, between CH3Hg+ and inorganic/organic reduced sulfur, and between Hg2+ and FeS. However, HgS dissolution occurs through the following pathways: it can be enhanced by polysulfide, and cysteine the transformation of S2– in HgS into soluble or volatile S species results ina release of Hg2+ and the displacement of Hg2+in HgS by thiophile metals (e.g., Cu+) can lead to HgS dissolution. Also, microorganisms can mediate the dissolution of HgS.
Remediation based on the transformation of Hg to HgS might be risky because HgS can be mobilized. Recently, the formation of HgSe received considerable attention due to its extremely lower solubility (Ksp=∼10−58) relative to HgS (Ksp=∼10−52). Dang et al. (Citation2019) reviewed the mechanism of MeHg and Se interactions. They reported that the mechanism of Se addition to mitigate MeHg risk is mainly through the decrease of Hg availability via the formation of HgSe nanoparticles, with the effectiveness of remediation depending on Se concentrations, Se species, redox conditions, and sulfate levels. They pointed out that more studies are needed to address the availability of HgSe nanoparticle to microbial methylators, mechanism of Se impacts microbial methylators and MeHg demethylation and evaporation (Dang et al., Citation2019).
3. Conclusions and future research needs
To achieve the aims of the Minamata Convention on Hg reduction, researchers highlighted the importance of the Hg cycle in forest ecosystems, Hg transformation and transportation, Hg0 emission control technologies, and controlling of Hg pollution. With concerted efforts on the global Hg cycle and remediation studies, we still have realistic chances to reach the goals of the Minamata Convention on Hg. The information provided here will be helpful for scientists, policy makers, and engineers who deal with Hg pollution issues.
Although a number of papers on Hg have been published during the last five years, there are emerging concerns in enhanced natural and human perturbations on the biogeochemical cycle, and the risk and remediation of Hg in the environment. Future studies should focus on the entire-ecosystem budget and the specific role of forest ecosystems in Hg retention under natural and human perturbations. Mercury contamination in water surrounding artisanal and small-scale gold mining sites should be further studied, as more sources might contribute to Hg contamination. More studies are needed to identify and explore microorganisms responsible for Hg methylation and de-methylation, and elucidate the mechanism of Hg methylation by microorganisms. To further optimize Hg0 removal technologies, it is essential to study the role of flue gas components in affecting Hg behavior. One should be aware of the possibility of re-dissolution and methylation of HgS in soils during remediation of Hg-contaminated sites through HgS precipitation. The alterative remediation solution by transformation of Hg into HgSe is promising, but the mechanisms of Se on MeHg should be further explored. These aspects should be further studied using advanced analytical tools including Hg stable isotope measurements and models.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Balasundaram, K., & Sharma, M. (2019). Technology for mercury removal from flue gas of coal based thermal power plants: A comprehensive review. Critical Reviews in Environmental Science and Technology, 49(18), 1700–1736. https://doi.org/10.1080/10643389.2019.1583050
- Beckers, F., & Rinklebe, J. (2017). Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Critical Reviews in Environmental Science and Technology, 47(9), 693–794. https://doi.org/10.1080/10643389.2017.1326277
- Chen, Y., Yin, Y., Shi, J., Liu, G., Hu, L., Liu, J., Cai, Y., & Jiang, G. (2017). Analytical methods, formation, and dissolution of cinnabar and its impact on environmental cycle of mercury. Critical Reviews in Environmental Science and Technology, 47(24), 2415–2447. https://doi.org/10.1080/10643389.2018.1429764
- Dang, F., Li, Z., & Zhong, H. (2019). Methylmercury and selenium interactions: Mechanisms and implications for soil remediation. Critical Reviews in Environmental Science and Technology, 49(19), 1737–1768. https://doi.org/10.1080/10643389.2019.1583051
- Dietz, R., Letcher, R. J., Aars, J., Andersen, M., Boltunov, A., Born, E. W., Ciesielski, T. M., Das, K., Dastnai, S., Derocher, A. E., Desforges, J.-P., Eulaers, I., Ferguson, S., Hallanger, I. G., Heide-Jørgensen, M. P., Heimbürger-Boavida, L.-E., Hoekstra, P. F., Jenssen, B. M., Kohler, S. G., … Sonne, C. (2022). A risk assessment review of mercury exposure in Arctic marine and terrestrial mammals. The Science of the Total Environment, 829, 154445. https://doi.org/10.1016/j.scitotenv.2022.154445
- Feng, X., Li, P., Qiu, G., Wang, S., Li, G., Shang, L., Meng, B., Jiang, H., Bai, W., Li, Z., & Fu, X. (2008). Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou Province, China. Environmental Science & Technology, 42(1), 326–332. https://doi.org/10.1021/es071948x
- Helmrich, S., Vlassopoulos, D., Alpers, C. N., & O’Day, P. A. (2021). Critical review of mercury methylation and methylmercury demethylation rate constants in aquatic sediments for biogeochemical modeling. Critical Reviews in Environmental Science and Technology, 1–26. https://doi.org/10.1080/10643389.2021.2013073
- Kocman, D., Horvat, M., Pirrone, N., & Cinnirella, S. (2013). Contribution of contaminated sites to the global mercury budget. Environmental Research, 125, 160–170. https://doi.org/10.1016/j.envres.2012.12.011
- Liu, Y., Liu, G., Wang, Z., Guo, Y., Yin, Y., Zhang, X., Cai, Y., & Jiang, G. (2021). Understanding foliar accumulation of atmospheric Hg in terrestrial vegetation: Progress and challenges. Critical Reviews in Environmental Science and Technology, 1–22. https://doi.org/10.1080/10643389.2021.1989235
- Ma, M., Du, H., & Wang, D. (2019). Mercury methylation by anaerobic microorganisms: A review. Critical Reviews in Environmental Science and Technology, 49(20), 1893–1936. https://doi.org/10.1080/10643389.2019.1594517
- Moreno-Brush, M., McLagan, D. S., & Biester, H. (2020). Fate of mercury from artisanal and small-scale gold mining in tropical rivers: Hydrological and biogeochemical controls. A critical review. Critical Reviews in Environmental Science and Technology, 50(5), 437–475. https://doi.org/10.1080/10643389.2019.1629793
- Sun, R., Sun, G., Kwon, S. Y., Feng, X., Kang, S., Zhang, Q., Huang, J., & Yin, R. (2021). Mercury biogeochemistry over the Tibetan Plateau: An overview. Critical Reviews in Environmental Science and Technology, 51(6), 577–602. https://doi.org/10.1080/10643389.2020.1733894
- UNEP. (2013). Minamata convention on mercury.
- Wang, K., Liu, G., & Cai, Y. (2021). Possible pathways for mercury methylation in oxic marine waters. Critical Reviews in Environmental Science and Technology, 1–19. https://doi.org/10.1080/10643389.2021.2008753
- Wang, X., Yuan, W., Lin, C.-J., & Feng, X. (2021). Mercury cycling and isotopic fractionation in global forests. Critical Reviews in Environmental Science and Technology, 1–24. https://doi.org/10.1080/10643389.2021.1961505
- Zhou, J., Du, B., Shang, L., Wang, Z., Cui, H., Fan, X., & Zhou, J. (2020). Mercury fluxes, budgets, and pools in forest ecosystems of China: A review. Critical Reviews in Environmental Science and Technology, 50(14), 1411–1450. https://doi.org/10.1080/10643389.2019.1661176