Abstract
The chemical, physical, and biological conditions of soil and growing media can be substantively improved by the addition of compost. Compost contains many plant essential nutrients (e.g. N, P, and K) and can also be a source of organic matter. However, concerns persist over composts with a high concentration of soluble salts and their effects on soil fertility, plant growth, and yields. Soluble salts refer to soluble ions such as Ca2+, K+, Mg2+, and Na+ in compost and are measured indirectly and cumulatively through electrical conductivity (EC). Specifically, compost salinity is commonly measured using a method referred to as EC5 whereas soils are measured using an ECe. The use of a variety of non-standardized methodologies often make interpreting results between studies difficult. A compost with an EC5 >5 dS m−1 could be the result of high concentrations of Na+ or other ions, which can be detrimental to plants due to their ability to accumulate in plant tissue and interfere with root uptake of water. Thus, reducing soluble salts, specifically Na+ and Cl− in composts is of high importance. Other soluble salts present in compost (e.g. K+ and Ca2+) are mineral nutrients required for plant growth and can aid in reducing soil sodicity. In appropriate proportions, quality compost with a high EC5 mixed with soil or media can enhance plant growth and yields. Composts with a high EC5 are adept at aiding in soil remediation by facilitating soil particle flocculation, helping leach Na+ deeper into the soil profile and raising soil cation exchange capacity in support of increased soil fertility. Labeling composts and developing appropriate application methods could allay concerns associated with composts where EC5 > 5 dS m−1and promote compost use and sustainable farming practices.
Introduction
Compost is a product produced through controlled aerobic, biological decomposition of organic materials. The product undergoes microbial action at both mesophilic and thermophilic temperatures, which significantly reduces the viability of pathogens (Association of American Plant Food Control Officials Citation2017). Compost has a wide range of chemical characteristics that include nutrient composition, pH, and bulk density, that are a result of the composting method applied and the composition and origin of the biodegradable materials used (Asses et al. Citation2018; Grigatti, Cavani, and Ciàvatta Citation2011; Michel and Reddy Citation1998). Traditionally, compost was comprised of waste plant material. More recent composting operations, however, include agricultural waste, yard waste, source-separate food waste, municipal organic waste, biosolids, and even human or animal manures (Gomez Citation1998; Zhang et al. Citation2018; Yadav, Tare, and Ahammed Citation2010). These wastes are being produced at increasing rates and their potential to pollute the air, water, and soil highlight the need to dispose of or utilize them in an environmentally friendly fashion (Ahel et al. Citation1998; Düring and Gäth Citation2002; Kumar et al. Citation2004; Mor et al. Citation2006). Incorporating these organic wastes into compost allows composters to recycle and reuse elements with agronomic value that would otherwise end up in landfills and be of no reuse value. Recycling of these organic wastes and the production of compost reduces dependency on finite resources and promotes sustainable agricultural practices (Gomez Citation1998; Qadir and Oster Citation2004).
The primary nutrients within compost are forms of N, P, and K. Plants assimilate nitrogen in the form of nitrate (NO3−) and ammonium (NH4+) and these nutrients can be interconverted from the various forms of nitrogen present within compost or fertilizer. Orthophosphate (H2PO4−) is the most available form of P for plant absorption in soils, and potassium oxide (K2O) for K (Natural Resources Conservation Service Citation2007; Pennsylvania State University Citation2017). In addition to these primary nutrients, salts, micro- and macro-nutrients, heavy metals, and other contaminants may also be present within composts.
Application of compost can alter the physical and chemical properties of soils which enhance the soil’s ability to promote vegetative growth (Chang, Chung, and Tsai Citation2007; Tejada et al. Citation2006; Weindorf, Zartman, and Allen Citation2006). Although compost typically possesses many beneficial plant nutrients, it is not considered a fertilizer because of the high variability (non-uniformity) of nutrients throughout the various compost products (United States Composting Council (USCC) and Test methods and parameters. Accessed Citation2010). Despite the documented benefits of applying compost for plant growth, some compost use has been met with skepticism due to its possible high salt concentration. There is a misunderstanding that the application of composts with high concentrations of soluble salts can lead to reduced soil quality and plant growth. Regions that are experiencing escalating problems with soil salinity (e.g. salinization) and sodicity may be reluctant to apply compost to their fields because of the phytotoxicity associated with particular soluble salts such Na+ and Cl− (Mahmoodabadi et al. Citation2013; Reddy and Crohn Citation2012; Wu, Ma, and Martinez Citation2000)
Sodicity refers to the amount of Na+ held in the soil and is commonly expressed via sodium adsorption ratio (SAR) or exchangeable sodium percentage (ESP) (Weil and Brady Citation2017). SAR is the proportion of Na+ relative to Ca2+ and Mg2+, whereas ESP is expressed as Na+ relative to total soil cation exchange capacity (CEC). By contrast, salinity refers to the measurement of soluble salts in the soil (commonly salts more soluble than gypsum in water). The limiting growth factor for plants growing in low- or non-sodic environments could be nutrient availability, including salts, while plants growing in environments rich in nutrients may be limited by high salinity, high sodicity, or both (Bernstein Citation1975). The relationship between EC and SAR is depicted in and the properties of saline, sodic, and saline-sodic soils are given in .
Figure 1. Relationship between electrical conductivity (EC) and sodium adsorption ratio (SAR) (Naidu and Rengasamy Citation1993).
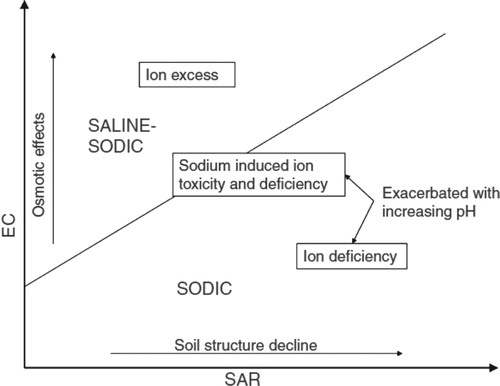
Table 1. General characteristics of saline, sodic and saline-sodic soils.
High salinity in soil can reduce the uptake of nutrient molecules into plants and therefore limits vegetative growth and yield (Grattan and Grieve Citation1998); osmotic effects are also a concern. Sharpley et al. (Citation1992) found that the presence of excess salts decreases the overall P in plant tissues resulting in reduced plant vigor and crop yield. NaCl accumulation in soil results in sodic soils, and plants grown in them suffer from burnt leaf margins that reduce overall plant vigor and crop yield (Grattan and Grieve Citation1998). These studies suggest an optimal nutrient concentration range is ideal for vegetative growth; importantly, such ranges are species specific and are dependent upon soil type and water potential. When nutrients are outside of this optimal range, the plant can experience nutrient-induced deficiencies or nutrient-induced toxicity (Grattan and Grieve Citation1998). Unfortunately, defining the range of optimal nutrient concentrations is problematic due to the variability present within soils, water potential, plant salt tolerances, and the form of available nutrients (Grattan and Grieve Citation1998; Reddy and Crohn Citation2012).
Thus, concern over high salt concentrations in compost having a negative impact on soil and agricultural yield is understandable, especially in arid and semiarid regions. Therefore, the objective of this article is to review current literature to evaluate which soluble salts are present in compost, the effect of those salts upon soil and plants, identify gaps in knowledge and sources of confusion regarding soluble salts and compost. The overarching goal of this review is to provide a reference for compost producers and users so they can make informed decisions regarding compost production and application.
Electrical Conductivity in Soil and Compost
A variety of ionic elements found in soil and compost may exist as soluble salts. Soil and compost salinity are commonly influenced by the presence of Na+, K+, Ca2+, Mg2+, Cl−, SO42+, CO32-, HCO3−, NO3− ions and some micronutrients. Much of the fundamental research on salinity was performed by the United States Salinity Laboratory Staff (Citation1954) in the quintessential text “Diagnosis and Improvement of Saline and Alkali Soils.” Today, Rhodes (Citation1996), Soil Survey Staff (Citation2019), and United Nations Food and Agriculture Organization (Citation2019) provide a litany of information on salinity.
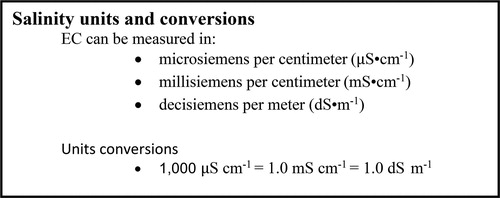
One commonly employed method for determining the soluble salt content of compost or soil is via electrical conductivity (EC). In this method, soil or compost are mixed with water to form a saturated paste or slurry. Electrical current is then passed between two electrodes immersed in the paste/slurry. The higher the content of soluble salts, the stronger the electrical transmission will be between electrodes. Readings are expressed in decisiemens per meter (dS m−1), millisiemens per cm (mS cm−1), or microsiemens per cm (µS cm−1). A summary of conversion between units is given in . Importantly, EC measurement does not identify the type of salt present in the sample, only the total cumulative concentration of the soluble salts present is determined. Thus, while NaCl may be one of the more common soluble salts encountered, there are many more that are present in various composts and soils that can result in a high EC.
Figure 2. Salinity units and conversions adapted from Birchall, Dillon, and Wrigley (Citation2008).
Confusion regarding EC levels in compost and soil arises due to the different methodologies used to measure them and the inability to directly compare the measured results (McLachlan et al. Citation2004). The EC of soils and soils amended with composts are commonly measured using a saturated paste extract (ECe) (Weil and Brady Citation2017), while compost EC is measured using a method referred to as EC5. EC5 is measured using extracts from a 5:1 (v/v) water-to-compost mixture to compensate for the elevated concentrations of soluble salts in compost (Thompson et al. Citation2001). Both of the aforementioned methods produce a measurement of EC; however, they are not comparable and thus must be converted to be compared. In addition, methods using other ratios by volume of water:medium have been used in various studies (e.g. 1:1, 1:2.5) (Sonmez et al. Citation2008; ). The development of equations to convert ECe to EC5 or to other dilutions have not been perfected due to the variation present in soil characteristics (Reddy and Crohn Citation2012). For example, clay has the ability to retain/adsorb ions and its presence in a soil sample can make converting results from one method to another less accurate (Sonmez et al. Citation2008; ). Thus, the variable methods of determining soil EC are comparable, but require suitable interpretation with the understanding that converting results from different methods may result in some degree of accuracy loss. Furthermore, investigations of compost feedstocks and their effects on various EC measurement methodologies have not been thoroughly documented to date. summarizes the EC of composts derived from variable feedstocks and methods used to estimate electrical conductivity in several published research articles that are reviewed in this article.
Figure 3. Relationships between different soil:water ratio used to measurements of electrical conductivity and soil type. Adapted from Sonmez et al. (Citation2008).
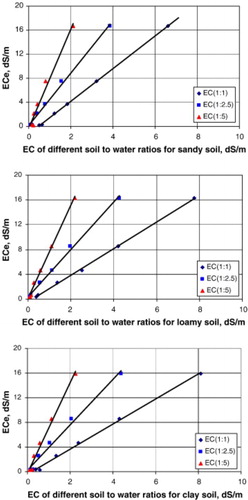
Table 2. Salinity of compost, associated feedstocks, and methodologies employed in determining such in several research studies.
Chemical analysis can further determine the specific types of salts causing a given EC reading (Colorado State University Citation2015). For example, Weindorf et al. (Citation2018) used portable X-ray fluorescence (PXRF) spectrometry for salinity determination of compost while simultaneously reporting many of the fundamental elements/ions responsible for such; PXRF has the advantage of also using elemental data to predict cation exchange capacity (CEC) (Sharma et al. Citation2015) and base saturation percentage (Rawal et al. Citation2019). Other chemical analyses, such as inductively coupled plasma-optical emission spectrometry (e.g. Soltanpour et al. Citation1996), various titrations (e.g. organic C via K2Cr2O7; Nelson and Sommers Citation1996), and flame photometry (e.g. Wright and Stuczynski Citation1996) can be used to determine the chemical composition of compost, soil, and water. After identifying and determining the specific concentrations of soluble salts present in a compost, an agricultural index (commonly referred to as Ag Index) can be calculated.
The Ag Index is an analytical measurement of a compost micronutrient mass (g) (N + P2O5 + K2O) divided by its NaCl mass (g) (Na + Cl) (Crohn Citation2016). Composts with an Ag Index > 10 are considered high quality due to the high ratio of micronutrients to NaCl. Values of ≤ 2 are considered poor quality due to the high concentration of NaCl or lack of micronutrients in the compost. Reference to the Ag Index of compost can alleviate some of the concerns about soluble salts in composts by indicating that the presence of detrimental soluble salts is limited and therefore should not be harmful to applied soils and plant yields (Reddy and Crohn Citation2012). There also may be a lack of understanding about the Ag Index, and therefore, educating compost end-users and farmers on how to reference the Ag Index would be beneficial. In addition, requiring composters to properly label their compost with a corresponding Ag Index or a range of the nutrient concentrations would further promote awareness of the compost composition and its benefits.
The effects of high soil salinity and sodicity on plants and their ability to tolerate these conditions has been the focus of research for some time (Liang et al. Citation2018; Deinlein et al. Citation2014; Mudgal, Madaan, and Mudgal Citation2010). In general, highly saline or saline-sodic soils affect a plant in two ways. First, a plant’s ability to imbibe water is greatly hindered when the plant is grown in saline or saline-sodic soil. This is referred to as the osmotic or water-deficit effect of salinity or and ultimately leads to a reduction in growth rate (Parihar et al. Citation2015). Second, plants grown in these soils are likely to accumulate an immoderate amount of soluble salts throughout their cells which will ultimately cause harm. This excessive accumulation is referred to as the salt-specific or ion-excess effect of salinity (Parihar et al. Citation2015). The ability of plants to tolerate these environmental stressors is referred to as salt tolerance and is defined as the plant’s relative yield plotted as a function of the average root-zone salinity. The variability in salt tolerance among plants is a result of the species specific evolutionary history.
Applcation of High EC Compost to Saline-Sodic Soils
As aforementioned, concerns exist over applying compost with high EC to saline or saline-sodic soils. Will such applications result in detrimental effects to the soil and/or the plants grown therein? Saline soils and saline-sodic soils are characterized as having an ECe > 4.0 dS m−1 while high salinity in soil can be defined as soils with an ECe ≥16 dS m−1 (). Typically, the salinity and sodicity of saline and saline sodic soils are likely to characterized as having a high pH and extreme ratios of Na+/Ca2+, Na+/K+, Mg2+/Ca2+ and Cl−/NO3− (Qadir and Schubert Citation2002). These nutrient imbalances reduce the cellular processes of plants and ultimately lead to a reduction in vegetative growth and yield. The excess of Na+ causes clay to swell and disperse, which causes a decrease in soil permeability, available water capacity, and infiltration rate (Lakhdar et al. Citation2009). In addition, saline and saline-sodic soils are likely to be deficient in organic matter and sources of N. Low organic matter can also lower soil porosity and CEC, thus limiting vegetative growth. These soil conditions commonly stem from geologic sources, spills of brine waters, improperly—managed fertilizer application, and/or irrigation with poor quality water high in soluble salts (Qadir and Oster Citation2004). These adverse conditions are amplified in arid regions where high-quality irrigation water is scarce and water evaporation occurs quickly (Mahmoodabadi et al. Citation2013; Qadir and Oster Citation2004). In addition, saline and saline-sodic soils are becoming increasingly abundant worldwide; salinization poses a serious threat to agricultural systems by reducing land fertility (Mahmoodabadi et al. Citation2013; Qadir and Oster Citation2004). As a result, there is an increasing need to reclaim these soils and return them to a more favorable condition for crop production.
Table 3. General degrees of salinization in soils.
The main principle to reclaiming sodic soils is to reduce the overall Na+ concentration by replacing the exchangeable Na+ with Ca2+. This is commonly done by applying one or some combination of amendments including gypsum (CaSO4), sulfuric acid (H2SO4), and organic matter. The application of sulfuric acid to calcareous sodic soil provides a soluble source of Ca2+ through the reaction of calcite to the acid. Ca2+ can then replace Na+ on the exchange complex of clays given its higher affinity on the lyotropic series (Mahmoodabadi et al. Citation2013). The addition of organic matter to soils increases the CEC as humic and fulvic acids are negatively charged colloids fostering ample sorption sites for cations. Subsequently, more nutrients become available for plants as the CEC increases (Diacono and Montemurro Citation2010). The increase in soil CEC also increases the chelating ability (i.e. the ability to form several bonds with a metal ion) of Ca2+ and Mg2+ in the soil, which enables them to replace Na+ from the cation exchange complex. This decreases soil sorption of Na+ in favor of higher affinity cations (Lakhdar et al. Citation2009). Once Na+ is displaced from soil exchange sites, it can be leached deeper in the soil profile (below the rooting zone). Polyvalent cations, such as Ca2+ from gypsum, replaces Na+ on the soil exchange sites and promotes soil flocculation and re-aggregation over time. Thus soil structural aggregation is enhanced, porosity increases, and the soil regains normal physical condition. However, the application of these amendments can be expensive; and, environmental concerns over the fate of Na+ deeper in the soil profile may also persist (Hao and Chang Citation2003). Furthermore, some instances in which gypsum application caused a decrease in available P and micronutrients have been documented (Mahmood Citation1982; Swarup Citation1986). Interest in environmentally friendly and low-cost alternatives to soil amendment has prompted the question of whether or not compost could be used instead.
Tazeh et al. (Citation2013) investigated the effects of applying municipal organic waste compost and cow manure to a saline-sodic soil in a controlled soil column experiment. Soil columns are commonly used in laboratories because the spatial and temporal aspects of solute movement in soils can be difficult to monitor and evaluate in-situ (Mahmoodabadi et al. Citation2013). The EC (measured with a 1:2.5 water:medium ratio) of the compost and manure were 19.6 and 16.7 dS m−1, respectively. The ECe and SAR of the soil were 15 dS m−1 and 20.4, respectively. The soil and amendments were thoroughly mixed together and packed into PVC columns that were 50 cm in length and 15 cm in diameter. The ECe and SAR were monitored over a five-month period where various treatments of leaching were applied. Both amendments reduced the ECe and SAR of the soil to ∼5 dS m−1 and 13, respectively. In most instances, the rate of leaching resulted in no significant difference in the ECe between the amendments. Thus, Tazeh et al. (Citation2013) demonstrated that high EC (1:2.5 water:medium dilution used to calculate EC) organic amendments did not result in an overall increase in ECe of the soil. On the contrary, organic amendments reduced the ECe and SAR. Other long-term studies lasting nine years or more have also demonstrated that compost application reduces Na+ concentrations and maintains or increases organic matter and essential nutrients (K, N, and P) in the soil (Diacono and Montemurro Citation2010; Miller, Beasley, and Drury Citation2013). A more detailed review of the effectiveness of compost use in salt-affected soils is given by Lakhdar et al. (Citation2009).
Planting crops with high salt tolerance, such as wheat and barley, can be beneficial to the soil reclamation process. Crop propagation may provide a salable crop and some degree of bioremediation. Qadir and Oster (Citation2004) suggest that roots will improve soil aggregation and hydraulic properties of soils, which will enhance root respiration. Furthermore, the symbiotic relationship between soil bacteria and roots may make Ca2+ more available, which will enable more Na+ leaching from the soil (Qadir and Oster Citation2004). Qadir, Qureshi, and Ahmad (Citation1997) showed that plots planted with either sesbania (Sesbania aculeata), sorghum (Sorghum bicolor), or kallar grass (Leptochloa fusca) exhibited an increase in P, Zn, and Cu; N decreased, however, in all plots except for those where N-fixing sesbania was planted. In conclusion, coupling compost application with the planting of salt-tolerant crops may expedite the soil remediation process and produce a more profitable crop over time.
After compost application, technologies such as electromagnetic induction (EMI) can be used to determine the spatial extent of salinity via the measurement of apparent electrical conductivity (ECa) at large scales. For example, Doolittle and Brevik (Citation2014) characterized soil variability and the location of saline seeps within an area of dryland farming in north central Montana using ECa from EMI. EMI allows users to monitor temporal increases in soil salinity as well as identifying spatial hotspots for remediation. With the spatial extent of salinity well established by EMI, farmers can use variable rate technology to reduce fertilizer application in impacted locations or target areas that need remediation via compost application.
Application of High EC Compost and the Effects on Plants
The effects of high soil salinity and sodicity on plants and their ability to tolerate these conditions has been the focus of research for some time (Liang et al. Citation2018; Deinlein et al. Citation2014; Mudgal, Madaan, and Mudgal Citation2010). In general, highly saline or saline-sodic soils affect a plant in two ways. First, a plant’s ability to imbibe water is greatly hindered by the osmotic or water-deficit effect of salinity or and ultimately leads to a reduction in growth rate (Parihar et al. Citation2015). Second, plants grown in these soils are likely to accumulate an immoderate amount of soluble salts throughout their cells which will ultimately cause harm and make it difficult to metabolize mineral nutrients. This excessive accumulation is referred to as the salt-specific or ion-excess effect of salinity (Parihar et al. Citation2015). The ability of plants to tolerate these environmental stressors is referred to as salt tolerance and is defined as the plant’s relative yield plotted as a function of the average root-zone salinity. The preferred soluble salt concentration is species specific, with deference to irrigation water quality and soil type (USCC, 2001). For example, ECe values from 2 to 4 dS m−1 can significantly reduce plant growth and potentially kill salt sensitive crops, such as strawberries (Fragaria) and lettuce (Lactuca). Salt tolerant crops such as wheat (Triticum) and rye (Secale) can tolerate ECe values as high as 7 dS m−1 (Mass and Grattan Citation1999). The application of composts can increase the electrical conductivity, alter the soluble salt composition and change the soil characteristics of the soils there are applied to. Therefore, determining the ideal compost to apply is highly dependent on the type of crop being grown and the soil to which it is applied. Studies reviewed in this section demonstrate that properly mixing or applying high EC5 compost with other medias/substrates does not induce phytotoxic effects. Rather, they enhance plant growth and yield.
Herrara et al. (Citation2008) investigated the effects of different combinations of peat and composted municipal solid wastes (CMSW) on plants and found that a mixture of CMSW with peat performed better than other mediums for growing tomato (Lycopersicon esculentum). The compost used during this three-year study featured EC5 values of 11.4 to 19.8 dS m−1. At study initiation, the ECe of the nursery substrates ranged from 1.0 to 22.0 dS m−1; following the growing period, ECe of the nursery substrate treated with CMSW ranged from 1.0 to 5.4 dS m−1. Cai et al. (Citation2010) conducted a similar study and found that mixed media composed of composted biosolids and leached composted biosolid resulted in similar growth characteristics as plants grown in the control media, which was composed of commercial peat and perlite.
Chang, Chung, and Tsai (Citation2007) studied the effects of compost and fertilizers on plant growth and soil characteristics over a three-year period. Composts with a range of EC5 of 4.6 to 18.7 dS m−1 were applied to plots at different rates and tilled into the soil at a depth of 15 cm. After tilling, a variety of 24 vegetable crops were transplanted to plots designated with different treatments. Crops grown in plots treated with inorganic fertilizers outgrew and produced higher yields than the crops grown in compost-treated plots during the first year of the study. However, during year two and three, crops grown in compost-treated plots grew more and produced higher yields than crops grown in plots treated with inorganic fertilizers (Chang, Chung, and Tsai Citation2007). This suggests that the benefits of adding compost are not always immediate and longer observation periods are required to observe their benefits. This is likely due to various interacting factors such as increased water retention, increased CEC, and increased microbial activity that allow the slow release of nutrients from the compost (Miller and Miller Citation2000). The experimental plots with the highest rate of compost application exhibited ECe values > 4 dS m−1 and the plants grown in these plots did not express any negative effects from the elevated ECe values; rather, they grew vigorously and obtained high yields. It was also observed that the growth of these plants was not significantly higher than plants grown in plots with a one-quarter to one-half reduction in compost application (Chang, Chung, and Tsai Citation2007). In a similar study of variable compost rates applied to soils in Texas, Weindorf, Zartman, and Allen (Citation2006) noted that soil water content significantly increased with increased rates of compost application. Thus, it is plausible that the additional moisture retention effectively dilutes salinity associated with certain compost products.
These studies and others suggest that soils or media amended with compost with ECe values in the range of 1 to 5 dS m−1 are suitable for many plants (Cai et al. Citation2010; Chang, Chung, and Tsai Citation2007, Herrara et al. Citation2008; Reddy and Crohn Citation2012; Walker and Bernal Citation2008). Note that the EC of soils amended with compost is commonly determined using the ECe method. Applying compost with EC5 values of ≥ 5 dS m−1 to soils and media has been shown to enhance the growth and yield of plants grown therein. Therefore, the literature suggests that the application of compost with high EC5 values and limited Na+ and Cl− concentrations will not induce negative effects as long as the soils or media they are applied to do not exceed an overall ECe of 5 dS m−1 after application (Cai et al. Citation2010; Chang, Chung, and Tsai Citation2007; Herrara et al. Citation2008; Reddy and Crohn Citation2012). In excess of such, soil can be irrigated with high quality water to leach excessive salts. Further scientific study examining how overall ECe of soil mixed with composts with high EC5 impacts soil ECe, plant growth, and yields is needed, especially as it relates to seedling germination in-situ vs. plant growth/vigor post-transplant and the long-term trends of soil ECe.
Compost applicators should also consider the substrates to which composts are being applied and the species of plans cultivated within them (Reddy and Crohn Citation2012). Studies examining the phytotoxicity of compost with an EC >5 dS m−1 have shown that when these composts are not diluted in other media or properly irrigated, the plants grown therein do experience negative effects from salt stress (Cai and Gao Citation2011; Chong Citation2000). Germination of most seeds should be done in soils that have an ECe of ≤ 4 dS m−1 to optimize the rate and overall emergence of seedlings. There are likely exceptions to these findings due to the high variability of salt tolerance amongst plant species (e.g. Salicornia europaea; Tamarix aphylla). Proper timing and application of compost are critical to optimizing its benefits while avoiding negative impacts including environmental effects.
Reducing EC in Compost
In general, the composting process reduces the EC associated with the feedstocks (Gao et al. Citation2010; Zhang et al. Citation2016). This decrease is likely due to the release of volatile organic sulfur compounds, the precipitation of mineral salts (ammonia, phosphates, magnesium, and sulfur ions), microbial consumption of salts, and leaching of compost piles during the decomposition process (Chang, Chung, and Tsai Citation2007; Gao et al. Citation2010; Wu et al. Citation2010). Said-Pullicino, Erriquens, and Gigliotti (Citation2007) found that the EC decreased from 7.1 to 5.0 dS m−1 after 250 d of pile composting under aerobic conditions. Despite the reduction of EC due to composting, it is important to consider the proportion of various feedstocks, their chemical properties, and the environmental conditions in which the compost is produced. Zhang et al. (Citation2018) noted that increasing the organics in municipal waste increased the EC of the compost. Therefore, increasing the proportion of feedstock with a higher EC will likely result in a compost with a higher EC. However, increases in compost EC due to the addition of high EC feedstock (e.g. poultry litter) can be countered by adding a bulking agent (e.g. sawdust, corn stalks, rice husks, grass clippings, hay, wood chips) to the compost, increasing the aeration rate during the composting, or both (Guidoni et al. Citation2018; Guo et al. Citation2012; Michel and Reddy Citation1998; Li-Xian et al. Citation2007). The addition of a bulking agent allows for increased airflow throughout the composts and ensures aerobic conditions are present. Other advantages from the addition of a bulking agent include a reduction in gaseous emissions and leachate production (Guidoni et al. Citation2018; Michel and Reddy Citation1998).
Composting is dependent upon temperature and moisture content within the compost. Various studies have suggested that the ideal temperature for composting is between 52 and 60 °C (Liang, Das, and McClendon Citation2003). Temperatures >55 °C reportedly eliminate pathogenic organisms within the compost and are recommended when using feedstocks known for pathogenic contamination such as cow manure (Bernal, Alburquerque, and Moral Citation2009). Moisture is necessary for metabolic and physiological activities of microbial communities and is also a medium that is capable of transporting dissolved nutrients throughout the compost (Liang, Das, and McClendon Citation2003). However, excessive moisture can result in anaerobic conditions, reduce the temperature, and therefore reduce decomposition (Kumar, Ou, and Lin Citation2010). Optimal moisture ranges are 50–70% however, it is dependent upon feedstock nutrients characteristics (particularly the carbon to nitrogen ratio) and aeration rate (Guo et al. Citation2012)
Recently, the incorporation of other materials (e.g. zeolite) into compost and their potential to reduce EC have been investigated (Turan Citation2008). Zeolite is a microporous crystalline composed of hydrated aluminosilicate of alkali and alkaline earth cations. The crystalline structure of the zeolite allows it to readily absorb cations; thus, it has a high CEC (Ramesh and Reddy Citation2011).
Chan, Selvam, and Wong (Citation2016) investigated the potential of zeolite in reducing EC in composted food waste supplemented with Mg and P salts. The addition of Mg and P salts conserved the N levels in the compost by forming struvite (NH4MgPO4 • 6H2O); however, EC5 increased as well. By adding zeolite (10% dry weight), the EC5 was reduced to 2.82 dS m−1 and NH4+ ion adsorption increased, resulting in higher total N content in the final product. Compost supplemented with Mg and P salts not treated with zeolite had an elevated EC5 of 6.45 dS m−1 and compost with neither Mg and P salts nor zeolite treatment also had an elevated EC5 of 3.6 dS m−1 in comparison to the compost treated with zeolite Turan (Citation2008) also found that the addition of zeolite reduced the EC5 of compost derived from poultry litter, a feedstock classically high in salinity. The composted poultry litter EC5 was reduced from 15.7 to 5.24 and dS m−1 by adding 5% to 10% zeolite (v/v), respectively.
Reduction in compost EC can be facilitated by both producers and end consumers. If high EC levels in compost are a concern, leaching compost with good-quality water toproduce a leachate can reduce the salts present therein (Ksheem et al. Citation2015; Pant et al. Citation2012; Qadir and Oster Citation2004). Additionally, thorough irrigation of soils amended with compost with high quality water can help alleviate vegetative salt stress during planting (Qadir and Oster Citation2004).
Leachate is generated when a composter captures the water that has percolated through the compost. Fornes et al. (Citation2010) leached three different composts with an initial EC5 of 8.3, 4.82, and 7.19 dS m−1. After eight leaching events, the EC5 of these composts were reduced to 0.35, 0.35 and 0.90 dS m−1 , respectively. Irrigation, in combination with organic amendments such as compost, can then help leach excess salts in saline or saline-sodic soils deeper into the soil. Pearson et al. (Citation2017, Citation2018) further extended the use of portable X-ray fluorescence for analysis of brackish and metal-laden waters, potentially making it a promising tool for both compost and leachate analysis on-site.
One concern of leaching compost is the loss of N, P, and K in the process. Ksheem et al. (Citation2015) found that most Na was leached in the first few leaching events, while Ca and Mg (divalent; higher affinity on the Lyotropic series) were mostly retained in the compost. Further investigation of how the nutrient composition of various composts change in response to leaching events could be beneficial. Discarding the first few leachates in a way that does not negatively affect the environment through surface and/or groundwater contamination presents a problem. Many composting facilities address this problem by re-applying the leachate to compost or fresh feedstocks to enhance its degradation (G. Kleinhienz, personal communication, April 22, 2019; Ming et al. Citation2008). Other compost users may dilute the leachate and then apply it as a fertilizer or weed control (Ksheem et al. Citation2015; Romero et al. Citation2013).
However, these options may not be appropriate for large composting facilities where an increasing amount of leachate is produced with high nutrient concentrations (e.g. NH4, PO4, and NO3) and a high chemical and biological oxygen demand (Bakhshoodeh et al. Citation2017; Brown et al. Citation2013). Ammonium is of particular concern due to its high toxicity to fish. In addition, a high chemical and biological oxygen demand of a leachate can create anoxic conditions if discarded into a body of water, which can cause fatalities of plants, fish and other aquatic organisms (Randall and Tsui Citation2002).
A common approach to address this problem is to discard the leachate in a constructed wetland (Vymazal Citation2007). Bakhshoodeh et al. (Citation2017) demonstrated that horizontal flow constructed wetlands planted with vetiver grass (Vetiveria zizanioides) can significantly reduce the electrical conductivity, biological and chemical oxygen demand, and concentration of nutrients such as nitrate and ammonia present in the leachate. This approach is a low-cost solution because it does not require extensive maintenance or high amounts of energy (Brown et al. Citation2013). However, this approach may not be as efficient as others and the constructed wetlands may require large amounts of space (Brown et al. Citation2013).
Roy et al. (Citation2018) reviewed the efficacy and applicability of biofilters, anaerobic bioreactors, membrane bioreactors, electro-coagulation/flotation, filtration and advanced oxidation processes to treat leachates. Roy et al. (Citation2018) concluded that the most efficient and cost-efficient treatments are biological treatments including membrane bioreactors and filtration. The downfall to membrane bioreactors and filtration processes such as reverse osmosis is that the filters become fouled and need to be replaced. Despite advances, further investigation and evaluation of these methods and various combinations of pre- and post-treatments are needed. Roy et al. (Citation2018) also suggested that one of the more practical and sustainable solutions in treating leachate would result in a product usable as fertilizer or weed control.
Management Implications
One of the biggest hindrances to thoroughly understanding the relationships between compost, EC, soil, and vegetative growth is the use of various EC methods across the literature. Measurement of EC values for soils and composts should be standardized so that EC measurements can be directly compared across the literature (McLachlan et al. Citation2004; Reddy and Crohn Citation2012). Such standardization could be incorporated into Test Method for the Examination of Composting and Compost (TMECC) and align with the Seal of Testing Assurance (STA) guidelines for laboratories conducting compost analysis. Also, integration of new methodologies and guidelines for the use of new technologies such as portable X-ray fluorescence into TMECC and STA will enable compost laboratories and producers to investigate compost salinity, CEC, heavy metals, and other fundamental chemistry parameters rapidly and in-situ.
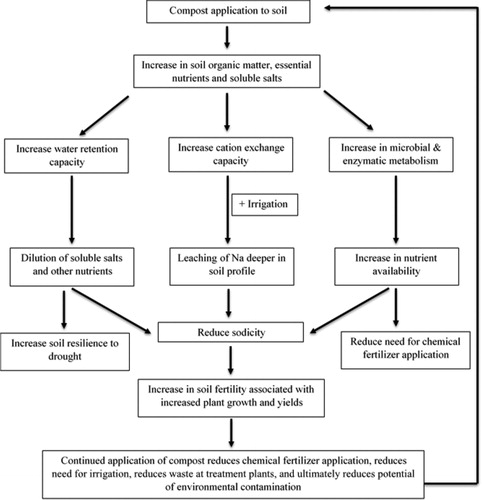
Despite inconsistent testing methods, the literature on soluble salts in compost suggests that composts should not be limited to an EC5 value of ≤5 dS m−1 (Chang, Chung, and Tsai Citation2007, Herrara et al. Citation2008; Reddy and Crohn Citation2012; Walker and Bernal Citation2008). Composts with a high EC5 can serve as an effective soil amendment that provides essential nutrients to plants and enriches soil health. Also, high quality composts with high EC5 not associated with Na2+ or Cl− can be used to remediate saline-sodic soils and return them to a more fertile state; remediating saline-sodic soils to a fertile state is of the utmost importance in regions suffering from desertification and increasing salinization (). The production of compost with high EC5 values should also relieve overtaxed waste processing facilities and reduce the potential of these wastes polluting the environment. The benefits from compost application to soils, particularly saline-sodic soils, and the externalized benefits to society are illustrated in .
Figure 4. Cumulative of effects from compost application to soils, particularly soils affected by salinization. Adapted from Lal (Citation2006).
The negative effects of high concentrations of Na+ and Cl− have been well documented (Liang et al. Citation2018; Mudgal, Madaan, and Mudgal Citation2010). Thus, limiting their concentration within compost is essential. Including an Ag Index on composts with an EC5 value >5 dS m−1 will be advantageous to the consumer and for developing best application practices. By doing so, the United States Composting Council and other composting organizations can decouple low-EC5 composts from the concerns associated with high-EC5 composts and restore confidence in the benign properties of low-EC5 composts. Educating farmers and compost users about the Ag Index and the benefits associated with appropriately applying composts with a high EC5 will likely prove advantageous to increasing the amount of composts applied. Proper labeling of high EC5 compost should be encouraged or potentially standardized in an attempt to educate agriculturists on the negative effects of high EC5 compost if improperly applied.
By labeling composts with an EC5 of ≥5 dS m−1 with an Ag Index, the industry acknowledges that these composts should be managed in a similar (yet non-regulated) manner as inorganic fertilizers. The International Plant Nutrition Institute states in their Plant Nutrition Manual (2012) that fertilization needs to follow the “Four R’s,”: the right nutrient, at the right rate, at the right time, and in the right place for the selected crop. With these guidelines in mind, the creation of best-use protocols will need to be developed to ensure that composts are properly applied and managed. These best-use protocols will need to be applicable to various soils and must consider access to high quality irrigation water and crop tolerance of salinity and/or sodicity. Some progress has been made in these areas by Reddy and Crohn (Citation2012), who developed a prediction method to estimate how the application of compost will affect soil ECe. The expansion of these predictive models to other soils will prove beneficial to those concerned with plant growth and yield and soil remediation.
The application and use of compost, and predictive models coupled with new technologies will become useful tools to address the increasing amount of saline-sodic soils worldwide in a timely and sustainable manner. Remediating saline-sodic soil and enhancing soil fertility hold large promise for human health by enhancing food security. Other methods to address these problems such as cultivating plants with higher salt tolerances have been met with limited success due to the genetic complexity of salt tolerant genes (Flowers Citation2004) and aversion by some to genetic modification (Dale Citation1999). Genes that enable plants to tolerate higher salt concentrations have been identified; however, there is much work to be done before genetic engineering can transfer these genes into crops so that they will be salt tolerant and still produce high yields (Deinlein et al. Citation2014). Therefore, enhancing and restoring soils to a more fertile state appears to be a viable option and can be achieved by the proper application of quality composts.
The overall feedstocks used in production of compost significantly impact the physicochemical properties of compost (Zhang et al. Citation2018). Compost producers should also consider the environmental variables present during composting (Guo et al. Citation2012; Kumar, Ou, and Lin Citation2010; Michel and Reddy Citation1998). Careful consideration of the feedstocks used and optimization of the environmental variables involved will enhance the production of quality compost. The primary function of composting is to decompose complex organic molecules while recycling nutrients present in the waste and provide a value-added end use for organic waste streams. Therefore, optimizing these variables so that larger quantities of waste products can be incorporated into the compost is a high priority.
The addition of a bulking agent, zeolite, or creating a leachate are effective strategies at reducing the EC of composts and should be considered when attempting to produce a compost with a specific EC5 (Chan, Selvam, and Wong Citation2016; Fornes et al. Citation2010). Additionally, numerous studies have shown that the addition of zeolite to compost made with municipal organic waste decreased the concentration of heavy metals present in the final compost (Kosobucki, Kruk, and Buszewski Citation2008; Turan and Ergun Citation2008; Zorpas et al. Citation2000). Zeolite is able to reduce the salinity and heavy metals during composting due to its high cation exchange capacity, yet its effectiveness is limited at low pH (Chan, Selvam, and Wong Citation2016). Metals transform into an inert form when they are retained with the crystalline structure of the zeolite. Thus, incorporating zeolite into compost appears to be beneficial by means of reducing the EC and the potential for metal contamination. Further study on the long-term effects of applying compost with zeolite to fields is needed to better elucidate temporal and spatial changes in nutrient and metal concentrations (Ramesh and Reddy Citation2011).
The production of compost is a natural, sustainable, and viable method to reduce waste disposal while producing a value-added product. However, it is a biological process that relies on proper conditions for the process to work correctly. Improper composting operations can lead to the production of noxious odors and gaseous emissions and decrease the public perception of the process. It is imperative that operators are educated and kept informed on contemporary best practices in order to achieve compost production success and end user satisfaction. Additionally, compost producers need to be able to educate end-users on the proper application of their product, as well as the limitations to ensure proper usage and end user satisfaction. Ultimately, end user satisfaction will drive demand, advance compost research and development, and encourage compost operation expansion.
Conclusions
Determining whether or not soluble salts present in compost will be disadvantageous or beneficial to applied soils and plant growth requires an understanding of which soluble salts are present. The disproportionate presence of Na+ and Cl− in soils and compost degrades agronomic production. However, the presence of other soluble salts such as K+, Ca2+, SO42+, CO32-, and NO3− promote soil health and vegetative growth. Therefore, limiting the presence of Na+ and Cl− and/or documenting their presence in compost, via an Ag Index, would benefit compost producers and end users. End users should also consider water availability and crop salt tolerance when selecting a compost for application.
Compost producers should carefully monitor the production methods and feedstocks to ensure the quality of their product and limit the presence of NaCl. Progress within compost science would be facilitated by the standardization of methods to determine the EC of compost and soils. This standardization would ensure that results are directly comparable across the literature and would alleviate confusion surrounding the different dilutions used to determine EC.
The application of compost to saline-sodic soil is a viable method to decrease the soils EC and SAR while simultaneously increase nutrient availability, organic matter, and improve soil physical characteristics. Compost characterized by a high EC should be properly mixed with soils or other medias to ensure that it does not cause salt stress to the plants grown therein. When done properly, the application of composts with high EC can enhance vegetative growth and plant yields to the same degree or better than inorganic fertilizers. Thus, compost application can increase the efficiency of nutrients used in agricultural and municipal systems and reduce the need to apply inorganic chemical fertilizers. Adoption and use of new technologies (e.g. EMI and PXRF) can help determine areas that are in need of remediation and may also curb the excessive use of inorganic fertilizers. Overall, the potential of compost to alleviate problems regarding organic recycling and reuse, saline-sodic soils, and environmental pollution by recycling nutrients back into the soil and agricultural systems is promising. The proper application of composts will increase plants yields, restore soil health and encourage farmers to use more sustainable farming practices.
Acknowledgments
The authors gratefully acknowledge the contributions of the Compost Council Research and Education Foundation (CCREF) and the BL Allen Endowment in Pedology at Texas Tech University in conducting this review. The authors would also like to thank the staff at the Environmental Research and Innovation Center (ERIC) at UW Oshkosh for sharing guidance and experience in the production and testing of compost.
References
- Association of American Plant Food Control Officials. 2017. AAPFCO product label guide. Accessedd March 18, 2019. http://www.aapfco.org/pdf/product_label_guide.pdf.
- Ahel, M., N. Mikac, B. Cosovic, E. Prohic, and V. Soukup. 1998. The impact of contamination from a municipal solid waste landfill (Zagreb, Croatia) on underlying soil. Water Science and Technology 37 (8):203–10. doi: 10.2166/wst.1998.0326.
- Asses, N., A. Farhat, S. Cherif, M. Hamdi, and H. Bouallagui. 2018. Comparative study of sewage sludge co-composting with olive mill wastes or green residues: Process monitoring and agriculture value of the resulting composts. Process Safety and Environmental Protection 114:25–35. doi: 10.1016/j.psep.2017.12.006.
- Bakhshoodeh, R., N. Alavi, M. Majlesi, and P. Paydary. 2017. Compost leachate treatment by a pilot-scale subsurface horizontal flow constructed wetland. Ecological Engineering 105:7–14. doi: 10.1016/j.ecoleng.2017.04.058.
- Bernal, M. P., J. A. Alburquerque, and R. Moral. 2009. Composting of animal manures and chemical criteria for compost maturity assessment. A Review. Bioresource Technology 100 (22):5444–53. doi: 10.1016/j.biortech.2008.11.027.
- Bernstein, L. 1975. Effects of salinity and sodicity on plant growth. Annual Review of Phytopathology 13 (1):295–312. doi: 10.1146/annurev.py.13.090175.001455.
- Birchall, S., C. Dillon, and R. Wrigley. 2008. Effluent and manure management database for the Australian dairy industry. Accessed March 19, 2019. http://www.dairyingfortomorrow.com.au/wp-content/uploads/salinity.pdf.
- Brown, K., A. J. Ghoshdastidar, J. Hanmore, J. Frazee, and A. Z. Tong. 2013. Membrane bioreactor technology: A novel approach to the treatment of compost leachate. Waste Management (New York, N.Y.) 33 (11):2188–94. doi: 10.1016/j.wasman.2013.04.006.
- Cai, H., T. Chen, H. Liu, D. Gao, G. Zheng, and J. Zhang. 2010. The effect of salinity and porosity of sewage sludge compost on the growth of vegetable seedlings. Scientia Horticulturae 124 (3):381–6. doi: 10.1016/j.scienta.2010.01.009.
- Cai, H., and D. Gao. 2011. Phytotoxicity of salts in composted sewage sludge and correlation with sodium chloride, calcium nitrate, and magnesium nitrate. Journal of Plant Nutrition 34 (12):1788–96. doi: 10.1080/01904167.2011.600406.
- Chan, M. T., A. Selvam, and J. W. Wong. 2016. Reducing nitrogen loss and salinity during ‘struvite’ food waste composting by zeolite amendment. Bioresource Technology 200:838–44. doi: 10.1016/j.biortech.2015.10.093.
- Chang, E., R. Chung, and Y. Tsai. 2007. Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population. Soil Science and Plant Nutrition 53 (2):132–40. doi: 10.1111/j.1747-0765.2007.00122.x.
- Chong, C. 2000. Relationship of soluble salts content in MSW compost media and rooting of evergreen cuttings. Compost Science & Utilization 8 (1):29–35. doi: 10.1080/1065657X.2000.10701747.
- Colorado State University. 2015. Salts: Soils and compost. Accessed March 26, 2019. http://www.extsoilcrop.colostate.edu/Soils/powerpoint/compost/soluble_salts.pdf.
- Crohn, D. M. 2016. Assessing compost quality for agriculture. doi:10.3733/ucanr.8514. Retrieved from https://escholarship.org/uc/item/4v1576f8
- Dale, P. J. 1999. Public concerns over transgenic crops. Genome Research 9 (12):1159–62. doi: 10.1101/gr.9.12.1159.
- Deinlein, U., A. B. Stephan, T. Horie, W. Luo, G. Xu, and J. I. Schroeder. 2014. Plant salt-tolerance mechanisms. Trends in Plant Science 19 (6):371–8. doi: 10.1016/j.tplants.2014.02.001.
- Diacono, M., and F. Montemurro. 2010. Long-term effects of organic amendments on soil fertility. A review. Agronomy for Sustainable Development 30 (2):401–22. doi: 10.1051/agro/2009040.
- Doolittle, J. A., and E. C. Brevik. 2014. The use of electromagnetic induction techniques in soils studies. Geoderma 223–225:33–45. doi: 10.1016/j.geoderma.2014.01.027.
- Düring, R., and S. Gäth. 2002. Utilization of municipal organic wastes in agriculture: Where do we stand, where will we go? Journal of Plant Nutrition and Soil Science 165 (4):544–56. doi: 10.1002/1522-2624(200208)165:4<544::AID-JPLN544>3.0.CO;2-#.Q
- Flowers, T. J. 2004. Improving crop salt tolerances. Journal of Experimental Botany 55 (396):307–19. doi: 10.1093/jxb/erh003.
- Fornes, F., C. Carrion, R. Garcia-de-la-Fuente, R. Puchades, and M. Abad. 2010. Leaching composted lignocellulosic wastes to prepare container media: Feasibility and environmental concerns. Journal of Environmental Management 91 (8):1747–55. doi: 10.1016/j.jenvman.2010.03.017.
- Gao, M., F. Liang, A. Yu, B. Li, and L. Yang. 2010. Evaluation of stability and maturity during forced-aeration composting of chicken manure and sawdust at different C/N ratios. Chemosphere 78 (5):614–9. doi: 10.1016/j.chemosphere.2009.10.056.
- Gomez, A. 1998. The evaluation of compost quality. TrAC Trends in Analytical Chemistry 17 (5):310–4. doi: 10.1016/S0165-9936(98)00013-2.
- Grattan, S. R., and C. M. Grieve. 1998. Salinity–mineral nutrient relations in horticultural crops. Scientia Horticulturae 78 (1–4):127–57. doi: 10.1016/S0304-4238(98)00192-7.
- Grigatti, M., L. Cavani, and C. Ciàvatta. 2011. The evaluation of stability during the composting of different starting materials: Comparison of chemical and biological parameters. Chemosphere 83 (1):41–8. doi: 10.1016/j.chemosphere.2011.01.010.
- Guidoni, L. L. C., R. V. Marques, R. B. Moncks, F. T. Botelho, M. F. da Paz, L. B. Corrêa, and É. K. Corrêa. 2018. Home composting using different ratios of bulking agent to food waste. Journal of Environmental Management 207:141–50. doi: 10.1016/j.jenvman.2017.11.031.
- Guo, R., G. Li, T. Jiang, F. Schuchardt, T. Chen, Y. Zhao, and Y. Shen. 2012. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresource Technology 112:171–8. doi: 10.1016/j.biortech.2012.02.099.
- Hao, X., and C. Chang. 2003. Does long-term heavy cattle manure application increase salinity of a clay loam soil in semi-arid southern Alberta?. Agriculture, Ecosystems & Environment 94 (1):89–103. doi: 10.1016/S0167-8809(02)00008-7.
- Herrara, F., J. E. Castillo, A. F. Chica, and L. L. Bellido. 2008. Use of municipal solid waste compost (MSWC) as a growing medium in the nursery production of tomato plants. Bioresource Technology 99 (2):287–96.
- International Plant Nutrition Institute 4R Plant Nutrition Manual. 2012. International Plant Nutrition Institute, Norcross, GA, USA. Accessed August 9, 2019. http://www.ipni.net/4r.
- Ksheem, A. M., J. M. Bennett, D. L. Antille, and S. R. Raine. 2015. Towards a method for optimized extraction of soluble nutrients from fresh and composted chicken manures. Waste Management 45:76–90. doi: 10.1016/j.wasman.2015.02.011.
- Kosobucki, P., M. Kruk, and B. Buszewski. 2008. Immobilization of selected heavy metals in sewage sludge by natural zeolites. Bioresource Technology 99 (13):5972–6. doi: 10.1016/j.biortech.2007.10.023.
- Kumar, S. A., A. V. Gaikwad, P. S. Shekdar, P. S. Kshirsagar, and R. N. Signh. 2004. Estimation method of national methane emission from solid waste landfills. Atmospheric Environment 21:3481–7.
- Kumar, M., Y. Ou, and J. Lin. 2010. Co-composting of green waste and food waste at low C/N ratio. Waste Management (New York, N.Y.) 30 (4):602–9. doi: 10.1016/j.wasman.2009.11.023.
- Lal, R. 2006. Enhancing crop yields in the developing countries through restoration of the soil organic carbon pool in agricultural lands. Land Degradation & Development 17 (2):197–209. doi: 10.1002/ldr.696.
- Lakhdar, A., M. Rabhi, T. Ghnaya, F. Montemurro, N. Jedidi, and C. Abdelly. 2009. Effectiveness of compost use in salt-affected soil. Journal of Hazardous Materials 171 (1–3):29–37. doi: 10.1016/j.jhazmat.2009.05.132.
- Liang, C., K. C. Das, and R. W. McClendon. 2003. The influence of temperature and moisture contents regimes on the aerobic microbial activity of a biosolids composting blend. Bioresource Technology 86 (2):131–7. doi: 10.1016/S0960-8524(02)00153-0.
- Liang, W., X. Ma, P. Wan, and L. Liu. 2018. Plant salt-tolerance mechanism: A review. Biochemical and Biophysical Research Communications 495 (1):286–91. doi: 10.1016/j.bbrc.2017.11.043.
- Li-Xian, Y., L. Guo-Liang, T. Shi-Hua, S. Gavin, and H. Zhao-Huan. 2007. Salinity of animal manure and potential risk of secondary soil salinization through successive manure application. The Science of the Total Environment 383 (1-3):106–14. doi: 10.1016/j.scitotenv.2007.05.027.
- Mahmood, S. 1982. Effect of soil reclamation treatments on available micronutrients (Cu, Zn, Mn, and Fe) of Kurrianwala soil series. M.Sc. thesis., Department of Soil Science, University of Agriculture, Faisalabad, Pakistan.
- Mahmoodabadi, M., N. Yazdanpanah, L. R. Sinobas, E. Pazira, and A. Neshat. 2013. Reclamation of calcareous saline sodic soil with different amendments (I): Redistribution of soluble cations within the soil profile. Agricultural Water Management 120:30–8. doi: 10.1016/j.agwat.2012.08.018.
- Mass, E. V., and G. R. Grattan. 1999. Crop yields as affected by salinity. In Agricultural drainage monograph No. 38, eds. R.W. Skaggs, J. van Schilfgaarde, 55–108.Madison, WI: ASA-CSSA-SSSA.
- McLachlan, K. L., C. Chong, P. R. Voroney, H. Liu, and B. E. Holbein. 2004. Variability of soluble salts using different extraction methods on composts and other substrates. Compost Science & Utilization 12 (2):180–4.
- Michel, F. C., and C. A. Jr., Reddy. 1998. Effect of oxygenation level on yard trimmings composting rate, odor production, and compost quality in bench-scale reactors. Compost Science & Utilization 6 (4):6–14. doi: 10.1080/1065657X.1998.10701936.
- Miller, D. M., and W. P. Miller. 2000. Ch. 9. Land application of wastes. In Handbook of soil science, ed. M. Sumner. New York: CRC Press.
- Miller, J. J., B. W. Beasley, and C. F. Drury. 2013. Transport of residual soluble salts and total sulfur through intact soil cores amended with fresh or composted beef cattle feedlot manure for nine years. Compost Science & Utilization 21 (2):99–109. doi: 10.1080/1065657X.2013.836067.
- Ming, L., P. Xuya, Z. Youcai, D. Wenchuan, C. Huashuai, L. Guotao, and W. Zhengsong. 2008. Microbial inoculum with leachate recirculated cultivation for the enhancement of OFMSW composting. Journal of Hazardous Wastes 153 (1–2):885–91.
- Mor, S., K. Ravindra, R. P. Dahiya, and A. Chandra. 2006. Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environmental Monitoring and Assessment 118 (1–3):435–56. doi: 10.1007/s10661-006-1505-7.
- Mudgal, V., N. Madaan, and A. Mudgal. 2010. Biochemical mechanisms of salt tolerance in plants: A review. International Journal of Botany 6 (2):136–43. doi: 10.3923/ijb.2010.136.143.
- Naidu, R., and P. Rengasamy. 1993. Ion interactions and constraints to plant nutrition in Australian sodic soils. Soil Research 31 (6):801–19. doi: 10.1071/SR9930801.
- Natural Resources Conservation Service. 2007. Manure chemistry–nitrogen, phosphorus and carbon. Manure Management Information Sheet 7. http://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_043440.pdf (verified 9 Aug. 2019).
- Nelson, D. W., and L. E. Sommers. 1996. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis—Part 3. Chemical Methods. Soil Science Society of America, Madison, WI.
- Pant, A. P., T. J. K. Radovich, N. V. Hue, and R. E. Paull. 2012. Biochemical properties of compost tea associated with compost quality and effects on pak choi growth. Scientia Horticulturae 148:138–46. doi: 10.1016/j.scienta.2012.09.019.
- Parihar, P., S. Singh, R. Singh, V. P. Singh, and S. M. Prasad. 2015. Effect of salinity stress on plants and its tolerance strategies: a review. Environmental Science and Pollution Research International 22 (6):4056–75. doi: 10.1007/s11356-014-3739-1.
- Pearson, D., S. Chakraborty, B. Duda, B. Li, D. C. Weindorf, S. Deb, E. C. Brevik, and D. P. Ray. 2017. Water analysis via portable X-ray fluorescence spectrometry. Journal of Hydrology 544:172–9. doi: 10.1016/j.jhydrol.2016.11.018.
- Pearson, D., D. C. Weindorf, S. Chakraborty, B. Li, J. Koch, P. Van Deventer, J. de Wet, and N. Yaw Kusi. 2018. Analysis of metal-laden water via portable X-ray fluorescence spectrometry. Journal of Hydrology 561:267–76. doi: 10.1016/j.jhydrol.2018.04.014.
- Pennsylvania State University. 2017. Compost analysis report. Agriculture Analytical Services Laboratory. Accessed March 26, 2019. http://blackgoldcompost.net/PDF/CompostAnalysis_04042017.pdf.
- Qadir, M., R. H. Qureshi, and N. Ahmad. 1997. Nutrient availability in a calcareous saline-sodic soil during vegetative bioremediation. Arid Soil Research and Rehabilitation 11 (4):343–52. doi: 10.1080/15324989709381487.
- Qadir, M., and J. D. Oster. 2004. Crop and irrigation management strategies for saline-sodic soils and waters aimed at environmentally sustainable agriculture. The Science of the Total Environment 323 (1–3):1–19. doi: 10.1016/j.scitotenv.2003.10.012.
- Qadir, M., and S. Schubert. 2002. Degradation processes and nutrient constraints in sodic soils. Land Degradation & Development 13 (4):275–2002. doi: 10.1002/ldr.504.
- Ramesh, K., and D. D. Reddy. 2011. Zeolites and their potential uses in agriculture. Advances in Agronomy 113:219–41.
- Randall, D. J., and T. K. N. Tsui. 2002. Ammonia toxicity in fish. Marine Pollution Bulletin 45 (1–12):17–23.
- Rawal, A., S. Chakraborty, B. Li, K. Lewis, M. Godoy, L. Paulette, and D. C. Weindorf. 2019. Determination of base saturation percentage in agricultural soils via portable X-ray fluorescence spectrometer. Geoderma 338:375–82. doi: 10.1016/j.geoderma.2018.12.032.
- Reddy, N., and D. M. Crohn. 2012. Compost induced soil salinity: A new prediction method and its effect on plant growth. Compost Science & Utilization 20 (3):133–40. doi: 10.1080/1065657X.2012.10737038.
- Rhodes, J. D. 1996. Salinity: Electrical conductivity and total dissolved solids. In Methods of soil analysis: Part 3 Chemical methods, eds. D.L. Sparks. Madison, WI: Soil Science Society of America.
- Romero, C., P. Ramos, C. Costa, and C. Màrquez. 2013. Raw and digested municipal waste compost leachate as potential fertilizer: Comparison with a commercial fertilizer. Journal of Cleaner Production 59 (15):73–8. doi: 10.1016/j.jclepro.2013.06.044.
- Roy, D., A. Azaïs, S. Benkaraache, P. Drogui, and R. D. Tyagi. 2018. Composting leachate: Characterization, treatment and future perspectives. Reviews in Environmental Science and Biotechnology. doi: 10.1007/s11157-018-9462-5.
- Said-Pullicino, D., F. G. Erriquens, and G. Gigliotti. 2007. Changes in the chemical characteristics of water-extractable organic matter during composting and their influence on compost stability and maturity. Bioresource Technology 98 (9):1822–31. doi: 10.1016/j.biortech.2006.06.018.
- Sharma, A., D. C. Weindorf, D. D. Wang, and S. Chakraborty. 2015. Characterizing soils via portable X-ray fluorescence spectrometer: 4. Cation exchange capacity (CEC). Geoderma 239–240:130–4. doi: 10.1016/j.geoderma.2014.10.001.
- Sharpley, A. N., J. J. Meisinger, J. F. Power, 1992. and, and D. L. Suarez. Root extraction of nutrients associated with long-term soil management. In Advances in soil science, ed. B. Stewart, vol. 19, 151–217.
- Soil Survey Staff. 2019. Salinity in agriculture. Accessed March 19, 2019. http://www.nrcs.usda.gov/wps/portal/nrcs/detailfull/national/water/quality/tr/?cid=nrcs143_010914.
- Soltanpour, P. N., G. W. Johnson, S. M. Workman, J. B. Jones, and R. O. Miller. 1996. Inductively coupled plasma emission spectrometry and inductively coupled plasma-mass spectrometry. In Methods of Soil Analysis—Part 3. Chemical Methods. Madison, WI: Soil Science Society of America.
- Sonmez, S., D. Buyuktas, F. Okturen, and S. Citak. 2008. Assessment of different soil to water ratios (1:1, 1:2.5, 1:5) in soil salinity studies. Geoderma 144 (1–2):361–9. doi: 10.1016/j.geoderma.2007.12.005.
- Swarup, A. 1986. Effect of gypsum, pyrites, farmyard manure and rice husk on the availability of zinc and phosphorus to rice in submerged sodic soil. Journal of Indian Society of Soil Science 34:844–8.
- Tazeh, E. S., E. Pazira, M. R. Neyshabouri, F. Abbasi, and H. Z. Abyaneh. 2013. Effects of two organic amendments on EC, SAR, and soluble ions concentration in a saline-sodic soil. International Journal of Biosciences 3 (9):55–68.
- Tejada, M., C. Garcia, J. L. Gonzalez, and M. T. Hernandez. 2006. Use of organic amendment as a strategy for saline soil remediation: Influence on the physical, chemical and biological properties of soil. Soil Biology and Biochemistry 38 (6):1413–21. doi: 10.1016/j.soilbio.2005.10.017.
- Thompson, W., P. Leege, P. Milner, 2001. and, and M. Watson. Test methods for the examination of composting and compost. In The US composting council, US government printing office, ed. J. Smith. Bethesda, MD: U.S. Composting Council.
- Turan, N. G. 2008. The effects of natural zeolite on salinity level of poultry litter compost. Bioresource Technology 99 (7):2097–101. doi: 10.1016/j.biortech.2007.11.061.
- Turan, N. G., and O. N. Ergun. 2008. Improving the quality of municipal solid waste compost by using expanded perlite and natural zeolite. CLEAN—Soil, Air, Water 36 (3):330–4. doi: 10.1002/clen.200700135.
- United Nations Food and Agriculture Organization. 2019. Management of salt affected soils. Accessed March 19, 2019. http://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/salt-affected-soils/en/.
- United States Composting Council (USCC). 2001. Field guide to compost use. Accessed March 26, 2019. http://content/plugins/wppdfupload/pdf/1330/Field_Guide_to_Compost_Use.pdf.
- United States Composting Council (USCC). 2010. Test methods and parameters. Accessed March 26, 2019. compostingcouncil.org/test-methods-parameters/.
- United States Salinity Laboratory Staff. 1954. Diagnosis and improvement of saline and alkali soils. Agricultural Handbook No. 60. Accessed March 19, 2019. http://www.ars.usda.gov/ARSUserFiles/20360500/hb60_pdf/hb60complete.pdf.
- Vymazal, J. 2007. Removal of nutrients in various types of constructed wetlands. The Science of the Total Environment 380 (1–3):48–65. doi: 10.1016/j.scitotenv.2006.09.014.
- Walker, D. J., and M. P. Bernal. 2008. The effects of olive mill waste compost and poultry manure on the availability and plant uptake of nutrients in a highly saline soil. Bioresource Technology 99 (2):396–403. doi: 10.1016/j.biortech.2006.12.006.
- Weil, R. R., and N. C. Brady. 2017. The nature and properties of soils. 15th ed. New York: Pearson.
- Weindorf, D. C., R. Zartman, and B. L. Allen. 2006. Effect of compost on soil properties in Dallas, Texas. Compost Science & Utilization 14 (1):59–67. doi: 10.1080/1065657X.2006.10702264.
- Weindorf, D. C., S. Chakraborty, B. Li, S. Deb, A. Singh, and N. Y. Kusi. 2018. Compost salinity assessment via Portable X-ray fluorescence (PXRF) spectrometry. Waste Management 78:158–63. doi: 10.1016/j.wasman.2018.05.044.
- Wright, R. J., and T. Stuczynski. 1996. Atomic absorption and flame emission spectrometry. In Methods of soil analysis—Part 3. Chemical methods. Madison, WI: Soil Science Society of America.
- Wu, L., L. Q. Ma, and G. A. Martinez. 2000. Comparison of methods for evaluating stability and maturity of biosolids compost. Journal of Environmental Quality 29 (2):424–9. doi: 10.2134/jeq2000.00472425002900020008x.
- Wu, T., X. Wang, D. Li, and Z. Yi. 2010. Emission of volatile organic sulfur compounds (VOSCs) during aerobic decomposition of food wastes. Atmospheric Environment 44 (39):5065–71. doi: 10.1016/j.atmosenv.2010.09.019.
- Yadav, K. D., V. Tare, and M. M. Ahammed. 2010. Vermicomposting of source-separated human faeces for nutrient recycling. Waste Management (New York, N.Y.) 30 (1):50–6. doi: 10.1016/j.wasman.2009.09.034.
- Zhang, S., Z. Chen, Q. Wen, L. Yang, W. Wang, and J. Zheng. 2016. Effectiveness of bulking agents for co-composting penicillin mycelial dreg (PMD) and sewage sludge in pilot-scale system. Environmental Science and Pollution Research International 23 (2):1362–70. doi: 10.1007/s11356-015-5357-y.
- Zhang, D., W. Luo, Y. Li, G. Wang, and G. Li. 2018. Performance of co-composting sewage sludge and organic fraction of municipal solid waste at different proportions. Bioresource Technology 250:853–69. doi: 10.1016/j.biortech.2017.08.136.
- Zorpas, A. A., T. Constantinides, A. G. Vlyssides, I. Haralambous, and M. Loizidou. 2000. Heavy metal uptake by natural zeolite and metals partitioning in sewage sludge compost. Bioresource Technology 72 (2):113–9. doi: 10.1016/S0960-8524(99)00110-8.
