ABSTRACT
Background
Cervical spine mobilizations may differentially modulate both components of the stress response, consisting of the autonomic nervous system and hypothalamic pituitary adrenal-axis, depending on whether the target location is the upper or lower cervical spine. To date, no study has investigated this.
Methods
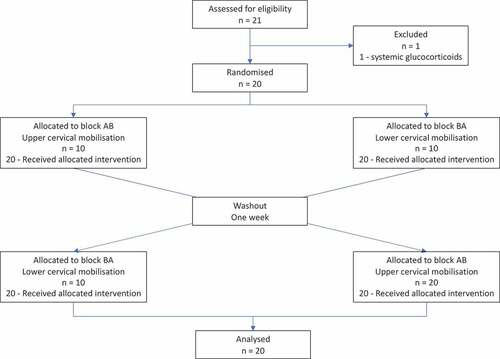
A randomized, crossover trial investigated the effects of upper versus lower cervical mobilization on both components of the stress response simultaneously. The primary outcome was salivary cortisol (sCOR) concentration. The secondary outcome was heart rate variability measured with a smartphone application. Twenty healthy males, aged 21–35, were included. Participants were randomly assigned to block-AB (upper then lower cervical mobilization, n = 10) or block-BA (lower than upper cervical mobilization, n = 10), separated by a one-week washout period. All interventions were performed in the same room (University clinic) under controlled conditions. Statistical analyses were performed with a Friedman’s Two-Way ANOVA and Wilcoxon Signed Rank Test.
Results
Within groups, sCOR concentration reduced thirty-minutes following lower cervical mobilization (p = 0.049). Between groups, sCOR concentration was different at thirty-minutes following the intervention (p = 0.018).
Conclusion
There was a statistically significant reduction in sCOR concentration following lower cervical spine mobilization, and between-group difference, 30 min following the intervention. This indicates that mobilizations applied to separate target locations within the cervical spine can differentially modulate the stress response.
Introduction
The body’s stress response has both central and peripheral components [Citation1–3]. The peripheral components consist of two interrelated systems: the autonomic nervous system (ANS) and the hypothalamic pituitary adrenal-axis (HPA-axis) [Citation1–3]. For the purpose of this study, ‘stress response’, refers to the peripheral components. Once exposed to a stressor, be that physical, physiological, or psychological, homeostasis of the human body is threatened, and the stress response functions to return the body to equilibrium [Citation4]. The stress response initially activates the fast acting, but short lasting ANS response, which then communicates with the HPA-axis for the slower acting but longer lasting endocrine response [Citation1,Citation4–6]. Communication between the ANS and HPA-axis is interchangeable and bidirectional [Citation7]. Consequently, the stress response either heightens or dampens its response [Citation1,Citation4–6]. A heightened response may present with an increased heart rate and hyperarousal, whereas a dampened response may present with a reduced heart rate and hypoarousal [Citation8].
Manual therapy is a common treatment modality used in the physiotherapy, osteopathy, and chiropractic professions. Despite demonstrating clinical efficacy and effectiveness [Citation9–11], the underlying mechanisms of spinal manual therapy are not fully understood [Citation12,Citation13]. One hypothesis is that manual therapy may exert effects on the ‘stress axis’ or ‘stress response’ [Citation14]. Manual therapy applied to the cervical spine has been shown to modulate isolated components of the stress response; either the ANS [Citation15–22] or the HPA-axis [Citation23,Citation24]. However, manual therapy targeting the thoracic spine [Citation25,Citation26] or craniosacral region [Citation27] has been shown to modulate both components of the stress response in unison. Evidence also suggests that manual therapy applied to distinct anatomical levels within the cervical spine can elicit a differential ANS [Citation28–30] or HPA-axis [Citation24] reaction. Currently, no study has investigated the physiological effects of cervical spine mobilization on both components of the stress response simultaneously, depending on the segments targeted.
A commonly used way to measure the HPA-axis response is by measuring cortisol, a stress hormone released from the adrenal cortex [Citation31]. Salivary cortisol (sCOR) measures represent a noninvasive, convenient, and reliable way to measure HPA-axis activity [Citation32]. HRV is a proxy measure of the cardiac ANS, more specifically the parasympathetic nervous system (PSNS) modulation on the heart, otherwise known as vagal tone [Citation33–35]. Root mean squared of successive differences of RR intervals (rMSSD) is the chosen HRV metric for this study as it has been shown to be a reliable indicator of PSNS activity [Citation36].
The aim of this work was to explore the physiological effects of mobilization to the upper versus lower cervical spine in healthy males, by comparing: (1) HPA-axis response measured with sCOR, and (2) the ANS response measured with HRV. This study will provide a deeper understanding of the mechanisms of cervical spine mobilization and has important implications for clinicians, whom may be able to selectively modulate the stress response depending on their target location within the cervical spine.
Methods
Study design
A randomized, crossover, repeated measures trial was conducted to evaluate the time-course effects of upper versus lower cervical mobilization on sCOR concentration and HRV. A crossover design was selected to minimize potential confounding by individuals’ specific covariates as each individual acts as their own control [Citation37]. The study was reported in accordance with the CONSORT 2010 statement: extension to randomized crossover trials [Citation37] (Appendix 1), and TIDieR checklist (Appendix 2). Ethical approval was obtained from the University of Otago Human Ethics Committee (H21/086). A protocol was registered a priori with Australia New Zealand Clinical Trials Registry (ACTRN12621001035819). All participants signed informed consent prior to enrollment in the study.
Participants
Healthy males, aged between 21 and 35 were recruited from the local community. The age range was selected due to maturation of the ANS before the age of 20 [Citation38], and decline of the endocrine system after the age of 35 [Citation39]. Participants were required to have a smart phone. Participants were excluded if they were taking systemic glucocorticoid or cardioactive medication; had an endocrine, central nervous system, cardiovascular, mental health, co-morbid musculoskeletal, or history of serious pathologic or psychiatric disorder. Participants were also excluded if they had any contraindications, and on a case-by-case basis if they had any precautions, to manual therapy [Citation40]. Females were excluded due to the differential effect of sex [Citation41,Citation42], and the menstrual cycle [Citation43–45], on our outcomes of interest.
Intervention
A mobilization technique was performed on all participants. These techniques consisted of low frequency, mid-range, bilateral, and alternating oscillations of either the upper cervical spine (C0–1 and C1–2) or lower cervical spine (C6–7 and C7-T1). Low frequencies have been associated with ANS processing and mobilizations can be performed within this frequency range [Citation46,Citation47]. Therefore, a frequency of 3 Hz was selected for each mobilization [Citation47]. Each level was mobilized for three sets of two-minutes, separated by a 30-s rest period. All interventions were performed by the same operator, a certified musculoskeletal physiotherapist specialist with over 30 years clinical experience. To control for potential contextual influences, each intervention was performed in the same room at the School of Physiotherapy, University of Otago [Citation48], at the same time [Citation8,Citation49–51] for each participant, between 1300 and 1700. Temperature was accounted for, and no natural light was allowed to enter the room.
Outcome measures
Salivary cortisol
Cortisol is a hormone released into the blood stream by the adrenal cortex and is the end product of the HPA-axis and a proxy measure of HPA-axis activity [Citation52,Citation53]. Whole saliva samples were collected with SalivaBio’s 2 mL cryovials and Saliva Collection Aids (Salimetrics, State College, PA) via the unstimulated passive drool technique [Citation32] (Appendix 3). This technique is gold standard and is also noninvasive and the most reliable method for collecting sCOR samples [Citation32]. Our primary outcome was the sCOR concentration at 30-min post-intervention. Concentrations of cortisol peak within the blood 10–30 min following a stressor, and then peak in the saliva 2–3 min following saturation in the blood [Citation54].
Heart rate variability
Heart rate variability:
HRV is the beat-to-beat variation of R-R intervals from the cardiac cycle [Citation35]. HRV provides a noninvasive measure of cardiac ANS activity, predominately vagal tone [Citation35,Citation55]. An iPhone application, Camera HRV (A.S.M.A. B.V.), was used to measure HRV with a technique called photoplethysmography (PPG). PPG uses the smartphones light to illuminate the skin and detect blood volume variation during a cardiac cycle [Citation56]. PPG is a reliable way to measure HRV and is validated when compared to electrocardiogram, the gold standard of HRV collection [Citation57–59]. Many metrics exist to quantify HRV [Citation34,Citation35]. For this study, the rMSSD was chosen as the primary time domain metric to quantify vagal tone [Citation36]. rMSSD measured with PPG has a low standard error [Citation60]. Please refer to appendix 4 for factors accounted for which can affect the outcomes.
Outcome measures data collection
Salivary cortisol
Participants collected sCOR samples at 14 timepoints. Eight were collected at home, and six were collected in clinic (). Cortisol exhibits a diurnal decline, with peak concentrations in the morning 30–45 min after awakening and a gradual decline over the course of the day, reaching a minimum around midnight [Citation61–63]. Baseline cortisol samples were collected to [Citation1]; plot the diurnal decline of cortisol concentrations [Citation2,Citation61] assess the accuracy of measurements with baseline and age-matched normative values [Citation51,Citation64]. Midnight sCOR samples have the highest reliability when assessing chronic HPA-axis tone [Citation65]. Hence, sCOR was collected the night after each intervention (post-pm) to assess the response of sCOR to the intervention. sCOR was collected in the seated position.
Figure 1. Timing of outcome measurement. During each intervention, sCOR concentrations and HRV data were collected in clinic at three timepoints, baseline or five-minutes prior to the intervention (pre-5), then five-minutes (post-5) and thirty-minutes (post-30) following the intervention. Baseline sCOR samples were collected at home on day five and six between the hours of 0600–0800 (baseline-am), 1400–1600 (baseline-afternoon), and 2200–0000 (baseline-pm). sCOR samples were then collected the night following each intervention between the hours of 2200–0000 (post-pm). HRV data was collected each morning on waking, at home, between the hours of 0600–0800. Day two to seven, and day nine to 14, were baseline HRV data (baseline-am). Day eight and 15 were post-intervention HRV data (post-am).

Heart rate variability
Participants collected HRV data at 21 different timepoints. Fifteen were collected at home, and six were collected in the clinic (). Baseline measurements were collected over six consecutive days prior to each intervention [Citation66,Citation67]. The day one measurement was omitted from analysis as a practice measurement. The gold standard for HRV measurement is in the middle of the night while the individual is sleeping, as this is when vagal tone is at its highest [Citation68]. This timing is not possible when using a smartphone application, hence our measurements were taken first thing in the morning upon waking, when the participant should still be in a vagally dominant state [Citation49,Citation50]. Participants were asked to breath spontaneously [Citation34], and measure their HRV for two-minutes [Citation34,Citation69]. All HRV measures were taken in supine [Citation34,Citation58], prior to sCOR collection, to minimize the autonomic effect of position change on HRV [Citation70].
Salivary cortisol data analysis
sCOR samples were analyzed with a Salimetrics ELISA immunoassay kit by a physiologist at a University of Otago physiology laboratory in accordance with the standard procedures (Appendix 5).
HRV data analysis
Raw RR intervals were exported, deidentified, stored, processed, and cleaned through Kubios HRV Standard version 3.5.0 (Biosignal Analysis and Medical Imaging Group, Kuopio, Finland) (Appendix 6).
Procedure
Potential participants attended an enrollment appointment and were screened against the inclusion and exclusion criteria. Included participants were provided with the saliva collection kit and smart phone application, along with detailed verbal and written instructions. Participants were requested to fill out an individual diary, accounting for various factors which can influence HPA-axis and ANS activity (Appendix 4). Perceived stress influences the HPA-axis and ANS [Citation6,Citation71]. To account for this, participants were requested to fill out the perceived stress scale (PSS-10) on day 1, 7, and 14. The PSS-10 has high reliability and validity, with higher scores indicating higher levels of perceived stress [Citation72].
Following baseline measurements, participants’ interventions were separated by a conservative one-week washout (). Considering our study’s healthy population, and comparable studies reporting a response no greater than minutes to hours after manual therapy techniques [Citation13,Citation24,Citation26,Citation73], a one-week washout period was ample. Upon arrival, participants were screened for any change in health status, or adverse reaction following the previous intervention. For environmental acclimatization, participants lay supine with extended legs, and avoided moving, speaking, or using their phone, for 5 min prior to pre-intervention measures [Citation34,Citation74]. The intervention was performed on the target location, followed by post-intervention measures.
Randomization and blinding
To prospectively code saliva collection vials, each participant selected a sealed opaque envelope containing their participation number upon enrollment. A research assistant, independent to the outcome assessor, used a computerized random number generator to develop a 1:1 ratio randomization table linking the participation number with their intervention block. It is not possible to blind the treating clinician [Citation75] and is unlikely the participants were blinded to which intervention they received. The outcome assessor only analyzed de-identified data.
Power calculations
We are unaware of any studies investigating upper or lower cervical mobilizations on our primary outcome, and the minimally clinically important difference for cortisol is currently unknown [Citation76]. The target location of mobilizations are thought to be anatomically separate, therefore we anticipated a large effect size of d = 0.8 [Citation77]. The upper cervical spine is in close proximity to the brainstem and PSNS [Citation78,Citation79], whereas the lower cervical spine is in close anatomical proximity to the inferior cervical (or stellate) ganglion, which are paravertebral ganglia of the sympathetic nervous system [Citation80]. An a-priori sample size calculation was performed using G*Power (Version 3.1.9.7). A sample size of n = 15 was required to provide 0.8 power at an a = 0.05 with a two-tailed test for a repeated measures crossover trial. Estimating a 20% drop out, we require 20 participants for even samples of 10 in each group.
Statistical analyses
Participant characteristics and outcome variables were presented with descriptive statistics. Data normality and skewness were checked by visual inspection of histograms, and formally with the Shapiro–Wilk test [Citation81]. Data and residuals were non-normally distributed, despite data transformation and outlier correction [Citation82]. Outliers were assessed through box and whisker plots and were winsorized if correlated with a confounding factor from participants clinical diary [Citation83]. Non-parametric tests were used for our non-normally distributed data [Citation84], from a small sample size [Citation85]. Within the clinic, a Friedman’s two-way ANOVA tested the within-group factor (time) on the outcomes [Citation86]. A Bonferroni correction adjusted for multiple post-hoc comparisons [Citation86] and accounted for a type 1 error [Citation87]. The Wilcoxon-signed rank test was used for the post hoc, between-group, pairwise comparison [Citation87]. A pairwise comparison was performed with the Wilcoxon-Signed Rank Test for the within-group measurements taken at home [Citation88]. Appropriate statistical analyses were performed to rule out any carryover, sequence, or period effects [Citation89]. Effect size was calculated with the formula r = z/√N [Citation87]. Missing data was minimal (3.5% for sCOR, and 2.8% for rMSSD) and was handled with pairwise deletion [Citation90]. Data analysis was performed using IBM SPSS Statistics version 28.0 (IBM Corporation, Armonk, NY).
Results
The flow of participants is presented in [Citation37]. Data collection occurred between the 13th of September and 13th of October 2021. Twenty males participated in this study. Participant characteristics are described in . No participants were lost from this study. Two HRV datasets were excluded due to technical and operator error. Eleven individual measures were lost due to sample contamination, operator and clinician error. No adverse reactions were reported following any manual therapy interventions.
Table 1. Baseline participant characteristics (n = 20).
Salivary cortisol
Clinic
Within groups, there was a statistically significant change in sCOR levels following lower cervical mobilization, X2 [Citation2] = 6.303. A Bonferroni correction revealed a statistically significant reduction in sCOR levels from pre-5 (Md = 0.194) to post-30 (Md = 0.194). There was no statistically significant change in sCOR levels following upper cervical mobilization, X2 [Citation2] = 4.900.
Between groups, there was a statistically significant difference in sCOR levels between the upper and lower cervical mobilization groups at post-30, z = 2.374. The median score of sCOR was lower following upper cervical (Md = 0.163) compared to lower cervical mobilization (Md = 0.194). There was no statistically significant difference in sCOR levels following upper cervical and lower cervical mobilization at pre-5, z = 1.847, or post-5, z = 1.254 (, ).
Figure 3. Clinic measurements of salivary cortisol and rMSSD.
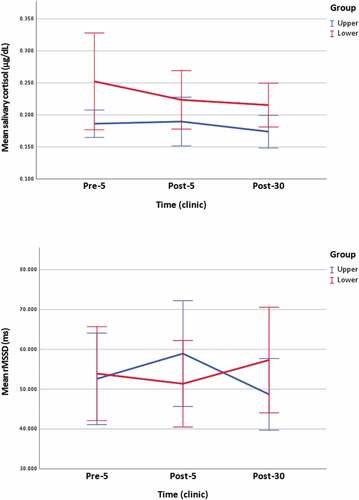
Table 2. Clinic measurements of salivary cortisol and rMSSD.
Home night
Within groups, there was no statistically significant change in sCOR levels from baseline-pm to post-pm following upper cervical, z = 0.747, or lower cervical mobilization, z = 1.067.
Between groups, there was no statistically significant difference in sCOR levels post-pm following upper cervical and lower cervical mobilization, z = 0.849 (, ).
Figure 4. Home measurements of salivary cortisol and rMSSD.
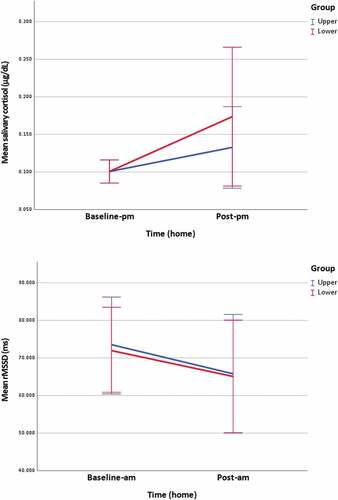
Table 3. Home measurements of salivary cortisol and rMSSD.
Heart rate variability: rMSSD
Clinic
Within groups, there was no statistically significant change in rMSSD following upper cervical, X2 [Citation2] = 4.353, or lower cervical mobilization, X2 [Citation2] = 1.529.
Between groups, there was no statistically significant difference in rMSSD following upper cervical and lower cervical mobilization at pre-5, z = −0.310, post-5, z = −1.396, or post-30, z = 0.118 (, ).
Home morning
Within groups, there was a statistically significant change in rMSSD from baseline-am to post-am following upper cervical mobilization, z = −1.982. The median score of rMSSD reduced from baseline-am (Md = 70.398) to post-am (Md = 61.183). No statistically significant change was reported following lower cervical mobilization, z = −0.686.
Between groups, there was no statistically significant difference in rMSSD post-am following upper cervical and lower cervical mobilization, z = −0.402 (, ).
µg/dL: microgram per deciliter, ms: milliseconds, n: population, p: adjusted significance, r: effect size, SD: standard deviation, * statistically significant p < 0.05
Discussion
There is an indication of a differential physiological response between upper and lower cervical spine mobilization at different timepoints, reflecting the complexity of interactions between the ANS and HPA-axis within the stress response. These results will be discussed below.
Salivary cortisol (primary outcome)
There was a statistically significant within-group decrease in sCOR from pre-5 to post-30 following lower cervical mobilization, and a statistically significant between-group difference in sCOR at the post-30 timepoint (). This indicates a downregulation of cortisol secretion from the HPA-axis 30 min following lower cervical mobilization. In support of our findings, a thoracic manipulation performed on healthy men aged 18–45 reduced sCOR concentration at 5- and 30-min following the intervention [Citation26]. The thoracic spine has a close anatomical relationship with the sympathetic nervous system (SNS) [Citation91], as does the lower cervical spine with the stellate ganglion of the SNS [Citation80]. Considering the bidirectional and interchangeable communication between the peripheral components of the stress response [Citation7], the lower cervical mobilization and thoracic manipulation could have a similar effect on the HPA-axis.
Albeit non-significant, there is indication of a differential direction of response from pre-5 to post-5, where upper cervical mobilization increases sCOR, and lower cervical mobilization reduces sCOR. Despite basing the primary outcome on the average time it takes cortisol to enter saliva from blood [Citation54], it can enter plasma much quicker via the fast corticosteroid feedback loop. The corticosteroid feedback loop operates in at least three domains; fast (seconds to minutes), intermediate (2 to 10 h), and slow (hours to days) domain [Citation92]. Upper cervical mobilization may stimulate the superior cervical ganglion located at the level of the second and third cervical vertebrae, which is sympathetic [Citation80], more so than the PSNS via the brain stem located at the level of the first cervical vertebrae [Citation78,Citation79]. It is possible that our interventions activated the fast corticosteroid feedback loop, resulting in an increase in sCOR following upper cervical mobilization, and a decrease following lower cervical mobilization.
Plausibly, the differential response from pre-5 to post-5 may be due to the individuals baseline cortisol concentrations, not the underlying anatomy. Our results showed that a higher baseline cortisol in the lower group lead to a downregulation of the HPA-axis and reduction in sCOR concentration, whereas a lower baseline in the upper group lead to an upregulation of the HPA-axis and increase in sCOR concentration. These results are mirrored in another study, which concluded that an upper cervical manual therapy technique increased blood pressure in those with hypotension, and decreased blood pressure in those with hypertension [Citation93]. Cortisol is essential for the maintenance of normal blood pressure [Citation94,Citation95]. This differential response indicates that manual therapy, to restore homeostasis, will either upregulate or downregulate its response depending on the individual’s baseline. This highlights the function of the stress response, which works bidirectionally and interchangeably to maintain homeostasis through feedback loops [Citation7].
There was a trend for sCOR concentrations to increase the night following each intervention (). This increase was greater following lower cervical spine mobilization. The same trend was shown following a thoracic manipulation, where there was an initial decrease in salivary cortisol, followed by a reciprocal increase 6 h later [Citation26]. Our results mirror this for the lower cervical mobilization group, where there was an initial decrease in sCOR concentrations, followed by a reciprocal increase that night. This response has been termed the ‘rebound effect’ [Citation96]. Considering the increase in cortisol concentrations the night following the intervention, mobilizations applied to the cervical spine may be novel treatment for clinical populations characterized by low cortisol levels, such as persistent post-concussion symptoms [Citation97–101] and long-COVID [Citation102–104].
Heart rate variability (secondary outcome)
There was a statistically significant decrease in rMSSD the morning following upper cervical mobilization (). Although non-significant, the same trend was shown following lower cervical mobilization. A decrease in rMSSD represents an attenuation of ANS regulation and reduced ability to adjust the internal environment in response to stressors [Citation105,Citation106]. Considering the interconnected relationship between the ANS and HPA-axis [Citation7], and the trend for sCOR concentrations to increase in both groups on the night of the intervention, it may indicate that individuals are in a less parasympathetically dominant state due to PSNS withdrawal [Citation106,Citation107]. Our results indicate that this withdrawal was still present the morning following the intervention.
There was a trend for a differential response in rMSSD at post-5 and post-30, depending on the mobilizations target location (). These findings are corroborated by the function of ANS within the context of the stress response; the ANS being the fast-acting component [Citation1,Citation4–6]. The ‘rebound effect’ concept is supported by the upper cervical group’s response, where there is an initial increase at post-5, followed by a reduction at post-30 which continues until the following morning. Other studies, albeit statistically significant, support our findings of upper cervical mobilization stimulating the PSNS [Citation16,Citation29], and lower cervical mobilization stimulating the SNS [Citation29]. The statistically significant results found in these studies may be due to differing tools (ECG) and metrics (i.e. LF/HF, SDNN) used to measure HRV, different interventions (manipulation), or different populations (including females).
Strengths and limitations
A major strength of our study is its internal validity. A stringent inclusion criterion, and careful account/control of many variables shown to influence our outcomes of interest, help strengthen our results. This does, however, limit our study’s external validity. Baseline-pm sCOR concentrations fit within normative age matched values [Citation62,Citation108], and pre-5 concentrations fit within each individuals baseline-afternoon values and normative age matched values [Citation62], further supporting accuracy of our results. Missing data were minimal for each analyzed participant (3.5% for sCOR, and 2.8% for rMSSD) and there was no attrition of participants. Another strength of this study is that it examined both components of the stress response, the ANS and HPA-axis, in unison.
This study is not without limitations. Our study was powered for the primary outcome, sCOR, to detect a large effect size, but it was only moderate. This may have introduced a type II error. Further to this, a type II error is possible due to our small sample size and large variability between participants evident by the 95% confidence intervals (). The crossover design should reduce the requirement for a larger sample size by mitigating individual variability [Citation109,Citation110]. Regardless, future research should consider adequate sample sizes and account for the various factors which can modulate the ANS and HPA-axis (Appendix 4). Non-normally distributed data meant we could not perform statistical analyses to obtain a group x time interaction. Variability within our outcomes and small sample size likely accounts for the non-normal distribution. Considering the small sample size, it is possible that baseline (pre-5) sCOR variation accounted for the statistically significant results in clinic () [Citation111]. However, statistical analyses revealed no statistically significant difference in pre-5 sCOR concentrations between groups, and the pre-5 values correlated with the population’s afternoon-pm baseline values, and age matched normative values [Citation62].
The aim of this work was not to investigate efficacy, but to investigate whether there was a differential response, so there was no sham or control group. Specific to a crossover trial, and considering our small sample size, there is the possibility of a carryover, sequence, or period effect [Citation111]. Statistical analyses of our statistically significant results ruled this out [Citation89]. A non-stressed population may not detect the same magnitude or direction of response as it would in a stressed population, limiting the generalizability of our results.
Implications for clinical practice and future research
This study has provided a starting point for the rationale of using manual therapy more selectively to target specific mechanisms. Aligning differential responses to underlying pathophysiology opens the way to more efficient and effective treatment. For example, persistent post-concussion symptoms can be characterized by low cortisol, which has been associated with more symptoms, more severe symptoms, and delayed recovery [Citation97–101]. Based on the present study results, we could perform lower cervical mobilization to increase cortisol levels that night, which could potentially lead to clinical benefit. A logical next-step from this study would be investigating the differential response of cervical spine manual therapy on clinical populations characterized by a dysfunctional stress response, such as those with persistent post-concussion symptoms [Citation97–101], chronic pain [Citation112] or osteoarthritis [Citation113], and relating the physiological changes to clinical outcomes. Healthy patients and those with neck pain showed a differential HRV response to lower cervical manipulation [Citation29]. Additionally, future studies could build on this trial by measuring our outcomes over a longer period of time and comparing the time-course effects of cervical spine mobilizations with other modalities shown to modulate the stress response, such as exercise [Citation114], vagus nerve stimulation [Citation115], or breathing control [Citation116]. Cortisol is a pleiotropic hormone effecting all major homeostatic systems, such as the immune, cardiovascular, central nervous system, therefore its mechanisms of effect will likely be very widespread [Citation31]. Future research needs to investigate the symptoms relating to a dysfunctional stress response, and responders/non-responders to a mechanisms-based intervention, termed ‘clinical phenotyping’ [Citation117].
Conclusion
Our study has shown that manual therapy applied to separate target locations within the cervical spine can differentially modulate the stress response. Thirty minutes following lower cervical mobilization, there was a statistically significant within-group reduction in cortisol concentration. There was also a statistically significant difference in cortisol concentrations between groups at 30 min following upper and lower cervical mobilization. These findings highlight the intricate, time dependent, interaction between both components of the stress response and the complexity of investigating such phenomenon.
Author contribution
Concept development: GF, CCh, EK, KS, CCo, ST
Literature search: GF
Design: GF, MB, CCh, EK, KS, CCo, RK, ST
Recruitment: GF
Interventions and participant management: GF and ST
Data collection: GF and ST
Data analysis: MB, RK, GF
Manuscript preparation: GF
Commented/edited final manuscript: GF, MB, CCh, EK, KS, CCo, RK, ST
Supplemental Material
Download PDF (1.2 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
SUPPLEMENTARY MATERIAL
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10669817.2023.2177071
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
Notes on contributors
Gerard Farrell
Gerard Farrell is a PhD candidate at the Center for Health, Activity and Rehabilitation Research, School of Physiotherapy at the University of Otago. His research interests include the neuroendocrine mechanisms of manual therapy, the part a dysfunctional stress response has to play in the development and maintenance of persistent post-concussion symptoms, and the role manual therapy plays in the treatment of persistent post-concussion symptoms.
Matthew Reily-Bell
Matthew Bell is a PhD candidate at the Department of Physiology, HeartOtago, School of Biomedical Sciences. His PhD is investigating CRISPR/Cas13 as a tool for the knockdown of microRNA unregulated in diabetic heart disease.
Cathy Chapple
Dr. Cathy Chapple is a senior lecturer at the Center for Health, Activity and Rehabilitation Research, School of Physiotherapy at the University of Otago. Her research interests are in the management of people with osteoarthritis. She is also investigating manual therapy for osteoarthritis patients and other musculoskeletal conditions including the role of the cervical spine in persistent post-concussion symptoms.
Ewan Kennedy
Dr. Ewan Kennedy is a senior lecturer at the Center for Health, Activity and Rehabilitation Research, School of Physiotherapy at the University of Otago. His research interests are in musculoskeletal disorders, concussion, and clinical education. Recent work has explored overlap between cervical spine and concussion injuries, with a focus on improving health service delivery.
Kesava Sampath
Dr. Kesava Sampath is a senior lecturer at the Center of Health and Social Practice, Waikato Institute of Technology, Hamilton, Waikato, New Zealand. His research work focuses on mechanisms underpinning manual therapy practice. Currently, he leads a research project investigating the usage of bio-psycho-social model of care by New Zealand osteopaths while treating people with persistent musculoskeletal pain. He is a visiting research fellow at the University of Technology, Sydney, where he is part of a global osteopathic network and actively collaborates/contributes to other projects. He is also an adjunct researcher at the Ara Institute of Canterbury and supervises post-graduate nursing research students.
Angela Spontelli Gisselman
Dr. Angela Spontelli Gisselman is an assistant professor at Tufts University School of Medicine. Her main research interests are in the role of health metrics, such as heart rate variability (an index of the autonomic nervous system), and their ability to influence decision making in rehabilitation, such as post-concussion rehabilitation. In addition to this line of research, other areas of interest include heart rate variability and temporomandibular disorders; the use of thermal imaging in tendinopathy; examination and management of shoulder injuries; load monitoring technology and post-operative rehabilitation.
Chad Cook
Prof. Chad Cook is a clinical researcher, physical therapist, and profession advocate with a history of clinical care excellence and service. His passions include refining and improving the patient examination process and validating tools used in day-to-day physical therapist practice. Dr Cook has authored or co-authored three textbooks, has published over 250 peer-reviewed manuscripts, and lectures internationally on orthopedic examination and treatment. He is a fellow of the American Academy of Orthopaedic Manual Physical Therapists; has specialized in manual therapy for over 19 years. His main areas of research interest are in examination and conservative or surgical treatment of orthopedic-related conditions.
Rajesh Katare
Assoc. Prof. Rajesh Katare is an associate professor at the Department of Physiology, HeartOtago, School of Biomedical Sciences. His main research interests include investigating the molecular mechanisms involved in the development of cardiovascular complications in diabetes, the role of microRNAs in cardiovascular diseases, the development of novel genetic and stem cell therapies for the treatment of ischemic and non-ischemic cardiovascular complications.
Steve Tumilty
Assoc. Prof. Steve Tumilty. is one of the few clinician scientists in Physiotherapy Worldwide and one of the only nine Registered Physiotherapy Specialists in New Zealand. He has an interest in blurring the boundaries between the manual therapy professions. The majority of his clinical experience has been in the outpatient musculoskeletal practice setting in UK, Germany and New Zealand. He also has experience in professional sports and Occupational Health Physiotherapy. In 2002, he came to work at the School of Physiotherapy, Otago University and he has developed and now coordinates the specialist Masters degree for Sports and Orthopaedic Manipulative Therapy for which he provides teaching and clinical expertise. Dr Tumilty’s current research interests are in Tendinopathy, photobiomodulation, modulation of the Hypothalamus-Pituitary Axis using manual interventions, and the influence of the autonomic nervous system on musculoskeletal pain and healing.
References
- Agorastos A, Heinig A, Stiedl O, et al. Vagal effects of endocrine HPA axis challenges on resting autonomic activity assessed by heart rate variability measures in healthy humans. Psychoneuroendocrinology. 2019;102:196–203.
- Sawicki CM, Humeidan ML, Sheridan JF. Neuroimmune interactions in pain and stress: an interdisciplinary approach. Neuroscientist. 2020;27(2):113–128.
- Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865–871.
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381.
- Herman JP. Regulation of hypothalamo-pituitary-adrenocortical responses to stressors by the nucleus of the solitary tract/dorsal vagal complex. Cell Mol Neurobiol. 2018;38(1):25–35.
- Godoy LD, Rossignoli MT, Delfino-Pereira P, et al. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front Behav Neurosci. 2018;12:1–23.
- Schuurmans AAT, Nijhof KS, Cima M, et al. Alterations of autonomic nervous system and HPA axis basal activity and reactivity to acute stress: a comparison of traumatized adolescents and healthy controls. Stress. 2021;24(6):876–887.
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252.
- Dunning JR, Butts R, Mourad F, et al. Upper cervical and upper thoracic manipulation versus mobilization and exercise in patients with cervicogenic headache: a multi-center randomized clinical trial. BMC Musculoskelet Disord. 2016;17(1):64.
- Lerner-Lentz A, O’halloran B, Donaldson M, et al. Pragmatic application of manipulation versus mobilization to the upper segments of the cervical spine plus exercise for treatment of cervicogenic headache: a randomized clinical trial. J Man Manip Ther. 2021;29(5):267–275.
- Gross A, Langevin P, Burnie SJ, et al. Manipulation and mobilisation for neck pain contrasted against an inactive control or another active treatment. Cochrane Database Syst Rev. 2015;2015(9):CD004249.
- Bialosky JE, Beneciuk JM, Bishop MD, et al. Unraveling the mechanisms of manual therapy: modeling an approach. J Orthop Sports Phys Ther. 2018;48(1):8–18.
- Picchiottino M, Leboeuf-Yde C, Gagey O, et al. The acute effects of joint manipulative techniques on markers of autonomic nervous system activity: a systematic review and meta-analysis of randomized sham-controlled trials. Chiropr Man Therap. 2019;27(1). DOI:10.1186/s12998-019-0235-1
- Sampath KK, Katare R, Tumilty S. Stress axis and osteopathy: a dual hormone approach. Int J Osteopath Med. 2019;33-34:24–30.
- Bakris G, Dickholtz M, Meyer PM, et al. Atlas vertebra realignment and achievement of arterial pressure goal in hypertensive patients: a pilot study. J Hum Hypertens. 2007;21(5):347–352.
- Budgell B, Hirano F. Innocuous mechanical stimulation of the neck and alterations in heart-rate variability in healthy young adults. Auton Neurosci. 2001;91(1):96–99.
- Knutson GA. Significant changes in systolic blood pressure post vectored upper cervical adjustment vs resting control groups: a possible effect of the cervicosympathetic and/or pressor reflex. J Manipulative Physiol Ther. 2001;24(2):101–109.
- La Touche R, París-Alemany A, Mannheimer JS, et al. Does mobilization of the upper cervical spine affect pain sensitivity and autonomic nervous system function in patients with cervico-craniofacial pain?: a randomized-controlled trial. Clin J Pain. 2013;29(3):205–215.
- McGuiness J, Vicenzino B, Wright A. Influence of a cervical mobilization technique on respiratory and cardiovascular function. Man Ther. 1997;2(4):216–220.
- Petersen N, Vicenzino B, Wright A. The effects of a cervical mobilisation technique on sympathetic outflow to the upper limb in normal subjects. Physiother Theory Pract. 1993;9(3):149–156.
- Vicenzino B, Cartwright T, Collins D, et al. Cardiovascular and respiratory changes produced by lateral glide mobilization of the cervical spine. Man Ther. 1998;3(2):67–71.
- Vicenzino B, Collins D, Benson H, et al. An investigation of the interrelationship between manipulative therapy-induced hypoalgesia and sympathoexcitation. J Manipulative Physiol Ther. 1998;21(7):448–453.
- Valera-Calero A, Lluch Girbés E, Gallego-Izquierdo T, et al. Endocrine response after cervical manipulation and mobilization in people with chronic mechanical neck pain: a randomized controlled trial. Eur J Phys Rehabil Med. 2019;55(6):792–805.
- Plaza-Manzano G, Molina-Ortega F, Lomas-Vega R, et al. Changes in biochemical markers of pain perception and stress response after spinal manipulation. J Orthop Sports Phys Ther. 2014;44(4):231–239.
- Kovanur Sampath K, Mani R, Katare R, et al. Thoracic spinal manipulation effect on neuroendocrine response in people with achilles tendinopathy: a randomized crossover trial. J Manipulative Physiol Ther. 2021;44(5):420–431.
- Sampath KK, Botnmark E, Mani R, et al. Neuroendocrine response following a thoracic spinal manipulation in healthy men. J Orthop Sports Phys Ther. 2017;47(9):617–627.
- Fornari M, Carnevali L, Sgoifo A. Single osteopathic manipulative therapy session dampens acute autonomic and neuroendocrine responses to mental stress in healthy male participants. J Am Osteopath Assoc. 2017;117(9):559–567.
- Amoroso Borges BL, Bortolazzo GL, Neto HP. Effects of spinal manipulation and myofascial techniques on heart rate variability: a systematic review. J Bodyw Mov Ther. 2018;22(1):203–208.
- Win NN, Jorgensen AM, Chen YS, et al. Effects of upper and lower cervical spinal manipulative therapy on blood pressure and heart rate variability in volunteers and patients with neck pain: a randomized controlled, cross-over, preliminary study. J Chiropr Med. 2015;14(1):1–9.
- Welch A, Boone R. Sympathetic and parasympathetic responses to specific diversified adjustments to chiropractic vertebral subluxations of the cervical and thoracic spine. J Chiropr Med. 2008;7(3):86–93.
- Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10(2):213–219.
- Groschl M. Current status of salivary hormone analysis. Clin Chem. 2008;54(11):1759–1769.
- Amara CE, Wolfe LA. Reliability of noninvasive methods to measure cardiac autonomic function. Can J Appl Physiol. 1998;23(4):396–408.
- Laborde S, Mosley E, Thayer JF. Heart rate variability and cardiac vagal tone in psychophysiological research – recommendations for experiment planning, data analysis, and data reporting. Front Psychol. 2017;08:08.
- Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European society of cardiology and the north American society of pacing and electrophysiology. Circulation. 1996;93(5):1043–1065.
- Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol. 2014;5:5.
- Dwan K, Li T, Altman DG, et al. CONSORT 2010 statement: extension to randomised crossover trials. BMJ. 2019;l4378. DOI:10.1136/bmj.l4378
- Silvetti MS, Drago F, Ragonese P. Heart rate variability in healthy children and adolescents is partially related to age and gender. Int J Cardiol. 2001;81(2):169–174.
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocr. 2002;87(2):589–598.
- Hutting N, Kerry R, Coppieters MW, et al. Considerations to improve the safety of cervical spine manual therapy. Musculoskelet Sci Pract. 2018;33:41–45.
- Koenig J, Thayer JF. Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci Biobehav Rev. 2016;64:288–310.
- Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocr. 1996;81(7):2468–2473.
- Saeki Y, Atogami F, Takahashi K, et al. Reflex control of autonomic function induced by posture change during the menstrual cycle. J Auton Nerv Syst. 1997;66(1–2):69–74.
- Sato N, Miyake S, Akatsu J, et al. Power spectral analysis of heart rate variability in healthy young women during the normal menstrual cycle. Psychosom Med. 1995;57(4):331–335.
- Schmalenberger KM, Eisenlohr-Moul TA, Jarczok MN, et al. Menstrual cycle changes in vagally-mediated heart rate variability are associated with progesterone: evidence from two within-person studies. J Clin Med. 2020;9(3):617.
- Knyazev GG. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev. 2012;36(1):677–695.
- Piekarz V, Perry J. An investigation into the effects of applying a lumbar Maitland mobilisation at different frequencies on sympathetic nervous system activity levels in the lower limb. Man Ther. 2016;23:83–89.
- Karim N, Hasan J, Ali S. Heart rate variability - a review. J Basic Appl Sci. 2011;7:71–77.
- Van Eekelen APJ, Houtveen JH, Kerkhof GA. Circadian variation in cardiac autonomic activity: reactivity measurements to different types of stressors. Chronobiol Int. 2004;21(1):107–129.
- Massin MM. Circadian rhythm of heart rate and heart rate variability. Arch Dis Child. 2000;83(2):179–182.
- Kudielka BM, Gierens A, Hellhammer DH, et al. Salivary cortisol in ambulatory assessment-some dos, some don’ts, and some open questions. Psychosom Med. 2012;74(4):418–431.
- Strahler J, Skoluda N, Kappert MB, et al. Simultaneous measurement of salivary cortisol and alpha-amylase: application and recommendations. Neurosci Biobehav Rev. 2017;83:657–677.
- Sladek CD, Michelini LC, Stachenfeld NS, et al. Endocrine-autonomic linkages. Compr Physiol. 2015;1281–1323. DOI:10.1002/cphy.c140028
- Bozovic D, Racic M, Ivkovic N. Salivary cortisol levels as a biological marker of stress reaction. Med Arch. 2013;67(5):374–377.
- Del Paso Ga R, Langewitz W, Mulder LJM, et al. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50(5):477–487.
- Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28(3):R1–39.
- Plews DJ, Scott B, Altini M, et al. Comparison of heart-rate-variability recording with smartphone photoplethysmography, polar H7 chest strap, and electrocardiography. Int J Sports Physiol Perform. 2017;12(10):1324–1328.
- Pernice R, Javorka M, Krohova J, et al. Reliability of short-term heart rate variability indexes assessed through photoplethysmography. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:5610.
- Heathers JA. Smartphone-enabled pulse rate variability: an alternative methodology for the collection of heart rate variability in psychophysiological research. Int J Psychophysiol. 2013;89(3):297–304.
- Sahroni A, Hassya IA, Rifaldi R, et al. HRV assessment using finger-tip photoplethysmography (PulseRate) as compared to ECG on healthy subjects during different postures and fixed breathing pattern. Procedia computer science. 2019;161:535–543.
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroen-docrinology. 2009;34(10):1423–1436.
- Nater UM, Rohleder N, Schlotz W, et al. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 2007;32(4):392–401.
- Touitou Y, Haus E. Alterations with aging of the endocrine and neuroendocrine circadian system in humans. Chronobiol Int. 2000;17(3):369–390.
- Schlotz W. Ambulatory psychoneuroendocrinology: assessing salivary cortisol and other hormones in daily life. New York, US: Guilford Press; 2011.
- Golden SH, Wand GS, Malhotra S, et al. Reliability of hypothalamic–pituitary–adrenal axis assessment methods for use in population-based studies. Eur J Epidemiol. 2011;26(7):511–525.
- Plews DJ, Laursen PB, Kilding AE, et al. Heart rate variability in elite triathletes, is variation in variability the key to effective training? a case comparison. Eur J Appl Physiol. 2012;112(11):3729–3741.
- Plews DJ, Laursen PB, Kilding AE, et al. Evaluating training adaptation with heart-rate measures: a methodological comparison. Int J Sports Physiol Perform. 2013;8(6):688–691.
- Kim H-S, Yoon K-H, Cho J-H. Diurnal heart rate variability fluctuations in normal volunteers. J Diabetes Sci Technol. 2014;8(2):431–433.
- Esco MR, Flatt AA. Ultra-short-term heart rate variability indexes at rest and post-exercise in athletes: evaluating the agreement with accepted recommendations. J Sports Sci Med. 2014;13(3):535–541.
- Young FLS, Leicht AS. Short-term stability of resting heart rate variability: influence of position and gender. Appl Physiol Nutr Metab. 2011;36(2):210–218.
- van Eck MM, Nicolson NA. Perceived stress and salivary cortisol in daily life. Ann Behav Med. 1994;16(3):221–227.
- Smith KJ, Rosenberg DL, Timothy Haight G. An assessment of the psychometric properties of the perceived stress scale-10 (PSS10) with business and accounting students. Account Perspect. 2014;13(1):29–59.
- Cerritelli F, Cardone D, Pirino A, et al. Does osteopathic manipulative treatment induce autonomic changes in healthy participants? a thermal imaging study. Front Neurosci. 2020;14:14.
- Quintana DS, Alvares GA, Heathers JAJ. Guidelines for reporting articles on psychiatry and heart rate variability (GRAPH): recommendations to advance research communication. Transl Psychiatry. 2016;6(5):e803.
- Cook C, Sheets C. Clinical equipoise and personal equipoise: two necessary ingredients for reducing bias in manual therapy trials. J Man Manip Ther. 2011;19(1):55–57.
- Colombi T. The effects induced by spinal manipulative therapy on the immune and endocrine systems. Medicina (B Aires). 2019;55(8):448.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. ed. Hillside, NJ: Lawrence Erlbaum Associates; 1988.
- Damodaran O, Rizk E, Rodriguez J, et al. Cranial nerve assessment: a concise guide to clinical examination. Clin Anat. 2014;27(1):25–30.
- Bland JH, Boushey DR. Anatomy and physiology of the cervical spine. Semin Arthritis Rheum. 1990;20(1):1–20.
- Elias M. Cervical sympathetic and stellate ganglion blocks. Pain Physician. 2000;3(3):294–304.
- Mohd Razali N, Yap B. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, lilliefors and Anderson-Darling tests. J Stat Model Analytics. 2011;2:21–33.
- Lo S, Andrews S. To transform or not to transform: using generalized linear mixed models to analyse reaction time data. Front Psychol. 2015;6:1171.
- Lien D, Balakrishnan N. On regression analysis with data cleaning via trimming, winsorization, and dichotomization. Commun Stat B: Simul Comput. 2005;34(4):839–849.
- Putt ME, Chinchilli VM. Nonparametric approaches to the analysis of crossover studies. Stat Sci. 2004;19(4). DOI:10.1214/088342304000000611
- Koch GG. The use of non-parametric methods in the statistical analysis of the two-period change-over design. Biometrics. 1972;28(2):577–584.
- Pereira DG, Afonso A, Medeiros FM. Overview of friedman’s test and post-hoc analysis. Commun Stat B: Simul Comput. 2014;44(10):2636–2653.
- Pallant J SPSS Survival Manual. 2020. doi:10.4324/9781003117452.
- Woolson RF. Wilcoxon signed-rank test. Encyclopedia of Biostatistics. 2005. DOI:10.1002/0470011815.b2a15177
- Lim C-Y, In J. Considerations for crossover design in clinical study. Korean J Anesthesiol. 2021;74(4):293–299.
- Burke S. Missing values, outliers, robust statistics & non-parametric methods. Statistics and Data Analysis. 2001;2:19.
- Patel TR. Chapter 36 - anatomy of the sympathetic nervous system. In: Tubbs R, Rizk E, Shoja M, Loukas M, Barbaro N Spinner R, editors. Nerves and nerve injuries. San Diego: Academic Press; 2015. pp. 495–506.
- Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5(1):1–24.
- Torns S. Atlas vertebra realignment and arterial blood pressure regulation in 42 subjects. J Upper Cervical Chiropr Res. 2012;2:40–45.
- Whitworth JA, Williamson PM, Mangos G, et al. Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag. 2005;1(4):291–299.
- Kelly J, Mangos G, Williamson P, et al. Cortisol and hypertension. Clin Exp Pharmacol Physiol. 1998;25(S1):S51–6.
- Cunniffe B, Hore AJ, Whitcombe DM, et al. Time course of changes in immuneoendocrine markers following an international rugby game. Eur J Appl Physiol. 2010;108(1):113–122.
- Ives J, Alderman M, Stred S. Hypopituitarism after multiple concussions: a retrospective case study in an adolescent male. Vaccine. 2007;43(3):431–439.
- Ritchie EV, Emery C, Debert CT. Analysis of serum cortisol to predict recovery in paediatric sport-related concussion. Brain Inj. 2018;32(4):523–528.
- Tabor J, La P, Kline G, et al. Saliva cortisol as a biomarker of injury in youth sport-related concussion. J Neurotrauma. 2022;40(3–4):296–308.
- Villegas E, Hartsock MJ, Aben B, et al. Association between altered cortisol profiles and neurobehavioral impairment after mild traumatic brain injury in college students. J Neurotrauma. 2022;39(11–12):809–820.
- Di Battista AP, Rhind SG, Churchill N, et al. Peripheral blood neuroendocrine hormones are associated with clinical indices of sport-related concussion. Sci Rep. 2019;9(1):1–10.
- Hashim M, Athar S, Gaba WH. New onset adrenal insufficiency in a patient with COVID-19. BMJ Case Rep. 2021;14(1):e237690.
- Kanczkowski W, Evert K, Stadtmüller M, et al. COVID-19 targets human adrenal glands. Lancet Diabetes Endocrinol. 2022;10(1):13–16.
- Kanczkowski W, Beuschlein F, Bornstein SR. Is there a role for the adrenal glands in long COVID? Nat Rev Endocrinol. 2022;18(8):451–452.
- Thayer JF, Åhs F, Fredrikson M, et al. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–756.
- Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci. 2006;1088(1):361–372.
- Thayer JF. On the importance of inhibition: central and peripheral manifestations of nonlinear inhibitory processes in neural systems. Dose-Response. 2006;4(1):2–21.
- Aardal E, Holm AC. Cortisol in saliva-reference ranges and relation to cortisol in serum. Eur J Clin Chem Clin Biochem. 1995;33(12):927–932.
- Wellek S, Blettner M. On the proper use of the crossover design in clinical trials. Deutsches Aerzteblatt Online. 2012. DOI:10.3238/arztebl.2012.0276
- Burmeister E, Aitken LM. Sample size: how many is enough? Aust Crit Care. 2012;25(4):271–274.
- Metcalfe C. The analysis of cross-over trials with baseline measurements. Stat Med. 2010;29(30):3211–3218.
- Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. 2014;94(12):1816–1825.
- Carlesso LC, Sturgeon JA, Zautra AJ. Exploring the relationship between disease-related pain and cortisol levels in women with osteoarthritis. Osteoarthr Cartil. 2016;24(12):2048–2054.
- Vieluf S, Hasija T, Jakobsmeyer R, et al. Exercise-induced changes of multimodal interactions within the autonomic nervous network. Front Physiol. 2019;10:240.
- Kaniusas E, Kampusch S, Tittgemeyer M, et al. Current directions in the auricular vagus nerve stimulation I - a physiological perspective. Front Neurosci. 2019;13:854.
- Zaccaro A, Piarulli A, Laurino M, et al. How breath-control can change your life: a systematic review on psycho-physiological correlates of slow breathing. Front Hum Neurosci. 2018;12:353.
- Damian K, Chad C, Kenneth L, et al. Time to evolve: the applicability of pain phenotyping in manual therapy. J Man Manip Ther. 2022;30(2):61–67.