Abstract
The aim of this work was to encapsulate superoxide dismutase (SOD) into biodegradable microparticles by spray-drying technique. The nature of the organic solvent to dissolve the polymer, the method of incorporation of the drug in the organic phase (with or without a surfactant, namely sucrose ester of HLB = 6), the surfactant/polymer ratio, and the nature of the biodegradable polyesters were investigated as formulation variables. The polyesters investigated as matrix were poly(ε-caprolactone) (PCL), poly(d, l, lactide-co-glycolide) (PLG-RG756), and poly(d, l-lactide) (PLA-R207) of respective molecular weight 78.2 kDa, 84.8 kDa, and 199.8 kDa. At surfactant/polymer ratio of 1/10, the SOD-retained enzymatic activities were higher (>95%) for PLG-RG756 and PLA-R207 but relatively lower for the PCL (∼ 85%) probably due to the PCL relatively higher hydrophobicity. The obtained microparticles exhibited average volume mean diameter of 4–10 μ m, the smaller for PCL and the larger for PLG-RG756 polymeric matrix. The in vitro release profile showed that SOD was completely (100%) released from PLA-R207 in 48 hr and from PLG-RG756 and PCL within 72 hr. These results showed that spray-drying with incorporation of surfactant such as sucrose ester may efficiently encapsulate SOD into biodegradable microparticles. Such formulations may improve the bioavailability of SOD and similar biopharmaceuticals.
The utility of spray-drying to produce protein-loaded is evident. Spray-drying is a simple, reproducible, rapid, and easy to scale-up technique (Masters Citation1991). In this procedure, the drug is dissolved or suspended in a polymer/organic solvent solution and aerosolized via a nozzle to create microdroplets leading to microparticles after hardening (Bodmeier and Chien Citation1988). Previous reports stated the preparation of biodegradable microparticles containing proteins by spraying technique (Bittner et al. Citation1998; Cleland and Jones Citation1996; Gander et al. Citation1996; Lee Citation2002; Maa and Prestrelski Citation2000; Park, Alonso, and Langer Citation1994; Takada et al. Citation1994; Thomasin et al. Citation1996). But these formulations usually involve the use of protein in suspension (Bittner et al. Citation1998; Cleland and Jones Citation1996), w/o emulsion (Cleland and Jones Citation1996; Gander et al. Citation1996; Park et al. Citation1994), or solution such as mixture of water and acetonitrile (Thomasin et al. Citation1996), the polymer being dissolved in the organic solvent. During the processing, the protein may interact with the organic solvent prior to formation of the microspheres. This solvent–protein interaction may then lead to protein denaturation. The extent of protein aggregation, and/or inactivation during and after spray-drying, can be minimized by means of formulation measures, such as the inclusion of surfactants and stabilizing sugars. Provided suitable spray-drying conditions can be identified, this process is attractive as a method to stabilize proteins (Lee Citation2002). Moreover, superoxide dismutase (SOD) had successfully been entrapped in polylactide by reversed micelle solvent evaporation using sucrose esters as surfactants (Hayashi et al. Citation1994).
SOD and biodegradable polyesters were involved in this study for the following reasons. SOD is a family of metalloenzymes known to accelerate spontaneous dismutation of the superoxide radical to hydrogen peroxide and molecular oxygen (Fridovich Citation1978). The potential field of using this enzyme in therapy is very wide including treatment of various diseases with low erythrocyte SOD activity (e.g., iron-deficiency, malaria parasitemia, Fanconi's anemia), inflammatory diseases (e.g., rheumatoid arthritis), and ischemic injuries (in small intestine and other tissues) (Bannister, Bannister, and Rotilio Citation1987). Biodegradable polyesters such as polylactide, polyglycolide, and their copolymers (Lewis Citation1990) and poly(ε-caprolactone) (Pitt Citation1990) were selected as polymeric matrix in this study because of their excellent record of biocompatibility, biodegradability, and nontoxicity in human either in surgery or in drug delivery.
The purpose of this work was to encapsulate a model biological compound, SOD in biodegradable polymers, using a spray-drying method. Basically, the nature of the organic solvent used to dissolve the polymer, the method of incorporation of the drug in the organic phase (with or without a surfactant, namely sucrose ester of HLB = 6), the surfactant/polymer ratio, and the nature of the biodegradable polyesters were investigated as formulation variables.
MATERIALS AND METHODS
Materials
The biodegradable polyesters used as matrix were poly(ε-caprolactone) (PCL, molecular weight [Mw] = 84.8 kD) from Union Carbide (Benelux), Poly(d, l-lactide) or Resomer® R207 (PLA-R207, Mw 199.8 kD) and 75/25 poly(d, l-lactide-coglycolide) Resomer® RG756 (PLG-RG756, Mw 78.2 kD) from Boerhinger-Ingelheim (Germany). The Cu-Zn superoxide dismutase (SOD, 3500 U/mg) from bovine erythrocyte was purchased from Sigma Chemical (St. Louis, MO, USA). The sucrose ester was stearate-palmitate ester (Sisterna SP30, Sisterna N.V., Netherlands). Reduced adenine dinucleotide (β-NADH, disodium salt) was obtained from Boerhinger-Mannheim (Germany), while MnCl2·2H2O, ethylenedinitrilotetraacetic acid (EDTA) and 2-mercaptoethanol were supplied by Merck, Darmstadt (Germany). The following chemicals were used as received from the supplier: bovine serum albumin (BSA) fraction V, Folin-Ciocalteu reagent (Merck, Darmstadt, Germany), polyvinyl alcohol (PVA, 87-89% hydrolyzed, Mw 13-23 kDa, Aldrich Chemical, USA). Other chemicals were reagent grade and used as supplied.
Preparation of Microparticles by Spray-Drying
Two formulations strategies were used to disperse the SOD aqueous phase into the organic phase before the spray-drying process: either with or without the addition of the sucrose ester of HLB = 6. The first formulation strategy (without surfactant incorporation) was adapted from the one previously described (Gander et al. Citation1996). Typically 0.5 ml of a solution of SOD (1.75× 102 U/ml) in 50 mM phosphate buffer pH 7.4 was dispersed in a solution of 0.5% (w/v) of polyester in 100 ml of organic solvent using an Ultraturrax model T25 (Janke & Kunkel, IKA-Labor technik) at high speed (8,000 rpm) for 5 min at room temperature. In this case, the organic solvents investigated were methylene chloride (MC) and mixtures of MC with either n-pentane (PT) or diethyl ether (DET) in ratio (1:1), respectively. The second formulation strategy (with surfactant incorporation) was adapted from the one previously described (Hayashi et al. Citation1994). Typically, a micellar solution was prepared by dispersing the surfactant at different ratios with the polyester used (1/2, 1/5, and 1/10) in 100 ml of methylene chloride solution; 0.5 g of polymer was dissolved in this micellar solution; finally, 0.5 ml of the above solution of SOD was then dissolved in this micellar solution of polymer (0.5%, w/v) after a brief shaking.
The microparticles were obtained by spraying the dispersions obtained from both formulation strategies through the nozzle (0.5 mm diameter) of a spray-dryer (co-current flow type) model Mini Büchi 190 (Büchi Laboratoriums-Technik AG, Flawil, Switzerland). The process parameters were set as follow: inlet temperature (45 ± 2°C), outlet temperature (34 ± 2°C), aspirator setting (15), pump setting (4.5–5.5 ml/min.), air flow rate (500 liters/hr) and spray flow pressure (6 bars). We have investigated the nature of the organic solvent used to dissolve the polymer, the method of incorporation of the drug in the organic phase (with or without a surfactant, namely sucrose ester of HLB = 6), the surfactant/polymer ratio, and the nature of the biodegradable polyesters as formulation variables.
Morphological Analysis
The external morphology of the microparticles was analyzed by optical and scanning electron microscopy (Youan, Gillard, and Rollmann Citation1999). The optical microscope (Leitz Wetzlar, Germany) was used at magnification x1000. For scanning electron microscopy (SEM), the spray-dried particles were redispersed in water, air-dried, and coated with gold-palladium under argon atmosphere. Examination was carried out under an SEM (Hitachi S-570, Japan) equipped with an image analyzer.
Particle Size Analysis
After formulation, the spray-dried particles were redispersed in a 0.9% (w/v) sodium chloride solution and sized with a Coulter Multisizer II (Coulter Electronics, Luton, U.K.) equipped with a 100-μ m aperture (Youan et al. Citation2001; Youan et al. Citation1999). Microparticle size was expressed as volume mean diameter (Vmd) in μ m. In all cases, the free solvent was thoroughly filtered using filter with a 0.22-μ m diameter. Experiments were performed in triplicate.
Assessment of SOD Loading and Retained Activity
The protein extraction method was adapted from the one used previously (Hayashi et al. Citation1994). Briefly, 100 mg of the unwashed spray-dried microparticles, accurately weighted, were dispersed in 1 ml of methylene chloride solution. After polymer dissolution, the SOD was extracted by centrifugation from this organic solvent with 2 ml of 50 mM phosphate buffer solution (PBS). The supernatant was then used to assess the percent protein loading (%w/w) and the percent retained enzymatic activity (%RA) as follows. The SOD amount in this supernatant was determined by the colorimetric method of Lowry et al. (Citation1951). The latter amount related to particle weight gave the percent SOD loading (%w/w). The %RA in the supernatant was determined from data obtained by the spectrophotometric method developed by Paoletti et al. (Citation1986) as follows. All solutions were made up with deionizer water according to the scheme previously proposed by these authors.
For each sample, the decrease in absorbance at 37°C per min (Δ Do/min: slope of the curve) of the β-NADH was recorded for 15 min at 340 nm with a spectrophotometer Uvikon 930 (Kontron Instrument) connected to a recorder. The decrease of the rate of β-NADH oxidation is a function of enzyme concentration.
Thus, 50% inhibition corresponding to one unit of the enzyme is produced by ∼15 ng of pure superoxide. The %RA was computed by relating this measured enzymatic activity in the supernatant to the theoretical activity of the quantity of protein quantified in the extract. A similar approach was used to assess enzyme activity at selected time points in the release medium.
In Vitro Release Study
The in vitro release of SOD was performed on unwashed SOD-loaded biodegradable polyester particle formulations. The polyester used was PCL, Mw = 84.8 kD, PLA-R207, Mw 199.8 kD), and 75/25 PLG-RG756, Mw 78.2 kD. Typically, the spray-dried microparticles (200 mg) were suspended in 2 ml of PBS containing 0.02% Tween 80, at 37°C in a 10-ml sealed glass vial and shaken horizontally (Shaker model Grants Instruments, Cambridge, England) at 60 rpm. At predetermined time intervals, the amount of SOD released in the supernatant was assayed by the method of Lowry et al. (Citation1951). Experiments were performed in triplicate over 72 hr. After the release study, the amount of remaining SOD within the particle was determined similarly after dissolution of the polymer in 2 mL of methylene chloride and extraction with 4 mL of PBS.
RESULTS AND DISCUSSION
Influence of Organic Solvent on Particle Characteristics
The results of this study are summarized in . When methylene chloride (MC) was mixed with solvents such as n-pentane (PT) and diethyl ether (DET) in a ratio 1/1, there was a dramatic increase in the %RA of the protein. The increase in the %RA may be attributed to the effect of dilution of the methylene chloride that exhibited a higher enzyme denaturating effect. Our preliminary studies showed that PCL was not soluble in DET and PT alone; therefore, the mixture with MC could not be avoided for the solubilization of this polymer for subsequent processing. Moreover, the enzyme denaturating effect of those solvents for the enzyme was in the range DET < PT ≪ MC. The additional rationale for selecting these solvents for the spray-drying application is their closer boiling point (tb) to the outlet temperature (∼ 34°C). The tb of DET, PT, and MC are 34.5°C, 36.06°C, and 40°C, respectively.
Influence of the nature of organic solvent on particle characteristics
In addition, these solvents exhibit relatively lower enthalpy of vaporization at 25°C (Δ Hvap(25°C)) compared with that of water (43.98 kJ/mol). The (Δ Hvap(25°C)) of DET, PT, and MC was 27.10, 26.43, and 28.52 kJ/mol, respectively (Lide Citation1999). The outlet temperature of a spray dryer is known to be correlated with activity loss in the drying of heat-sensitive materials (Broadhead, Rouan, and Rhodes Citation1992; Lee Citation2002). Therefore, ideally, it may be desirable to use a solvent with very low boiling point and enthalpy of vaporization for the microencapsulation of heat sensitive material by spray-dryng.
It also was noteworthy that the mixture of MC with these solvents increased particle mean diameter. In the Buchi laboratory spray-dryer, the coupling of liquid feeding rate (vlf) with atomizing air velocity (vaa) and their influence on spray droplet size is defined by the so-called air/fluid mass ratio, na/f, which represents the energy available for atomization:
(1)
Increase in na/f represents more energy and therefore reduces spray-droplet size and consequently the size of the dried particles (Masters Citation1991). The viscosity (resistance to flow) of pure MC, DET, and PT is 0.413, 0.224, and 0.224 mPa.s, respectively. For instance, the density of pure MC and PT are 1.3255 and 0.6262 g/cm3 at 20°C (Lide Citation1999). The addition of the polymer may change the trend of these values. Since MC alone was a better solvent for PCL than either PT or DET alone, it was postulated that the overall energy available for atomization was decreased when the solvent mixture was used, leading to coarser original droplets of diameter, Dw. The diameter of a solid produced by spray-drying, Ds, depends on the diameter of the original spray-droplet, Dw, and also the total solids' content of the spray solution, w2 (Hickey et al. Citation1994; Lee Citation2002):
(2)
where ρ2 is the density of the solid. The foregoing facts may explain the obtention of coarser particles when the mixture of solvents were used. The spray-drying temperatures (Tinlet and Toultet) also are important process variables that determines droplet size in combination with the energy of atomization. A more systematic study of the combination of the foregoing factors is needed to confirm these observations. Overall morphological analysis showed the presence of polymer fibers in the formulation prepared by spraying drug-containing solvent mixture, which contributed to their broader size distribution observed by Coulter counter. For instance, size ranged from 4 to 54 μ m for MC alone, 11 to 57 μ m for MC/PT, and 5 to 48 μ m for MC/DET.
Incorporation of Sucrose Ester on Particle Characteristics
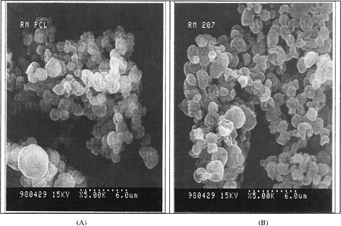
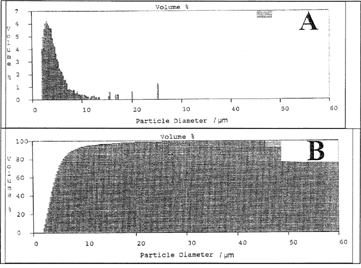
The main results of these experiments are shown in . The SOD-containing PCL particles were prepared by spray-drying with addition of sucrose ester of HLB = 6 in methylene chloride at different ratios (1/2, 1/5, 1/10 w/w). Methylene chloride was chosen because of its higher solvation power for the polymer based on preliminary results. This sucrose ester was selected among others based on promising results obtained during preliminary studies. At the highest sucrose ester/polymer ratio (1/2 w/w) the particle size distribution was relatively broader due to fiber formation as confirmed by morphological analysis and Coulter counter. It had been argued that the fiber formation occurs because of strong and extensive intermolecular bonds and stiff and adhering polymer chains. Thus, there was an inadequate force to disperse the filaments into droplets. The successful atomization into droplets was dependent on both the type of polymer used and, to a lesser extent, the viscosity of the spray solution (Bodmeier and Chien Citation1988). At the lowest ratio (1/10 w/w), the particles exhibited quite regular and spherical shape with narrow size distributions from 2.4 to 8.16 μ m as evidenced by the electron micrograph () and size distribution obtained by Coulter counter (). also shows that the %RA increased when the surfactant/polymer ratio decreased. Further investigations are needed to elucidate the latter observations.
Influence of the surfactant/polymer ratio on particle characteristics
Nature of Biodegradable Polyester on Particle Characteristics
The main results of these experiments are shown in and (release profile). The SOD-loaded biodegradable particles were prepared by spray-drying with the addition of sucrose ester of HLB = 6 in methylene chloride at ratio 1/10. The polyesters used were PLG-RG756, 78.5 kDa, PLA-R207, 199.8 kDa, and PCL, 84.8 kDa. The obtained microparticles exhibited average volume mean diameter of 4 to 10 μ m, the smaller from PCL and the larger from PLG-RG756 polymer. The difference in particle size could be explained by their overall effect on the energy of atomization according to equations 1 and 2. This may be due to the differences in their molecular weights (78.2–199.8 kDa), chemical composition (lactone, lactide, and glycolide units), and in their interaction parameters with the solvent and rheologic properties (Flory Citation1953). These observations also are consistent with previous work that showed polymer composition affected microsphere morphology prepared by spray-drying (Bittner et al. Citation1998).
Influence of the nature of the polyester on particle characteristics
The %RA was very high (> 95%) for lactide-derived polyesters (PLA-R207 and PLG-RG756) but relatively lower with the PCL (∼ 85%) probably because of relatively higher hydrophobicity of the polymer from the presence of longer hydrophobic backbone in its chemical structure (Pitt Citation1990).
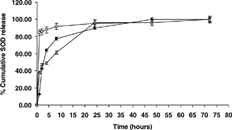
(in vitro release profile) showed that the SOD was completely (100%) released from PLA-R207 in 48 hr and from PLG-RG756 and PCL within 72 hr. Owing to the demonstrated stability of SOD in similar medium for 70 days (Hayashi et al. Citation1994), the continuous monitoring of the protein release was only assessed by colorimetric method of Lowry et al. (Citation1951). However, selected samples were withdrawn at some isolated time points to confirm the preservation of activity of the protein in the release medium. Beside the triphasic release of SOD from PCL particles, the particles exhibited generally a biphasic SOD release profile: burst effect (due to loosely encapsulated SOD present at particle/water interface) followed by continuous release of SOD. The PLA-R207 exhibited the lowest 1 hr burst effect and PLG-RG756, the highest burst effect. Because these experiments were performed on unwashed particles, at this stage it is impossible to explain these observations. Further investigations are needed to elucidate the exact release mechanism of SOD, including similar experiments with washed particle to minimize the burst effect in addition to concomitant analysis of the kinetics of polymer degradation.
3 In vitro release of SOD from biodegradable microparticles prepared from three different polyesters: □ = 75/25 PLG-RG756, 78.2 kDa; △ = PCL, 84.8 kDa; and • = PLA-R207, 199.8 kDa, as measured by Lowry as described in the Methods section. The release medium was 50 mM PBS, pH 7.4, containing 0.02% (w/v) Tween 80. Error bars refer to standard deviation (n = 3). Other particle characteristics are summarized in .
CONCLUSION
At surfactant/polymer ratio of 1/10, the retained enzymatic activities of SOD were higher (> 95%) with two lactide-derived polyesters (PLG-RG756 and PLA-R207) and relatively lower for the PCL (∼ 85%) probably due to the PCL's relatively higher hydrophobicity. In absence of sucrose ester, the retained enzymatic activity was relatively low. This observation was consistent with the general concept that spray-drying of protein solution without excipients can lead to unfolding, aggregation, and inactivation (Lee Citation2002). The obtained microparticles exhibited average volume mean diameter of 4–10 μ m, the smaller being from PCL and the larger from PLG-RG756 polymer. The in vitro release profile showed that SOD was completely (100%) released from PLA-R207 in 48 hr and from PLG-RG756 and PCL within 72 hr. These results showed that spray-drying with incorporation of surfactant such as sucrose ester may efficiently encapsulate SOD and similar protein drugs into biodegradable microparticles. Such formulations may improve the bioavailability of similar biotechnology derived drugs.
This work was supported by the Scientific Research Program (Cote d'Ivoire Government) and Texas Tech University Health Science Center, Amarillo, Texas, USA.
REFERENCES
- Bannister J. V., Bannister W. H., Rotilio G.. 1987. Aspects of the structure, function, and applications of superoxide dismutase. Crit. Rev. Biochem.. 22: 111–180
- Bittner B., Ronneberger B., Zange R., Volland C., Anderson J. M., Kissel T.. 1998. Bovine serum albumin loaded poly(actide-co-glycolide) microspheres: Influence of polymer purity on particle characteristics. J. Microencapsul.. 15: 495–514. [PUBMED], [INFOTRIEVE]
- Bodmeier R., Chien H.. 1988. Preparation of biodegradable poly(D, L-lactide). Microparticles using a spray-drying technique. J. Pharm. Pharmacol.. 40: 754–757. [PUBMED], [INFOTRIEVE]
- Broadhead J., Rouan E. S. K., Rhodes C. T.. 1992. The spray drying of pharmaceuticals. Drug Dev. Ind. Pharm.. 18(11&12)1169–1206
- Cleland J. L., Jones A. J.. 1996. Stable formulations of recombinant human growth hormone and interferon-gamma for microencapsulation in biodegradable microspheres. Pharm. Res.. 13: 1464–1475. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Flory P. J.. 1953; Principles of Polymer Chemistry. Ithaca, NY, Cornell University Press
- Fridovich I.. 1978. The biological activity of oxygen radicals. Science. 201: 875–880. [PUBMED], [INFOTRIEVE]
- Gander B., Johansen P., Nam Tran H., Merkle H. P.. 1996. Thermodynamic approach to protein microencapsulation into poly(D,L-lactide) by spray drying. Int. J. Pharm.. 129: 51–61. [CROSSREF]
- Hayashi Y., Yoshioka S., Aso Y., Li WanPo A., Terao T.. 1994. Entrapment of proteins in poly(L-lactide) microspheres using reversed micelle solvent evaporation. Pharm. Res.. 11(2)337–340. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Hickey A., Concessio N., Ort M., Platz R.. 1994. Factors influencing the dispersion of dry powders as aersols. Pharm. Technol., August. 58
- Lee G.. 2002. Spray-drying of proteins. Pharm. Biotechnol.. 13: 135–158. [PUBMED], [INFOTRIEVE]
- Lewis D. L.. 1990. Controlled release of bioactive agents from lactide/glycolide polymers. In Controlled Release of Bioactive Agents from Lactide/Glycolide Polymers, M., Chasin, R., Langer, New York, Marcel Dekker
- Lide D. R.. 1999; CRC Handbook of Chemistry and Physics, 80th ed., Boca Raton, FL, CRC Press
- Lowry O. M., Rosebrough N. J., Farr A. L., Randall R. J.. 1951. Protein measurement with Folin phenol reagent. J. Biol. Chem.. 193: 265–275. [PUBMED], [INFOTRIEVE]
- Maa Y. F., Prestrelski S. J.. 2000. Biopharmaceutical powders: Particle formation and formulation considerations. Curr. Pharm. Biotechnol.. 1(3)283–302. [PUBMED], [INFOTRIEVE]
- Masters K.. 1991; Spray Drying Handbook, 5th ed., New York, John Wiley & Sons
- Paoletti F., Aldinucci D., Mocali A., Capaprini A.. 1986. A sensitive spectrophotometric method for determination of superoxide dismutase activity in tissue extracts. Anal. Biochem.. 154: 536–541. [PUBMED], [INFOTRIEVE]
- Park T. G., Alonso M. J., Langer R.. 1994. Controlled release of proteins from poly(L-lactide acid) coated polyisobutylcyanoacrylate microcapsules. J. Appl. Polym. Sci.. 52: 1787–1807
- Pitt C. G.. 1990. Poly(ε-caprolactone) and its copolymers. In Biodegradable Polymers as Drug Delivery Systems, M., Chasin, R., Langer, New York, Marcel Dekker
- Takada S., Uda Y., Toguchi H., Ogawa Y.. 1994. Preparation and characterization of copoly(dl-lactic/glycolic acid) microparticles for sustained release of thyrotrophin releasing hormone by double nozzle spray drying method. J. Control. Rel.. 32: 79–85. [CROSSREF]
- Thomasin C., Corradin G., Men Y., Merkle H. P., Gander B.. 1996. Tetanus toxoid and synthetic malaria antigen containing poly(lactide)/poly(lactide-co-glycolide) microspheres: Importance of polymer degradation and antigen release for immune response. J. Control. Rel.. 41: 131–145. [CROSSREF]
- Youan B. B., Jackson T. L., Dickens L., Hernandez C., Owusu-Ababio G.. 2001. Protein release profiles and morphology of biodegradable microcapsules containing an oily core. J. Control. Rel.. 76(3)313–326. [CROSSREF]
- Youan B. B. C., Gillard J., Rollmann B.. 1999. Protein-loaded poly(epsilon-caprolactone) microparticles III. Entrapment of superoxide dismutase by (water-in oil)-in water solvent evaporation method. STP Pharma. Sci.. 9(2)175–181