Abstract
This study involves development of amphiphilic gels consisting solely of nonionic surfactants bearing cyclosporine and characterized for microstructure, gelation temperature, and in vitro drug release into dermis. The formulation is nonirritant and suitable for topical application. Gels consisting of cyclosporine were prepared using different methods by mixing the solid gelator (sorbitan or glyceryl fatty acid esters) and the liquid phase (liquid sorbitan esters or polysorbates) and heating them at 60°C to form a clear isotropic sol phase, and cooling this sol phase to form an opaque semisolid at room temperature. Gel microstructure was examined by phase contrast microscopy while gelation temperatures were measured by melting point apparatus and differential scanning calorimetry. These amphiphilic gels were evaluated in vitro for topical as well as transdermal delivery using rat skin mounted in a Franz diffusion cell. Gel microstructures consisted mainly of clusters of tubules of gelator molecules that had aggregated upon cooling of the sol phase, forming a 3D network throughout the continuous phase. The gels demonstrated thermoreversibility with robust gel network. At temperatures near the skin surface temperature, the gels softened considerably and moreover, it facilitated the drug to accumulate in dermis, thus making an ideal delivery vehicle of cyclosporine topically that can be used in treatment of psoriasis. Thus amphiphilic gels were demonstrated as the ideal vehicle for topical use of cyclosporine.
INTRODUCTION
Cyclosporine A (CsA, cyclosporine), a powerful immunosuppressive agent that selectively inhibits T helper cells, has revolutionized organ transplantation. It is a neutral, lipophilic (log P > 3), cyclic undecapeptide, with molecular weight 1202 Da and very low aqueous solubility (0.04 mg/ml at 25°C). Such poor water solubility and the absence of adequate formulations in which cyclosporine could be administered almost led to the drug being abandoned for clinical development (Borel and Kis Citation1997). Fortunately, this did not happen, lipid formulations were developed and cyclosporine remains the first-line immunosuppressant in organ and tissue transplantation. Also, cyclosporine was approved for the treatment of psoriasis (Lebwohl et al. Citation1998) and also is being investigated for many other disorders of the immune system, such as asthma (Rohatagi et al. Citation2004), moderate-to-severe eye diseases (Sall et al. Citation2000; Robert et al. Citation2001), inflammatory bowel disease (Sandborn Citation1996), and rheumatoid arthritis (Lee et al. Citation2001).
Different routes of drug administration, such as topical, inhalation, and ocular also are being investigated. A perfect treatment for psoriasis with ideal formulation characteristics is still lacking because the drug needs to accumulate in the dermal region rather than in blood. Therefore the amphiphilic gels, consisting solely of nonionic surfactants (Murdan, Ford, and Florence Citation1998), have been prepared for topical delivery of drugs (Jibry and Murdan Citation2001). A range of drugs can be solubilized in the gels, with the possibility of delivering them into and through the skin as the surfactants act as penetration enhancers (Jibry, Zaman, and Murdan Citation2001). These amphiphilic gels bearing cyclosporine were tested for their irritation potential to skin disguised in the form of patch.
The skin is comprised of different layers, each with distinct properties and functions. The main role of the outer layer, the epidermis, is to act as a barrier and prevent the ingress of harmful chemicals and microbes into the body, while restricting the loss of water and other ions from the body to the environment. Cells in the uppermost layer, the stratum corneum, are cornified and embedded in a lipid matrix (Elias Citation1983). It is this impervious layer that forms the barrier to drug penetration into the skin, as well as providing protection to the body from the external environment. It is this layer, therefore, that will be the first point of contact with any topically applied product.
Transdermal delivery offers a number of advantages such as the avoidance of the first-pass effect that usually follows oral delivery (Barry Citation1983); however, if the formulation circumvents drugs being released into blood circulation through topical delivery, it may be of utmost importance for certain skin ailments. The use of chemical penetration enhancers significantly increases the number of candidates suitable for such delivery by increasing skin permeability (Barry Citation1993), but a balance between their benefits and adverse effects is needed. Most penetration enhancers interact with skin constituents and might cause reversible or irreversible damage to the skin cells, which must be investigated. Damage to skin cells is a side effect of many, if not all, penetration enhancers, as an increase in permeation cannot be achieved without some perturbation to the skin barrier (Kanikkannan and Singh Citation2002). Skin irritation is defined as a nonimmunological local inflammatory reaction that is usually reversible. It is characterized by erythema and edema, following a single or repeated application of a chemical to the same cutaneous site (Jibry, Heenan, and Murdan Citation2004).
The gel state has been defined in many different ways. Hermans (Citation1949) defined the gel as a colloid disperse system that is solid-like in its mechanical properties and that consists of at least two components that extend themselves continuously throughout the whole system. Later, Flory (Citation1994) added that a gel must have a continuous structure, for example, well-ordered lamellar structures, and disordered physically aggregated polymer networks, covalent polymeric networks, and particulate structures. The gel state can be classified further depending on the nature of the bonds involved in the three-dimensional solid network, as well as on the nature of the liquid phase. Thus, chemical gels arise when strong covalent bonds hold the network together and physical gels when the hydrogen bonds and electrostatic and Vander Waal interactions maintain the gel network (Hermans Citation1949). When the liquid component is aqueous, the gel is termed a hydrogel and when the liquid is an organic medium, the gel is called an organogel.
The amphiphilic gels, where one surfactant causes the gelation of another, are being studied as delivery vehicles for drugs by the oral route. The gels are able to dissolve certain poorly water-soluble drugs such as cyclosporine and can therefore be used as vehicles for these “difficult” drugs. In vivo experiments in mice and dogs that were orally dosed with cyclosporine-containing gels showed high oral absorption in dogs (Murdan et al. Citation1999). Thus, an attempt was made to develop a formulation of amphiphilic gels carrying cyclosporine topically that can be targeted to infected cells of severe postural psoriasis.
Researchers thought that the surfactant nature of the gels would augment permeation of the active agents into and/or through the skin and that the gels could be used as topical/ transdermal carriers without causing significant irritancy to the skin. Indeed, in experiments in mice and in humans, we have shown that the gels, applied twice a day for 5 consecutive days, showed little irritancy to the skin (Jibry and Murdan Citation2004). In this article, we report on the formation and the physical characterization of amphiphilic gels carrying cyclosporine. Here an attempt has been made to understand the nature of the gels and their behavior when topically applied carrying cyclosporine to the skin as drug delivery vehicle.
MATERIALS AND METHODS
Span 40, 60, 80, Tween 20, 60, 80, and glyceryl monostearate were obtained from Sigma Chemicals (USA) and used as received. Sodium lauryl sulfate was obtained from BDH (UK). Labrasol was obtained as gift sample from Colorcon Asia Pacific Pvt. Ltd (Singapore) and used as received. Sodium chloride and potassium dihydrogen phosphate also were obtained from Sigma Chemicals. Polyethylene glycol was obtained from Aldrich Chemical Company (USA). Orthophosphoric acid was obtained from Qualigens (Mumbai India). All the solvents used in this study were of analytical grade.
HPLC Method for Estimation of Cyclosporine A
The HPLC system used for analysis was equipped with 10 ATVP binary gradient pumps (Shimadzu), a Rheodyne (Cotati, CA, USA) model 7125 injector with a 20 μl loop and SPD-M10 AVP diode array detector (Shimadzu). HPLC separation was achieved on a Lichrosphere Lichrocart CN column (250 mm, 4 mm, and 5 μm) (Merck). Column effluent was monitored at 210 and 205 nm. Data was acquired and processed using Shimadzu software. The mobile phase was a mixture of 0.01% trifluoroacetic acid and acetonitrile (45:55). Both the solutions were filtered and degassed before use. Chromatography was performed at a flow rate of 1.0 ml/min.
Extraction of Cyclosporine A in Formulations
The appropriate volume of formulations were taken into tubes were vortexed for 25 sec and left for 30 min at 37°C. Hexane (5 ml) was added to each tube. The tubes were again vortexed for 5 min and left to stand for 5 min. The hexane layer was separated and to the remaining solution 5 ml of ether was added. The tubes were again vortexed for 5 min, left to stand for 5 min, and dipped in liquid nitrogen for 2 min. The ether layer was decanted off and the solution was transferred to clean glass tubes. The ether extracts were evaporated using Heto vacuum concentrator and the residues were reconstituted in 1 ml of ethanol, and 25 μl of these solutions were injected onto HPLC column.
Extraction of Cyclosporine A in Skin
At the end of permeation experiments, determination of amount of drug in SC was evaluated by tape stripping (20 strips) and after that the surface of the skin was washed three times with distilled water to remove excess drug and formulation from the surface of skin, and then the skin tissues cut into small pieces. The tissue was further homogenized with 2 ml of water. The homogenates were transferred to a 10-ml conical tube and extracted with hexane so that the lipid or any impurities present in the hexane layer are removed. Then homogenates were extracted with 5 ml of ether. After shaking for 5 min and centrifuging for 5 min at 2000 rpm, the organic phase was transferred to a test tube for evaporation to dryness under a stream of nitrogen at 35°C. Residue was redissolved in 1 ml of ethanol, and shaken for 1 min. An aliquot of 20 μl was injected into HPLC for analysis of drug into dermal region (Jianxin et al. Citation2000).
Preparation of Gels
The gels were prepared by adding gelators in fluid surfactant to a minimum concentration at which gelator forms gels (minimum gelation concentration). Span 40, Span 60, and glyceryl monostearate were used as the gelators (solid component of the gel), and the fluid phases consisted of liquid Spans or liquid Tweens and Labrasol. The solid gelator was weighed into a glass vial and the required amount of liquid surfactant was added. The vial was then placed in a water bath at 60°C for 10 min with occasional vortexing. The solid gelator dispersed in the liquid surfactant and a sol phase (homogenous liquid) was produced. The sol phase was allowed to cool by standing at room temperature overnight. Gelation (defined as the transition from a sol to a gel state) was considered successful, if upon inversion of the vial, samples did not surge perceptibly (Jibry et al. Citation2004). The average minimum gelation concentration of different amphiphilic gels has been shown in .
TABLE 1 Minimum gelation concentration of different amphiphilic gels at room temperature
Determination of Phase Transition Temperatures (Tg) of Amphiphilic Gels
The gel-to-sol and/or sol-to-gel phase transition temperatures were measured using differential scanning colorimetry (DSC), as detailed below (Murdan, Andrysek, and Son Citation2005) and is presented in .
TABLE 3 Phase transition temperature of different gels by differential scanning calorimetry in °C measured on day 1 and on storage for 6 months
A DSC (Waters 10) was used to determine the phase transition of some gels. Samples of 200 to 300 mg were weighed into a stainless steel sample cell, heated at a rate of 1°C/min from 0°C to 60°C, and then cooled down to 0°C at the same speed, against a blank reference cell. The endothermic peak on the DSC trace, corresponding to the gel-to-sol phase transition, was used to determine the phase transition temperature. A blank run where both cells were empty also was conducted under the same conditions to obtain a baseline (Murdan et al. Citation2005).
Gel Microstructure
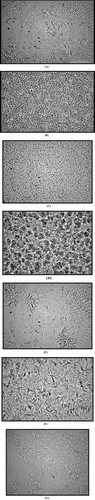
A thin smear of the gel was placed on a microscope (Nikon Eclipse E200) slide, covered with a cover slip, and observed under phase contrast slot at 40× and/or 100× equipped with a digital camera (Nikon, Japan). Light microscopy revealed that like Span 60 and Span 40 organogels, Span 60 and Span 40 amphiphilic gels consist of tubules that become more numerous with increasing gelator concentration. The tubules are clustered into star-or flower-shaped structures depending on the gel (). The clusters, dispersed throughout the continuous phase, are linked to one another forming a coherent network.
In Vitro Skin Permeation
Formulation Used in In Vitro Skin Permeation
Different amphiphilic gel preparations were used for in vitro skin permeation study given in (). These gels were prepared by same method described in the section on Skin Irritation Study. The drug CsA is incorporated into these gels by dissolving known amount of drug into gels. Approximately 10 mg of drug was incorporated into 1.3 gm of gel and from these 200 mg of the gel (∼1.5 mg CsA) was taken in to patch and applied onto the fresh rat skin. Then 30% paraffin wax with same amount of drug incorporated was used as control (Jibry and Murdan Citation2004).
TABLE 2 Amphiphilic gels used for in vitro skin permeation study
Fabrication of Patches
In a flexible double-sided adhesive tape 2.0 mm thickness, a circular hole of 1.5 cm2 area was cut in the center and fixed from one side on an aluminum foil of 2.5 cm, to act as a backing membrane. The weight of gel was uniformly filled in the whole area.
Preparation of Rat Skin
A portion of (3×3 cm) the full thickness skin of the abdominal region of rat was carefully excised freshly after humanely sacrificing the animal. The hair on the rat skin was removed first by clipping with a pair of scissor and then by gently rubbing the finger against the skin, followed by wiping off the undesirable matter on the skin with a cotton swab. The dermal side of the skin was carefully cleared off from any adhering subcutaneous tissue and blood vessels. The prepared skin was then washed with saline and used a fresh (Jianxin et al. Citation2000).
Across the Skin Permeation Study
In vitro skin permeation was performed using Franz diffusion cell. The patch bearing gel (containing the CsA) was applied over the stratum corneum surface of the skin and mounted in the cell (reservoir volume 25 ml, area exposed to the donor compartment 1.5 cm2). The receptor medium was 30% alcohol isotonic with 0.9% NaCl to maintain the sink condition and stirred by magnetic stirrer at 37 ± 2°C. Then 1 ml of receptor fluid was withdrawn periodically for 12 to 24 hr, replaced every time with the fresh fluid solution and samples were analyzed by HPLC. In vitro release profile was observed using different formulations and the same was carried out with free-CsA incorporated in a 30% paraffin wax.
Drug Retention Study in Stratum Corneum
For drug retention study in in vitro permeation experiments, the skin was removed from the diffusion cells after completion of experiments. The skin was cleaned with cotton soaked in isotonic buffer (phosphate buffer 7.4), and dried by gently pressing the skin between two filter papers. Stratum corneum (SC) layer was removed by tape stripping method, using Scotch tape. Stripping was carried out 15 times to remove SC according to previous studies (Jianxin et al. Citation2000). Cyclosporine present in the tape strips was extracted with ethanol. In 10 ml ethanol the strips were dissolved by stirring on a magnetic stirrer for 15 min. The obtained solution is then filtered with a membrane filter and evaporated to dryness. The residue was then redissolved in 1 ml of ethanol and shaken for 1 min. An aliquot of 20 μl was injected into HPLC for analysis.
Stability Studies
The formulations were prepared and stored in glass vials and kept at room temperature for 4 months. These formulations were studied for physical examination: syneresis, consistency, and appearance of phase separation. DSC of the samples were carried out and checked for deviation in phase transition temperature. No major changes were found in the phase transition temperature of the preserved gel samples except in gels in which syneresis occurred ().
Skin Irritation Study
Amphiphilic gels with the following compositions were prepared (Jibry and Murdan Citation2004, 2005)
Gel 1: Span 40/Tween 20 (20% gelator concentration).
Gel 2: Span 40/Span 80 (30% gelator concentration).
Gel 3: Control aqueous cream B.P. (30% paraffin wax).
The gels were prepared by heating the appropriate quantities of gelator (Span 40) and solvent (Tweens) in a water bath at 60°C to produce a clear homogenous sol phase. The latter was left to cool and set as an opaque semisolid gel at room temperature overnight.
Procedure
In skin irritation study, 24 hr prior to the gel application, the animal dorsal surface was shaved with the clippers. The skin was checked for any cuts, if present, and allowed to rest overnight. On day one, all rats were treated topically with respective formulations once daily for 2 days. Two patches were applied containing 100 mg of gel and aqueous cream BP in a 1.5 cm2 patch, respectively, on their backs at a distance of ∼5 cm. All treated sites were then covered with sterile gauze and secured with surgical tape to prevent grooming and removal of the formulation from the skin. At the end of the application time, the gauze was taken off and the treated area was gently wiped off and cleaned.
Visual Assessment and Topical Application
The skin irritation (erythema) was evaluated after each application according to the scale depicted in . Two rats were assigned and marked individually for each formulation group:
Group 1: Rat marked on head received Gel Span 40/Span 80 (20% of gelator concentration).
Group 2: Rat marked on tail received Gel Span 40/Tween 60 (20% of gelator concentration).
Group 3: Rat marked on abdomen received control.
TABLE 4 Visual erythema scoring scale
RESULTS AND DISCUSSION
The surfactant gels were prepared by mixing the increasing concentration of solid surfactant (gelator) in a liquid surfactant (solvent) in appropriate ratio. The lowest concentration of gelator that caused gelation at room temperature was taken as the minimum gelation concentration (MGC). The MGC seems to depend on hydrophilic lipophilic balance (HLB) of both surfactants, melting point of gelator and viscosity of solvent surfactant (Kanouni et al. Citation2002). Span 60 is good gelator as evidenced by its lower gelation concentration compared with Span 40. Span 60 demonstrated low value of MGC compared with Span 40 in all cases (). This could be attributed to the fact that Span 60 being lipophilic (HLB value 4.7) has a longer carbon chain compared with Span 40 (HLB value 5.7) and possesses low intermolecular affinities between gelator and solvent molecules; thus gelator molecules do not have much room in the solvent pool and gelate out at lower concentration. Similarly glyceryl monostearate (GMS) having HLB of 3.8 (lower than both Spans) showed even lower value than Span 60 and 40. The value of MGC follows: Span 60 > Span 40 > GMS. The lower gelation concentration is considered to be appropriate as maximum number of solvent molecules has room to interact per gelator. Higher melting point of GMS (61°C) than of Span 60 and 40 may be attributed to lower solubility in the solvent phase that could result in low gelator solvent interactions and gelate at lower concentration.
The prepared gel melts to the solution phase that can be cooled again to the gel state signifies its thermoreversible property. The gelation concentration observed in Tween 60 is less than other Tweens, which may be due to its viscous nature at room temperature. In case of labrasol the gelator concentration is due more to its low viscosity compared with other solvents (Lin, Kachar, and Weiss Citation1989).
The phase transition temperature (Tg), an indicator of temperature at which gel converts to sol and vice versa, increased as gelator concentration was increased. Furthermore, at the same gelator concentration, the melting points of both Span 60 and Span 40 gels increased with increasing HLB of the liquid component of the gel. Gel-to-sol transition takes place when cross-linked amphiphiles undergo breakdown followed by deaggregation (James Citation1986). The breakdown of cross-link and deaggregation of gel network does not take place simultaneously; therefore, a sharp phase transition temperature is not expected. The phase transition temperature of all the gels was determined by DSC and presented in .
The prepared gels were stored for 6 months and then evaluated for stability. The gels that showed fine aggregates were found to be more stable than larger aggregates. All the gels except Span 40/Span 80 and GMS/Tween 60 showed good stability. Span 40/Span 80 and GMS/Tween 60 gels showed syneresis that may be attributed to larger aggregates formed. Syneresis is caused by contraction of the solid network of tubules, as a result of which the fluid is pushed out and the two phases of gel system separate (Attwood and Florence Citation1983). Labrasol gels were found stable after 6 months due to high gelation concentration. The phase transition temperatures of these gels were noted, and there was no significant change in phase transition temperature on storage ().
Structures of these gels were observed microscopically. The gel network was found to disperse in the form of tubules and clusters. The clusters, dispersed throughout the continuous phase, linked to one another and formed a coherent network. Microscopy showed more clearly that the star-shaped clusters (GMS/Tween 60) are composed of fine tubules converging at a central point forming a channel nodule and that the tubular (Span 60/Tween 80) aggregates are linked together via junction points. In contrast to the star-shaped structure, clusters of tubules were observed in the majority of the amphiphilic gels. With the increase in gelator concentration, the network also increased. The microscopical features of the gels composed of Tween 60 exhibited fibrous segments rather than tubule clusters as in the case of other solvents (, , , , , , ). The fibrous structure observed in this case could be because highly viscous solvent surfactants molecules surround each gelator molecule and prevent intergelator connections. This effect thus results in fibrous structure of the gel and is in agreement with studies of (Barreiro-Iglesias, Alvarez-Lorenzo, and Concheiro Citation2001) who demonstrated decrease in viscosity of carbopol gels on addition of nonionic surfactants.
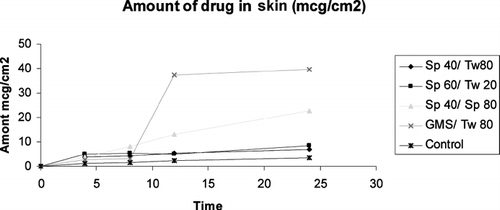
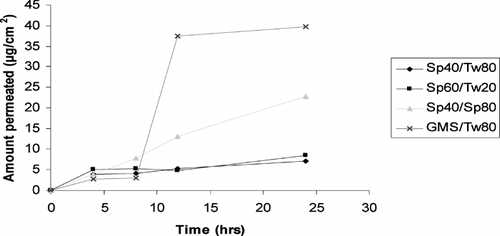
In vitro skin permeation studies were used to estimate transdermal/topical permeation, as the method is economical, readily available, and correlates well with in vivo performance. Therefore in the present investigation, in-vitro transdermal/topical permeation of CsA incorporated in amphiphilic gels was carried out. The area of the donor compartment was 1.766 cm2. Nearly 200 mg of gel bearing CsA was applied on freshly excised, cleaned, and shaved rat skin. In this study the amount of drug retained in SC as well as dermal region was determined. We observed that the drug was undetectable across the skin on application of various formulations. A long lag phase up to 12 hr was observed. The lag phase and poor release was not only due to the large molecular size of CsA, but also due to the fact that it has a ring structure and thus by its nature, a large diffusional cross-section. The amount of CsA in the skin and stratum corneum was determined and shown in ( and ).
Lipophilic drug such as CsA generally presents higher partition coefficient in SC. However, partition in viable epidermis is low presumably due to accumulation in SC. Different strategies can be used to increase the topical/transdermal delivery of the drugs. In our present study we verified whether the amphiphilic gels promoted the topical/transdermal delivery of CsA. We studied the effect of various amphiphilic gels made of different nonionic surfactants on delivery of CsA as a function of time and evaluated the retention of CsA in the SC compared with the control formulation. The preparation containing amphiphilic gels enhanced the topical delivery of the CsA within several hours. The CsA in receptor phase or across the skin was minute or undetectable during the first 12 hr.
Skin permeation study was performed by applying amphiphilic gels bearing CsA on rat skin. We found that on application of GMS/Tween80, the amount of drug found in dermis portion was (39.6 μg/cm2) almost 10-fold compared with the amount of drug present in the SC (i.e., 4 μg/cm2) 12 hr postapplication. In Span 40/Span80, the amount of drug in dermis region was ∼12 μg/cm2, and almost the same amount was found in SC that was 3-fold lower than GMS/Tween 80. In fact the lipophilic drug such as CsA presented high partition in SC; however, its partition in viable epidermis is low, presumably due to the accumulation in SC. These findings agree with the previous studies reporting that GMS acts as a penetration enhancer. Formulations containing GMS have been shown to increase topical delivery of urea and indomethacin (Ogiso, Iwaki, and Paku Citation1995). Additionally, cubic phases made of monoolein increase the permeation of several drugs including peptides (Carr, Earish, and Carrign Citation1997).
HLB value also plays a significant role in the skin permeation. Since partitioning seems to be effective in GMS/Tween 80, it could be due to a larger difference in the HLB value and could have resulted in better partitioning of the drug. In Span 40/Span 80, the amount observed in dermis region was 3-fold less compared with the GMS/Tween 80. This could be due to lipophilic nature of both Span 40 and Span 80 (with high HLB value). This could have resulted in high affinity with CsA and have prevented the drug to permeate beyond SC. In Span 60 and Span 40 the permeation in dermis is low, which could be due to hydrophobic interaction of CsA with high HLB Span 60 and Span 40.
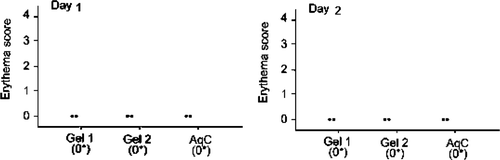
In the case of amphiphilic gels for skin compatibility measured with erythema (Jibry and Murdan Citation2004), Erythema was evaluated visually after each exposures of mouse skin to the different formulations. Erythema that is caused by the increased blood flow in dermis region enabled us to monitor the response of the preparation in that layer. The score for each rat at the end of the day 1 and 2 was found to be zero for all formulations (). Aqueous cream B.P did not cause any viable change on day 1 in any rat. However, no significant difference was found between the erythema score before treatment and on any day after treatment with the gels. There was no significant difference found in irritation potential of gel 1: Span 40/Tween 20 (30%) and gel 2: Span 40/Span 80 (30%) and between both gel and aqueous cream B.P. From these observations, we can say that amphiphilic gels were significantly nonirritant and have the same safety profile compared with aqueous cream B.P, a widely used moisturizer.
CONCLUSION
The gel is a colloidal disperse system that is solid-like in its mechanical properties and consists of at least two components that extend themselves continuously throughout the whole system. An attempt has been made to prepare amphiphilic gels composed solely of nonionic surfactants. Results indicated that gelation occurs on cooling the solution phase and gelator molecule self-assembles into tubular clusters or long fibers that form a network that immobilizes the fluid phase. A wide range of gels in which the gelator was Span 60, Span 40, GMS, the solvent phase was Tween 20, 40, 80, and Labrasol were tested. Where Span 60 forms gel at lower concentration, these gels are semisolid at rest and converted into solution form with increase in temperature in stability studies performed.
The skin permeation studies revealed on application of GMS/Tween 80 containing cyclosporine, the amount of drug found in the dermis portion was (39.6 μg/cm2) almost ten times compared with the amount of drug present in the SC (i.e., 4 μg/cm2) after 12 hr postapplication. In Span 40/Span 80 the amount of drug in the dermis region was ∼12 μg/cm2, and almost the same amount was found in SC that is three times lower than GMS/Tween 80. By this we can say the HLB value plays a significant role in skin permeation. Partitioning seems to be effective in case of GMS/Tween 80. In case of Span 40/Span 80, the amount observed in dermis region was less (about three times) compared with the GMS/ Tween 80. This could be due to lipophilic nature of both Span 40 and Span 80 (having high HLB value). In case of Span 60/Span 40 the permeation in dermis is low, which could be due to hydrophobic interaction of CsA with high HLB Span 60 and Span 40.
REFERENCES
- Attwood D., Florence A. T. Surfactant Systems, their Chemistry, Pharmacy, and Biology. Chapman and Hall, London 1983
- Barreiro-Iglesias R., Alvarez-Lorenzo C., Concheiro A. Incorporation of small quantities of surfactants as a way to improve the rheological and diffusional behavior of carbopol gels. J. Control. Rel. 2001; 77(1–2)59–75
- Barry B. W. Dermatological Formulations: Percutaneous Absorption. Marcel Dekker, New York 1983; 1–48
- Barry B. W. Vehicle effect: what is an enhancer?. Topical Drug Bioavailability, Bioequivalence, and Penetration, V. P. Shah, H. I. Maibach. Plenum Press, New York 1993; 261–276
- Borel J. F., Kis Z. L. The discovery and development of cyclosporine (Sandimmune). Transplant. Proc. 1997; 23: 1867–1874
- Carr M. G., Earish S., Carrign O. J. Drug delivery from a liquid crystalline base across visking and human stratum corneum. Int. J. Pharm. 1997; 157: 35–42
- Elias P. Epidermal lipids, barrier function and desquamation. J. Invest. Dermatol. 1983; 80: 44–49
- Flory P. J. Introductory lecture. Disc. Faraday Soc. 1994; 57: 7–18
- Hermans P. H. Gels. Colloid Science, H. R. Kruyt. Elsevier Publishing, Amsterdam 1949; 2: 483–651
- James K. C. Solubility and Related Properties. Marcel Dekkar, New York 1986
- Jianxin G., Qineng P., Guoqin S., Chunhong J. Lecithin vesicular carriers for transdermal delivery of cyclosporine A. Int. J. Pharm. 2000; 194: 201–207
- Jibry N., Murdan S. Preparation and characterization of novel amphiphilogels. British Pharmaceutical Conference, GlasgowScotland, UK, 2001
- Jibry N., Zaman A. A., Murdan S. Amphiphilogels: delivery vehicles for poorly-soluble drugs. British Pharmaceutical Conference, GlasgowScotland, UK, 2001
- Jibry N., Heenan R. K., Murdan S. Amphiphilogels for drug delivery: formulation and characterization. Pharm. Res. 2004; 21: 1852–1861
- Jibry N., Murdan S. In vivo investigation, in mice and in man, into the irritation potential of novel amphiphilogels being studied as transdermal drug carriers. Eur. J. Pharm. BioPharm. 2004; 58(1)107–119
- Kanikkannan N., Singh M. Skin permeation enhancement effect and skin irritation of saturated fatty alcohols. Int. J. Pharm. 2002; 248: 219–228
- Kanouni M., Rosano H. L., Naouli N. Preparation of a stable double emulsion (W1/O/W2): role of the interfacial films on the stability of the system. Adv Colloid Interface Sci. 2002; 99(3)229–254
- Lebwohl M., Ellis C., Gottlieb A., Koo J., Krueger G., Linden K., Shupack J., Weinstein G. Cyclosporine consensus conference: with emphasis on the treatment of psoriasis. J. Am. Acad. Dermatol. 1998; 39: 464–475
- Lee W. K., Lee J., Kim W. U., Cho C. S., Kim H. Y., Ahn H. J., Han S. H., et al. Combination therapy with cyclosporine and methotrexate in severe rheumatoid arthritis. A prospective, multicenter, 40-week study. Arthr. Rheum. 2001; 44: S1914
- Lin Y. C., Kachar B., Weiss R. G. Novel family of gelators of organic fluids and structure of their gels. J. Am. Chem. Soc. 1989; 11: 5542–5551
- Murdan S., Ford J., Florence A. T. Novel surfactant-in-surfactant amphiphilogels. J. Pharm. Pharmacol. 1998; 50: 151
- Murdan S., Arunothayanun P., Ford J., Florence A. T. Amphiphilogel systems as oral delivery vehicles for cyclosporine A: preliminary in vivo results. Proc. Symp. Lipid Surfactant Dispersed Sys. 1999; 237–238
- Murdan S., Andrysek T., Son D. Novel gels and their dispersions as oral drug delivery systems for cyclosporine. Int. J. Pharm. 2005; 300(1–2)113–124
- Ogiso T., Iwaki M., Paku T. Effect of various enhancers on transdermal penetration of indomethacin and urea, and relationship between penetration parameters and enhancement factors. J. Pharm. Sci. 1995; 84(4)482–488
- Robert P. Y., Leconte V., Oliv E. C., Ratsimbazafy V., Javerliat M., Adenis J. P. Cyclosporine A eyedrops: manufacturing, kinetics and indications in 2000. J. Fran. D′ Ophtalmol. 2001; 24: 527–535
- Rohatagi S., Calic F., Harding N., Ozoux M. L., Bouriot J. P., Kirkesseli S., DeLeij L., Jensen B. K. Pharmacokinetics, pharmacodynamics, and safety of inhaled cyclosporine A (ADI628) after single and repeated administration in healthy male and female subjects and asthmatic patients. J. Clin. Pharmacol. 2004; 40: 1211–1226
- Sall K., Stevenson O. D., Mundorf T. K., Reis B. L. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology 2000; 107: 631–639
- Sandborn W. J. A review of immune modifier therapy for inflammatory bowel disease: azathioprine, 6-mercaptopurine, cyclosporine, and methotrexate. Am. J. Gastroenterol. 1996; 91: 423–433