Abstract
For emergency contraceptive, the rapid delivery of levonorgestrel (LNG) to plasma is desirable, furthermore, a sustained delivery of LNG along with rapid absorption will be necessary. The pharmacokinetics and pharmacodynamics of LNG entrapped in different kinds of liposome formulations via nasal administration in rats were evaluated and compared with LNG suspension via the oral route. The relative bioavailabilities of these liposome formulations via nasal administration were 100% or higher than 100%. The Cmax and Tmax values of sterylglucoside (SG) and chitosan-contained formulations by nasal administration were 416.84 ng/mL and 1.02 hr, 227.97 ng/mL and 2.02 hr, respectively, compared with that of 334.94 ng/mL and 1.89 hr of oral suspension. Fully 100% contraception was observed for all the formulations. SG could promote the absorption of LNG via the nasal route and may provide a rapid onset of action of LNG for emergency contraception. Chitosan could retain LNG in the nasal cavity for long contact time to sustain delivery of LNG. The rapid onset and sustained delivery of LNG can be achieved via the nasal route using liposomes as the vehicle.
INTRODUCTION
Nasal route of drug delivery was conventionally used for treatment of local diseases such as nasal infections, nasal congestion, and nasal allergy. Recent studies have exploited the nasal route for the systemic delivery of drugs such as peptide and protein drugs, vaccine, polar drugs with small molecular weight, and some lipophilic drugs (Illum Citation2003). Lipophilic drugs, such as fentanyl, pentazocine, testosterone, and nifedipine, are well absorbed from the nasal cavity with the pharmacokinetic profiles often identical to those obtained after intravenous injection and bioavailabilities approaching 100%. It has also been shown that the bioavailability of progesterone, 17 β-estradiol, 17 α-ethinylestradiol, and norethisterone (Kumar et al. Citation1991; Hussain, Kimura, and Huang Citation1984) given via nasal route in rats is superior to that via the oral route. Levonorgestrel (LNG) is a synthetic progestin used as a progestin-only emergency contraceptive and either alone or in combination with an estrogen, as an oral contraceptive (Kook, Gabelnick, and Duncan Citation2002). LNG also has been the progestogen of choice for inclusion in drug delivery systems such as implants, intrauterine devices, and intravaginal rings. A study involving nasal administration of LNG also showed the superiority of the nasal route (Shahiwala and Misra Citation2004).
To aid emergency contraceptive, the rapid delivery of LNG to plasma is desirable, furthermore a sustained delivery of LNG along with rapid absorption is necessary. The sustained delivery of LNG can be accomplished via the use of transdermal LNG patches, but considerable time is generally required to reach a steady-state blood concentration of LNG from such patches. On the other hand, the rapid delivery of LNG can be achieved through the nasal route. Our objectives were to evaluate the absorption-promoting effect of sterylglucoside (SG)-contained liposomes on intranasally administered LNG. Chitosan also was incorporated as a mucoadhesive agent to retain the formulations at the nasal cavity and to enhance LNG bioavailability via the nasal route. The pharmacokinetic and contraceptive efficacy was evaluated in rats.
MATERIALS AND METHODS
Levonorgestrel (LNG) was provided as a gift from Zizhu Pharmaceutical (Beijing, China); egg phosphatidylcholine (EPC) was purchased from Engel Bio (Sichuan, China); cholesterol (Ch) was purchased from Wako Pure Chemical Industries (Tokyo, Japan); chitosan (MW 100, 000 Da and the deacetylation degree 85%) was purchased from Qingdao Scitech Co. (Shandong, China); sterylglucoside (SG) was supplied by Ryukakusan Co. (Tokyo, Japan). All other chemicals were of reagent grade.
Preparation of LNG Formulations
The liposome suspensions of EPC/Ch/LNG, EPC/SG/LNG, and EPC/SG/LNG/chistan were prepared in a drug : EPC : Ch or SG molar ratio of 2 : 2 : 1 and dissolved in chloroform-methanol. The organic solvent was thoroughly removed by nitrogen flow at room temperature to form dry membranes. Then 200 μL of 0.9% sodium chloride solution or 1% chitosan solution in 0.01% acetic acid was added into the dry membranes and subjected to bath sonication for 5 min. The final concentration of LNG in different formulations was maintained at 25 mg/mL. The mean particle size was 10–15 μm determined by microscopy.
LNG suspension was prepared in 0.9% sodium chloride solution by sonication with a concentration of 0.5 mg/mL, and the mean particle size was 10–15 μm determined by microscopy.
Pharmacokinetic Studies
Female Wistar rats (250 ± 20 g) obtained from the Institute of Zoology, Chinese Academy of Sciences (Beijing, China) were used unless otherwise specified. All animals were housed in polypropylene cages with free access to palletized chow and tap water, with a 12-hr light/dark cycle. For nasal administration, animals were partially anesthetized with anesthetic ether, and the formulations of EPC/Ch/LNG, EPC/SG/LNG, and EPC/SG/LNG/chistan were administered to the nasal cavity by means of micropipette. The dose of LNG was 500 μg/rat or 350 μg/rat, respectively. For oral administration, a 500 μg/rat LNG suspension was given by mouth with a 28-gauge long blunt needle.
For all animals, blood samples were withdrawn from the orbital vein prior to the administration and at 1, 2, 3, 4, 6, 8, and 12 h after the administration. The resulting plasma was separated by centrifugation at 4000 rpm for 10 min. The plasma samples (400 μL) were extracted with 500 μL of chloroform for twice. The organic phase was combined and evaporated under a stream of nitrogen at 40°C until dry. The residue was reconstituted in 100 μL of acetonitrile/water (70/30, v/v) solution and 50 μL of sample was subjected to HPLC. The HPLC system (HP series 1100) consisted of a model 1100 pump, a model 1100 detector with 240 nm, and a Hypersil ODS2 column (4.6 × 250 nm). The HPLC analysis was performed at ambient temperature with a mobile phase consisting of acetonitrile and water (70:30, v/v) at a flow rate of 1 mL/min.
Plasma concentration versus time profiles were evaluated by standard noncompartmental methods. The highest observed plasma concentration and the corresponding time were defined as the Cmax and Tmax values, respectively. The elimination rate constant (K) was obtained by linear regression from the best-fit slope of the terminal log-linear decline in plasma concentrations versus time profile. The half-life (t1/2) was obtained at 0.693/K. The area under the plasma concentration curve to the last quantifiable concentration (Ct) at time t (AUC0-t) was determined by linear trapezoidal integration. The relative bioavailability (F) of nasal route against oral route was calculated.
Pharmacodynamic Study
Only animals with proven fertility were selected for the study. In each group, 10 female estrous Wistar rats and 3 male Wistar rats (200 ± 20 g) were used for mating. Each female rat was inspected every morning for evidence of mating (the presence of vaginal plugs or sperm). As soon as copulation was detected (defined as day 1), rat was removed from the mating cage, LNG formulations were administered for a consecutive period of 4 days, with the LNG suspension via oral and liposome formulations via nasal route, at a dose of 350 μg per rat. The rats were killed on the postcoital day 12. The uterus was torn apart and the number of embryos was counted.
RESULTS
Pharmacokinetics
The plasma concentration–time curve of LNG is shown in , and the pharmacokinetic parameters of each curve were calculated and shown in .
FIG. 1 Mean levonorgestrel plasma concentrations versus time profiles for oral and nasal administration (mean ± SD, n = 4). ♦-LNG suspension by oral administration at 500 μg/rat; ▴-EPC/SG/LNG by nasal administration at 500 μg/rat; ▵-EPC/SG/LNG/chitosan by nasal administration at 500 μg/rat; •-EPC/SG/LNG by nasal administration at 350 μg/rat; ▪-EPC/Ch/LNG by nasal administration at 350 μg/rat.
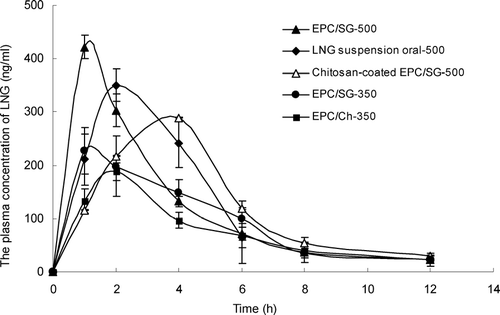
TABLE 1 Pharmacokinetic parameters of different levonorgestrel (LNG) formulations after oral or nasal administration in rats*
The F of EPC/Ch/LNG was 99.33%, while the F of EPC/SG/LNG (114.19% and 110.64% at the dose of 500 and 300 μg, respectively) and EPC/SG/LNG/chitosan (114.49%) were higher than that of EPC/Ch/LNG. Compared with the Cmax and Tmax of LNG suspension by oral administration, EPC/Ch/LNG showed a significantly decreased Cmax and Tmax, from 334.94 ng/mL to 263.70 ng/mL and 1.89 hr to 1.43 hr respectively. Substitution of cholesterol by SG increased the Cmax and decreased the Tmax markedly, indicating that EPC/SG/LNG have a better absorption promoting effect on LNG than EPC/Ch/LNG. When EPC/SG/LNG were suspended in 1.0% chitosan solution (EPC/SG/LNG/chitosan), the Cmax and Tmax were both lowered to 227.97 ng/mL and 2.02 hr, respectively.
Pharmacodynamics
Pharmacodynamic studies were carried out as described above. The administration scheme and the results are shown in . Fully 100% contraception was observed for all the formulations.
TABLE 2 Contraceptive effects with different levonorgestrel (LNG) formulations
DISCUSSION
Nasal route of drug delivery was conventional and effective for lipophilic drugs, as described in the Introduction. Although the lipophilic drugs can be easily and fully absorbed into the systemic circulation via nasal route, the generally rapid clearance of the administered formulation from the nasal cavity should be surmounted (Illum Citation2003). Bioadhesive nasal delivery systems provide a prolonged contact between the drug formulations and the absorption site in the nasal cavity by delaying the mucociliary clearance. This could maintain an effective drug concentration for prolonged periods of time with improved bioavailability (Soane et al. Citation1999). However, bioadhesive materials always lead to a delayed absorption of intranasally administered drug formulations because viscidity (Shahiwala and Misra Citation2004) was opposite to the initial aim of exploiting the advantage of rapid onset of action when administered via nasal route. Therefore, bioadhesive nasal delivery systems in combination with absorption promoting agents are possible to achieve a prolonged action time with improved bioavailability and without delaying the onset time.
Liposomes are phospholipids vesicles composed of lipid bilayers enclosing one or more aqueous compartments. Liposomes provide an efficient drug delivery system because they can alter the pharmacokinetics and pharmacodynamics of entrapped drugs. Liposomes are attracting great interest for drug delivery by the nasal and lung route. In fact, the absorption enhanced behavior of liposomes is related to the incorporation of phospholipids in the membrane, opening “new pores” in the paracellular tight junction (CitationIllum 1997).
In the present study, the lipid concentration in all liposome formulation were ∼75 mg/mL, thought to be so viscous that it can remain in the nasal cavity for sufficient time to allow complete absorption of LNG. Increased bioavailabilities of LNG were observed for EPC/SG/LNG and EPC/SG/LNG/chitosan formulations via nasal route than for the oral route and EPC/Ch/LNG formulation via nasal route. The increased Cmax and reduced Tmax of EPC/SG/LNG were significant compared with oral formulation. LNG was quickly absorbed into the systemic circulation and exhibited a rapid onset of action by nasal route. These changes were due to that the SG can promote drugs absorption. It has been reported that SG promotes insulin absorption and enhances the bioavailability of verapamil following nasal administration (Maitani et al. Citation2000). A possible explanation for SG to promote drug absorption was that it affects both paracellular pathway and transcellular pathways caused by perturbation of lipids (Maitani et al. Citation2000).
Chitosan has been shown to have mucoadhesive properties because of its viscosity and interaction of the positively charged amino group with the negatively charged sites on the mucosa surface (Artursson et al. Citation1994; Luessen et al. Citation1996). EPC/SG/LNG/chitosan significantly sustained the release and absorption of LNG when compared with EPC/SG/LNG. The fast-released LNG from EPC/SG/LNG has to traverse the viscous and hydrophilic chitosan gel to reach the nasal mucosa before absorption takes place. Although the Tmax was significantly increased to 2.02 hr compared with 1.00 hr of EPC/SG/LNG, there was no significant difference with 1.89 hr of LNG suspension given orally. Furthermore, the T1/2 was increased to 4.48 hr for the EPC/SG/LNG/chitosan, suggesting that chitosan could retain LNG in the nasal cavity for longer contact time for LNG transport across the nasal mucosa.
The pharmacodynamic study showed that both administration routes were effective for emergency contraception and prevent a high percentage of pregnancies when used within a few days after coitus.
CONCLUSION
Nasal administration of liposome formulations of LNG were developed and evaluated for their pharmacokinetic and pharmacodynamic efficacy. EPC/SG/LNG via nasal route can increase the bioavailability of LNG when compared with LNG suspension by oral route. EPC/SG/LNG turned out to facilitate LNG absorption greatly via the nasal route and may provide rapid onset of action of LNG for emergency contraception. Chitosan could retain LNG in the nasal cavity for longer contact time. Therefore, EPC/SG/LNG/chitosan via nasal route may serve as a sustained-release LNG delivery system with a rapid onset of action for emergent contraception.
We are very thankful to Prof. Liu Bin (Department of Histology & Embryology, School of Basic Medical Sciences, Peking University) for his guidance in the pharmacodynamic study of LNG.
REFERENCES
- Artursson P., Lindmark T., Daris S. S., Illum L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm Res. 1994; 11: 1358–1361
- Hussain A. A., Kimura R., Huang C. H. Nasal absorption of testosterone in rats. J. Pharm. Sci. 1984; 73: 1300–1301
- Illum L. Third Annual Meeting of the Nasal Drug Delivery Focus Group. American Association of Pharmaceutical Scientists, Boston, MA November 5, 1997
- Illum L. Nasal drug delivery-possibilities, problems and solutions. J. Control. Rel. 2003; 87: 187–198
- Kook K., Gabelnick H., Duncan G. Pharmacokinetics of levonorgestrel 0.75 mg tablets. Contraception 2002; 66: 73–76
- Kumar T. C., Shah R. S., Chitlange S. M., et al. Effects of intranasal administration of norethisterone on folliculogenesis, cervical mucus, vaginal cytology, endometrial morphology and reproductive-endocrine profile in women. Contraception 1991; 44: 245–67
- Luessen H. L., Leeuw B. J., Langemeyer M. W., Boer A. B., Verhoef J. C., Junginger H. E. Mucoadhesive polymers in peroral peptide drug delivery. IV. Carbomer and chitosan improve the intestinal absorption of the peptide drug buserelin in vivo. Pharm. Res. 1996; 13: 1668–1672
- Maitani Y., Nakamura K., Suenaga H., Kamata K., Takayama K., Nagai T. The enhancing effect of soybean-derived sterylglucoside and β-sitosterol β-d-glucoside on nasal absorption in rabbits. Int. J. Pharm. 2000; 200: 17–26
- Shahiwala A., Misra A. Nasal delivery of levonorgestrel for contraception: an experimental study in rats. Fertil. Steril. 2004; 81: 893–898, (supp l)
- Soane R. J., Frier M., Perkins A. C., Jones N. S., Davis S. S., Illum L. Evaluation of the clearance characteristics of bioadhesive systems in humans. Int. J. Pharm. 1999; 178: 55–65