Abstract
Ethylcellulose inserts of niridazole fabricated by casting were studied for in vitro release and in vivo clinical effectiveness. The in vitro drug release was steady and sustained for over 7 days and followed diffusion kinetics. Selected batch, EN3, was evaluated clinically in patients with periodontitis for 6 months. A significant improvement (α ≤ 0.05) in clinical indices from baseline was observed. Intergroup study revealed a significant (α ≤ 0.01) change in the bleeding index, gingival index, plaque index, calculus criteria, and pocket depth. Significant reduction in total bacterial count in gingival crevicular fluid was observed before and postdevice insertion, as well as between control and treatment groups.
Periodontal pockets are developed by pathogenic infection at gingival sulcus. Under severe conditions, the infection may aggravate leading to a loss of teeth. Bacteria in the periodontal pocket stimulate flow of serum ultrafiltrate or gingival crevicular fluid (GCF) that rapidly washes out materials put into the pocket. Periodic mechanical debridement of plaque and topical or systemically applied antimicrobials were advocated for treatment of periodontitis until now (Smith et al. Citation1998; Addy and Renton-Harper Citation1996). The effectiveness of the conventional treatment is, however, limited by the lack of accessibility of the periodontopathic organisms inside the periodontal pocket (Medlicott, Tucker, and Holborow Citation1994). The direct application of therapeutic drugs, or their introduction via a mouth rinse, does not achieve effective concentrations at the periodontal pocket for sufficient duration of time.
Systemic treatment shows peak concentrations in ∼2–3 hours after oral administration. This system theoretically reaches all periodontal pockets, but majority of drug concentrates in serum. This prompts repeated systemic administration, compromising patient compliance. Furthermore, several drawbacks are associated with systemic administration, such as high cumulative dose, severe side effects, and low availability of antimicrobial, at the site of action has limited this route of administration (O'Connor, Newman, and Wilson Citation1990; Larsen and Fiehn Citation1997).
Local drug delivery devices release small amounts of active drugs over a time directly to the site of infection, which overcomes the shortcomings of the conventional modes of antimicrobial application (Arrona et al. Citation1998; Ciancio Citation1999; Kinnane and Radvan 1999; Somayaji et al. Citation1998; Schwach-Abdellauoi, Vivien-Castioni, and Gurny 2000). The greatest advantage of intrapocket delivery systems is that treatment does not depend heavily on patient compliance. Also, the amount of drug required to achieve effective concentration in GCF is considerably less than systemic regimen. Many antimicrobial agents like tetracycline (Friesen et al. Citation2002), ampicillin (Catauro et al. Citation2006), metronidazole and amoxycillin (Ahuja, Ali, and Rahman Citation2006), ornidazole (Mastiholimath et al. Citation2006) and, minocycline (Lu and Chei Citation2005) have been reported for treatment of periodontitis.
Niridazole, a substituted nitrothiazole derivative, has been successfully used in schistosomiasis (Mahmoud Citation1977) and other helminthic infections. Following the discovery of praziquantel, a safer alternative, niridazole was used clinically as a second line drug (Andraus, Thomas, and Szubert Citation1983), and its use was slowly phased out following incidents of high dose-related side effects ranging from headache to convulsions and psychosis (Abdallah and Saif Citation1969; Coutinho and Barreto Citation1969). Wade and Addy (Citation1987) compared the in vitro activity of metronidazle, niridazole, and tetracycline against subgingival bacteria in chronic patients. Niridazole was consistently more effective than the other drugs against obligate anaerobes and exhibited activity against facultative organism. Based on the in vitro efficacy of niridazole against subgingival bacteria the authors suggested that niridazole is worthy of evaluation in periodontal patients. Further, the low minimum inhibitory concentration of niridazole (0.25 mg/L) for periodontopathogens (Hof et al. Citation1985; Hof and Stroder Citation1986) requires very low doses of niridazole for intrapocket delivery and consequently almost no systemic side effects are expected.
In an earlier article, we discussed niridazole inserts of resomer RG503H and RG858 for local long-term delivery at periodontal pocket (Barat et al. Citation2006); however, the clinical evaluation was limited to 1 month. The objective of this study was to develop film type inserts of niridazole using ethylcellulose for long-term treatment of periodontal disease. The inserts were prepared by casting and optimized to achieve inserts with desired physical properties. The properties of the films were studied and in vitro characteristics and long-term clinical trials of the inserts in patients suffering from periodontal disease were investigated.
MATERIALS AND METHODS
Niridazole was obtained as gift sample from Egis Pharmaceuticals Ltd. (Hungary). Ethylcellulose (degree of substitution 2.42–2.53, viscosity of 5% solution in 80:20 toluene: ethanol at 25°C was 14 cps) was procured from CDH chemicals (Mumbai, India). All other reagents used were of analytical grade.
Preparation of Inserts
Casting Method
An accurately weighed quantity of ethylcellulose (), with or without plasticizer namely dibutyl phthalate, was dispersed in chloroform in a beaker and stirred until a clear solution was obtained. Niridazole, sieved through #300, was dispersed into polymeric solution until it was homogeneous. The resultant polymeric mixture was deaerated under vacuum, cast on leveled glass moulds, and dried at room temperature under an inverted funnel for 36 hr. Upon drying, the films were wrapped in butter paper and stored in amber colored glass vials in a desiccator until further use. The films were subdivided into inserts (2 × 6 mm) by punching out.
TABLE 1 Composition and physicochemical characterization of the prepared inserts
Spraying Method
One batch of inserts (EN7) was prepared by spraying technique. Briefly, drug and polymer solution containing plasticizer (dibutyl phthalate) was sprayed at a rate of 10–12 ml/ hr with a spray gun (nozzle size-0.5 mm) on a Teflon plate under air pressure (760–765 mm of Hg). After spraying, the films were dried as described above for the casting method.
EVALUATION OF INSERTS
Thickness and Weight Uniformity Studies
Inter- and intrabatch variation in thickness and weight uniformity studies on fabricated inserts were performed. The thickness of the inserts was measured at five different randomly selected spots with a screw gauge. For uniformity of weight, 10 inserts from each batch were weighed individually and their average determined. The readings are an average of three trials.
Drug Content Uniformity
For determination of uniformity of drug content, 6 inserts from each batch were weighed individually and placed in a volumetric flask containing 3–5 ml of dimethyl formamide (DMF) to leach out the drug. After swirling for 1 hr, the volume was made up in McIlvaine's buffer pH 6.6. The resultant solution was filtered through a G-2 glass filter. An aliquot of the filtrate was suitably diluted and analyzed for NZ content at 365 nm (Shimadzu, UV-1601, Japan).
In Vitro Release Studies
Weighed inserts were placed in a stainless steel wire mesh holder of dimensions 2 × 4 × 6 mm and suspended in amber-colored vials containing 10 ml of McIlvaine's buffer pH 6.6 as the dissolution medium. The vials were stoppered and placed on a vial holder fitted in a water bath thermostated at 37 ± 1°C. At predetermined time intervals the dissolution medium was completely withdrawn and replaced with a fresh 10-ml portion of the prewarmed buffer to ensure sink conditions. The samples were analyzed for niridazole content on a UV/Vis-spectrophotometer at 365 nm (UV-1601, Shimadzu, Japan), using an appropriate blank.
Clinical Trials
Approval of the ethical committee of the Institute of Medical Science (Banaras Hindu University, India) was obtained prior to the commencement of study. The design of the trial followed a single blind parallel group with matched pair design. Prior to full-scale clinical trials, a pilot study was done on 12 patients (18–55 years of age, either sex). The patients, constituting 24 sites each in treatment and control, were selected from the outpatient department at Department of Dentistry, Institute of Medical Sciences, Banaras Hindu University. After satisfying about the general health status of patients, only those with at least 4 periodontal pockets with depth equal to or greater than 6 mm, that were not adjacent to each other were enrolled for clinical trials. Pregnant or lactating patients and those with a history of antibiotic and/or periodontal therapy within the past 6 months were excluded from the clinical study. The selected patients were briefed about the treatment types (full mouth scaling and periodontal therapy), treatment duration, number of follow-up (FU) visits, and the benefits and risks involved. After obtaining written consent from the patients to participate in the trial, oral hygiene instructions (OHI) were given.
Prescaling, depending upon the severity of calculus and plaque and accessibility of the subgingival sulcus, was carried out in 2–3 sittings at intervals of 7 days. The maximum time interval for complete supragingival scaling between initiation of prescaling and day 0 (baseline) was 21 days and the minimum was 14 days. Full mouth scaling was carried out using subgingival and supragingival scales.
The sites for the trial were randomly selected after a periodontal screening of the mouth. After screening, patients were divided into two group; treatment (scaling, OHI, and placement of drug-loaded insert into the periodontal pocket) and control group (scaling and OHI only). After microbiological sampling, the clinical parameters like gingival index (GI), bleeding index (BI), calculus criteria (CC), and plaque index (PI) were measured and scored from 0 to 3 on day 0 for baseline data. On the same day the drug-loaded periodontal inserts were placed at the selected sites of the treatment group. Patients were called for follow-up clinical evaluations on day 7, 14, 21, 28, 45, 60, 90, and 180, postplacement of the inserts.
Microbiological Study
The selected periodontal site (control -2,4,6,8,10,12; treatment -1,3,5,7,9,11) was dried using sterile cotton balls. A sterile absorbent paper point (Dia-Dent no. 40, Diamond Dental Industrial Co. Crongju, Korea), standardized for collecting 0.1 ± 0.07 ml of GCF, was inserted into the periodontal pocket. After 60 sec, they were removed and transferred aseptically into sterile fluid thioglycollate broth for anaerobic processing. Simultaneously subgingival plaque was collected with a sterile standardized curette (1 ml) and transferred to sterile saline solution. A loopful of sample was inoculated on McConkey's agar plate and incubated at 37 ± 1°C for 24 hr for further studies. Quantitative microscopy was carried out in a Leitz HM-Lux 3 model microscope (Leitz Wetzlar, Germany), with a micrometer objective (Erma, Tokyo, Japan).
Statistical Evaluation
Intragroup comparison (longitudinal) between baseline data and subsequent data was carried out by Wilcoxon's signed rank test, in case of nonparametric data. The in vitro data were analyzed using Student's t-test. p < 0.05 was considered to be significant.
RESULTS AND DISCUSSION
The fabricated inserts were of 0.2–0.4 mm thick. The physicochemical properties of the prepared inserts are shown in . The addition of dibutyl phthalate was necessary to obtain drug-loaded films with sufficient stability, flexibility, and smoothness and to allow subdivision of the films into inserts of uniform dimensions. Weight, thickness, and drug content varied within ± 10% for the prepared inserts.
Drug Release Studies
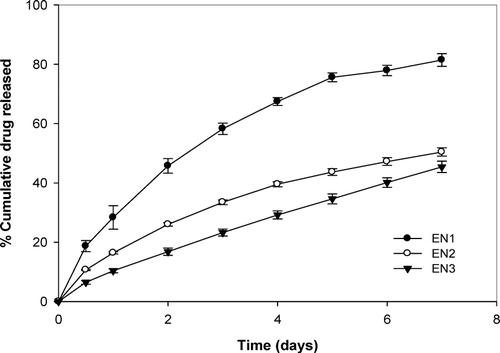
Inserts containing 7.4% w/w of niridazole (EN1) released drug at the rate of 3.1 μg/hr in 24 hr, which gradually decreased to 1.3 μg/hr during the next 7days. Similarly, EN3 (38.4% w/w of drug load) exhibited release rate that decreased from 3.96 μg/hr (day 1) to 2.48 μg/hr depleting ∼ 50% of drug at the end of dissolution study.
The release of niridazole from the inserts depended on drug loading as evident from . As the concentration of niridazole was increased from 0.25 mg/insert (EN1) to 1 mg/insert (EN3), the release rate decreased implying that lesser drug load exhibits faster release. The reason can be explained by the following concepts. Ethyl cellulose, a hydrophobic matrix forming material, is used in fabricating the inserts. Drug release from such systems depends on the pore formation in the matrix due to dissolution of drug. In these systems, the drug on the superficial layer dissolves and diffuses into the surrounding dissolution media creating pores in the hydrophobic polymer matrix. However, the low aqueous solubility of niridazole implies that lesser drug is leached from the matrix. With increasing concentration, lesser number of pores are formed, hindering diffusion of the media into the matrix and thus decreasing the release. The release of niridazole from the inserts was at a steady rate.
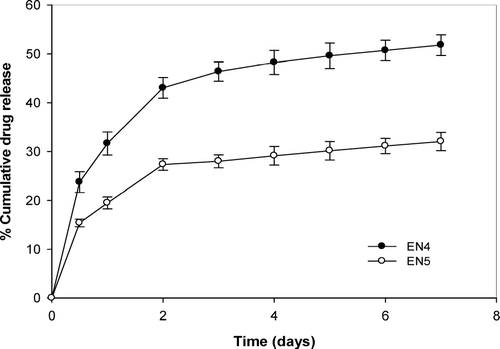
However, EN4 and EN5 with ethylellulose content of 65 and 75 mg/cm2, respectively, appear to give an initial rapid release followed by a steady release (). An early rapid release of niridazole presumably due to dissolution and diffusion of drug from the surface of the insert is followed by a steady second phase, in which subsequent release of drug is controlled by the elution of the dissolved drug from the matrix. Previously a study showed 20 and 30% chlorhexidine release in 205 days from ethylcellulose inserts containing 5 and 10% of initial drug load (Friedman and Golomb Citation1982), whereas faster release was observed in this study with inserts containing 5 and 10% of niridazole releasing 30 and 35% of drug load in 7 days.
Kinetic analysis of release profile gave release diffusion exponent of ≈ 0.5, indicating matrix release kinetics (). The mean dissolution time (MDT) increased with increment in the drug load in the inserts. Batch EN1 with an initial niridazole content of 250 μ g/insert had a MDT of 72.43 days. An increase in the initial niridazole content from 500 μ g/insert (EN2) and 1000 μ g/insert (EN3) increased the MDT to 182.84 and 204 days, respectively.
TABLE 2 Drug release kinetics from the fabricated inserts
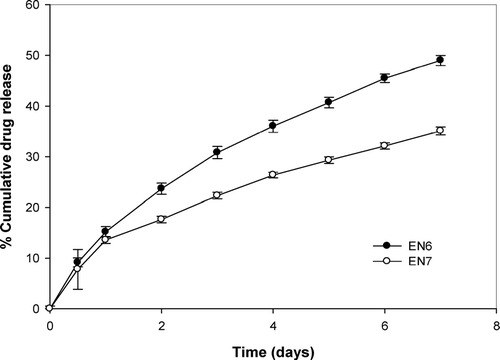
As shown in , the method of fabrication of inserts affects the release rate. The rate of release was generally slower from inserts fabricated by spraying in comparison to the corresponding inserts prepared by casting method. This can be attributed to the nature of the film with the incorporation of plasticizer. Dibutyl phthalate was used in this study to impart flexibility to inserts. At 40% w/w, the membrane bulk density might have increased causing reduced pore volume within the sprayed inserts. In this case, diffusion of drug through pore might be the prevailing mechanism for drug release (Ozturk et al. Citation1990; Wiljmans and Baker Citation1995). Diffusion of drug through the membrane followed water filled pores that decreased as the pore volume decreased (Sun, Wei-Fung, and Chih-Cheng Citation1999). However, solution-diffusion through the membrane becomes the dominating mechanism of drug permeation through the cast inserts. Incorporation of plasticizer increased the flexibility and free volume between the polymer chains for easier diffusion of drug, thus leading to faster release.
Clinical Evaluation
A pilot study of batch EN3 containing niridazole (1 mg/ insert) with release rate of 2 μ g/hr was conducted on 6 patients (constituting 12 sites). The minimum inhibitory concentration of niridazole is 0.25 mg/L (Hof and Stroder Citation1986; Hof and Sticht-Groh, Citation1984). Thus, the insert could raise about 4L to an effective bactericidal concentration over 7 days, which is considerably higher than the volume arising of gingival fluid flow of 10 μ l/hr (Cimasoni Citation1974) or 1.68 ml for 7 days. A full scale clinicalevaluation of batch EN3 was performed in patients with periodontitis. As shown in , the insert showed significant decrease in all the clinical indices (α ≤ 0.05) from baseline values. The pocket depth was significantly reduced from baseline values (α ≤ 0.05) and in comparison to control group (α = 0.05) at 6 months. Intergroup comparison between treatment and control rejected the null hypothesis that the groups are similar for plaque index and calculus criteria (α = 0.01) and for gingival index and bleeding index (α = 0.01). In the earlier clinical study with EC-chlorhexidine films (Stabholz et al. Citation1991), significant improvements of bleeding on probing and pocket depth were observed. However, the device was depleted of drug within 3 days thus frequent visits were needed to achieve the desired result. On the other hand, EC-niridazole inserts maintained drug release for 7 days and was depleted of only 50% of drug load. The mean dissolution time was calculated to be 200 days. Thus, the need to reinsert a device for the completion of the study period was eliminated.
TABLE 3 Full scale clinical evaluation of niridazole insert (Batch EN3) in patients for 6 months.
Variation in total bacterial count of GCF was observed before device insertion (day 0) and postdevice insertion (day 7). As shown in , treatment with niridazole-EC lead to a highly significant (α = 0.01) decrease in total bacterial count in the treatment group than control group. Surprisingly, the control group also exhibited a reduction in total bacterial count from baseline (day 0), which can be attributed to better oral hygiene maintenance and/or to Hawthorne effect (Goodson et al. 1989). On day 28, postdevice insertion showed a significant (α = 0.05) repopulation of periodontal pocket. Repopulation of bacteria is considered a risk factor for reoccurrence of the disease process. However, evaluation of the composition of bacteria showed an increase in relative proportions of Gram-positive organisms and a decrease in Gram-negative organisms. The human periodontal disease process is characterized by a changeover of the subgingival microflora from the facultative Gram-positive bacteria in healthy pockets to strictly anaerobic Gram-negative organisms in diseased pockets. Thus, though there has been an increase in total bacterial count, the higher proportion of Gram-positive organisms indicates regression in disease condition.
TABLE 4 Total bacterial count of gingival crevical fluid pre- and postdevice insertion
CONCLUSION
Niridazole-loaded ethylcellulose inserts have shown promising results both in vitro and in vivo evaluation. Based on in vitro drug release data, it is assumed that by altering a few film components (i.e., initial niridazole loading and matrix content) the desired release rate could be achieved. Long-term clinical evaluation in patients showed a significant improvement in the clinical indices. The subjects did not report any foreign body sensation or discomfort during the treatment with the niridazole-EC inserts. No loss of insert occurred during the period of follow-up studies. The study showed the usefulness of niridazole in treating periodontitis and this could provide a new life for niridazole.
The authors thank the Department of Science and Technology, Government of India, for funding the project.
REFERENCES
- Abdallah A., Saif M. Clinical evaluation of niridazole in schistosoma haematobium and mansoni infections. Ann. NY Acad. Sci. 1969; 160: 686–695
- Addy M., Renton-Harper P. Local and systemic therapy in the management of periodontal disease: an opinion and review of the concept. J. Oral Rehab. 1996; 23: 219–231
- Ahuja A., Ali A., Rahman S. Biodegradable periodontal intrapocket device containing metronidazole and amoxycillin: formulation and characterization. Pharmazie 2006; 61: 25–29
- Andraus P., Thomas M., Szubert P. R. Praziquantel. Med. Res. Rev. 1983; 3: 147–200
- Arrona G., DeRosa A., Rosso F., et al. A biocompatibility study of the effects of slow-release antibiotic materials in the treatment of periodontal disease-I. The biocompatibility of cellulose acetate charged with 25% tetracycline hydrochloride. A clinical and scanning microscopic study of a case. Minerva Stomatol. 1998; 47: 553–557
- Barat R., Srinatha A., Pandit J. K., et al. Niridazole biodegradable inserts for local long-term treatment of periodontitis: possible new life for an orphan drug. Drug Del. 2006; 13: 365–373
- Catauro M., Raucci M. G., Convertito C., et al. Characterization, bioactivity and ampicillin release kinetics of TiO2 and TiO24SiO2 synthesized by sol-gel processing. J. Mater. Sci. Mater. Med. 2006; 17: 413–420
- Ciancio S. G. Local delivery of chlorhexidine. Compend. Contin. Edu. Dent. 1999; 20: 427–432
- Cimasoni G. The crevicular fluid. The monograph in Oral Science, 3rd vol. ed., G. M. Whitford. Karger, New York 1974
- Coutinho A., Barreto F. T. Treatment of hepatosplenic schistosomiasis mansoni with niridazole: relationships among liver function, effective dose, and side effects. Ann. NY Acad. Sci. 1969; 160: 686–695
- Friedman M., Golomb G. New sustained release dosage form of chlorhexidine for dental use. J. Periodontal Res. 1982; 17: 323–328
- Friesen L. R., Williams K. B., Krause L. S., Killoy W. J. Controlled local delivery of tetracycline with polymer strips in the treatment of periodontitis. J. Periodontol. 2002; 73: 13–19
- Goodson J. M., Tanner A., McArdle S., et al. Multicenter evaluation of tetracycline fiber therapy. III Microbiological response. J. Periodontal Res. 1991; 26: 440–451
- Hof H., Sticht-Groh V. Antibacterial effects of niridazole: its effect on microaerophilic campylobacter. Infection 1984; 12: 36–39
- Hof H., Stroder J. Antibacterial activity of GO 10213, a nitroimidazole derivative. Antimicrob. Agents Chemother. 1986; 29: 953–954
- Hof H., Eisenbarth B., Denzler A., et al. Therapeutic activities of nitrothiazole derivatives in experimental infections with salmonella typhimurium and bacterioids fragilis. J. Antimicrob. Chemother. 1985; 6: 205–210
- Kinnane D. F., Radvan M. A six-month comparison of three periodontal local antimicrobial therapies in persistent periodontal pockets. J. Periodontol. 1990; 70: 1–7
- Larsen T., Fiehn N. E. Development of resistance to metronidazole and minocycline in vitro. J. Clin. Periodontol. 1997; 24: 254–259
- Lu H. K., Chei C. J. Efficacy of subgingivally applied minocycline in the treatment of chronic periodontitis. J. Perionotol. Res. 2005; 40: 20–27
- Mahmoud A. A. Current concepts: schistosomiasis. N. Engl. J. Med. 1977; 297: 329–1331
- Mastiholimath V. S., Dandagi P. M., Gadad A. P., et al. Formulation and evaluation of ornidazole dental implants for periodontitis. Ind. J. Pharm. Sci. 2006; 68: 68–71
- Medlicott N. J., Tucker I. G., Holborow D. W. Delivery systems for the administration of drugs to the periodontal pocket. Adv. Drug Del. Rev. 1994; 13: 181–203
- O'Connor B. C., Newman H. N., Wilson M. Susceptibility and resistance of plaque bacteria to minocycline. J. Periodontol. 1990; 61: 228–233
- Ozturk A. G., Ozturk S. S., Palsson B. O., et al. Mechanism of release from pellets coated with an ethylcellulose-based film. J. Control. Rel. 1990; 14: 203–213
- Schwach-Abdellaoui K., Vivien-Castioni V., Gurny R. Local delivery of antimicrobial agents for the treatment of periodontal diseases. Eur. J. Pharm. Biopharm. 2000; 50: 83–99
- Stabholz A., Soskolene W. A., Friedman M., Sela M. N. The use of sustained release chlorhexidine for the maintenance of periodontal pockets: 2-years clinical trial. J. Periodontol. 1991; 62: 429–433
- Smith S. R., Foyle D. M., Needleman I. G., Pandya N. V. The role of antibiotics in the treatment of periodontitis. Part I-systemic delivery. Eur. J. Prosthodont. Res. Dent. 1998; 3: 79–86
- Somayaji B. V., Jariwala U., Jayachandran P., et al. Evaluation of antimicrobial efficacy and release pattern of tetracycline and metronidazole using a local delivery. J. Periodontol. 1998; 69: 409–413
- Sun Y., Wei-Fung H., Chih-Cheng C. Spray coated and solution cast ethyl cellulose pseudolatex membranes. J. Membr. Sci. 1999; 157: 159–170
- Wade W. G., Addy M. Comparison of in vitro activity of niridazole, metronidazole and tetracycline against subgingival bacteria in chronic periodontitis. J. Applied Bacteriol. 1987; 63: 455–457
- Wiljmans J. G., Baker R. W. The solution-diffusion model: a review. J. Membr. Sci. 1995; 107: 1–21