Abstract
The study demonstrated that lipid microspheres (LM) containing rifampicin (LM-RFP) could deliver the drug to alveolar macrophages in vitro and in vivo, and that intranasal administration to animals could achieve preferential accumulation in the lungs with less effect on the liver. The LM-RFP particles had a mean diameter of 247.2 ± 75.7 nm, and their size remained stable when stored at 4°C or 25°C for at least 4 weeks. In vitro uptake of [3H]LM-RFP by alveolar macrophages was over 4 times higher than that of unencapsulated [3H]RFP, whereas the in vivo uptake was 30 times higher. Flow cytometric analysis and confocal laser scanning microscopy confirmed that LM could deliver the encapsulated drug effectively to alveolar macrophages in vitro and in vivo Intranasal administration of [3H]LM-RFP to normal mice resulted in preferential pulmonary uptake of the drug and lower levels in the blood and liver compared with administration of unencapsulated [3H]RFP. In conclusion, LM-RFP could be a promising preparation for delivery via the respiratory tract to tuberculosis (TB) and TB/HIV patients.
Tuberculosis (TB) is still one of the most important infectious diseases worldwide and its incidence is increasing due to the AIDS epidemic (Drobniewski et al. Citation2005). The most common form of TB is pulmonary disease in which a large number of bacteria reside in the alveolar macrophages (Russell Citation2001). Even if adequate blood levels of antituberculous drugs are achieved by long-term oral treatment, it is hard to kill all these bacteria, probably because the drugs are not efficiently taken up by alveolar macrophages. Also, a long-term oral therapy is known to cause hepatotoxicity (Tasdug et al. Citation2005). Rifampicin (RFP) is one of the most effective antituberculous agents, and is often used to treat HIV/TB patients in combination with anti-HIV agents (Justesen et al. Citation2004). RFP also is a strong inducer of hepatic microsomal enzyme CYP3A4 that leads to drug interactions with protease inhibitors. Accordingly, a RFP formulation that delivers the drug more efficiently to the target site might reduce the total dose and frequency of administration. It also might allow HIV/TB patients to take the drug simultaneously with protease inhibitors (Niemi et al. Citation2003).
Liposomes have long been proposed as an effective drug carrier (Doel and Khuller, Citation1997a, Citation1997b; Salem et al. Citation2005). Microcapsules formed from biodegradable polymers also have been extensively investigated (Suarez et al. Citation2001a; Dutt and Khuller Citation2001; Ul-Ain et al. Citation2003; Sharma et al. Citation2004; Pandey and Khuller Citation2004). Lipid microspheres (LM) are composed of soybean oil and lecithin and have a mean diameter of 200–300 nm. Drugs encapsulated in LM have been demonstrated to display potent pharmacological activity with a reduction of undesirable effects (Hoshi et al. Citation1986; Takenaga et al. Citation1993a, Citation1999), because LM are preferentially taken up by inflammatory cells (Shoji et al. Citation1985; Kiyokawa et al. Citation1987; Mizushima et al. Citation1990; Igarashi et al. Citation1996; Takenaga Citation1996). LM containing prostaglandin (PG)E1 already have been used clinically and have made a contribution to improvement of the health of patients with peripheral vascular disease (Hoshi et al. Citation1986). If antituberculous drugs were stably encapsulated in LM, more efficient pharmacological activity could be achieved.
LM are clinically used as injectable preparations, which tend to be trapped by the reticuloendothelial system in the liver and spleen after the systemic administration. Therefore, use of another route, such as the respiratory tract, would be desirable to reduce hepatic accumulation. Liposome emulsions and microcapsules containing antituberculous agents have already been investigated (Suarez et al. Citation2001b; Justo and Moraes Citation2003; Pandey et al. Citation2003), but an LM formulation containing RFP has not been studied yet. We already have shown that LM-encapsulated drugs can be delivered by aerosolized inhalation. For example, the inhalation of LM-encapsulated PGE1 delayed the onset and decreased the severity of dyspnea and bronchial irritation compared with inhalation of free PGE1 in animals and asthma patients (Mizushima et al. Citation1983). In addition, the inhalation of an LM-encapsulated TXA2antagonist (Takenaga et al. Citation1993b) or PAF antagonist (Takenaga et al. Citation2000) resulted in enhancement of their pharmacological activity.
The present study was designed to examine whether LM containing RFP (LM-RFP) could deliver the drug to alveolar macrophages and whether administration via the respiratory tract was an effective drug delivery route. To deliver precise doses of the drug, intranasal (distribution study) and intratracheal (uptake by alveolar macrophages) treatments were performed.
MATERIALS AND METHODS
Rifampicin was purchased from Wako Pure Chemicals Ltd. (Osaka, Japan). Soybean phosphatidylcholine (soybean PC) and soybean oil composed of triglycerides with C8–10 fatty acids were kindly provided by Nisshin Oilio Co. (Kanagawa, Japan). AdipoRed™ was purchased from Cambrex Bio Science (Walkersville, USA).
Lipid Microspheres
LM containing RFP (LM-RFP) were prepared as described previously with minor modifications (Hoshi et al. Citation1986). Soybean oil containing C8-10 fatty acids was used instead of oil containing C14-18 fatty acids, and soybean PC was used instead of egg PC.
RFP (20 mg) was dissolved in soybean oil (1 g), and then soybean PC (120 mg) was added. The mixture was homogenized for 5 min at 15,000 rpm in a Polytron homogenizer. Then an aqueous solution of 2.58% glycerol was added to the mixture with stirring to create a 10% (w/v) oil-in-water suspension. Further homogenization was done at 20,000 rpm for 20 min. The resultant emulsion was passed through a French Pressure Cell Press (SLM Instruments, IL, USA), and the procedure was repeated another five times to ensure complete emulsification and achieve a final RFP concentration of 2 mg/mL. The LM preparation was added to sterile glass ampoules under a stream of nitrogen gas, and the ampoules were stored.
The particle size and zeta potential were measured by an ELS-8000 analyzer (Otsuka Electronics Co., Tokyo, Japan). The respective formulation was diluted with distilled water (size) and phosphate buffered saline (pH7.2)(zeta potential) for measurement.
To make the LM preparation, radiolabeled RFP ([3H]RFP, 28.0 Ci/mmol, 29.4 μg/ml, Moravek Biochemicals, Brea, CA, USA) and Nile Red solution (AdipoRed™) were used in some experiments. AdipoRed was used as a marker for LM and/or the encapsulated drugs, because of a high affinity for triglyceride (oil).
Cells
Rat-derived alveolar macrophage cells (NR8383; Atcc no. CRL-2192: Sumisho Pharma International Co., Osaka, Japan) were cultured in Ham F-12K medium containing 15% fetal bovine serum.
Animals
Male Wistar rats weighing 210–230 g (SLC, Shizuoka, Japan) were used for the study of uptake by alveolar macrophages, while male ICR mice weighing 30–35 g (SLC) were used for the distribution study.
All animals were housed at a constant temperature (23 ± 1°C) and humidity (50–60%) with free access to a standard diet and water. The animal room had a 12-hr light/dark cycle. All the experimental procedures were done in accordance with the rules of the animal committee of St. Marianna University.
In Vitro Uptake of RFP
NR8383 cells (1 × 106/mL) were incubated with unencapsulated [3H]RFP or [3H]LM-RFP at a temperature of 4°C or 37°C under a 5% CO2/95% air atmosphere. An aliquot of LM (50 μL = 100 μg of RFP) was added to each well, while unencapsulated [3H]RFP was dissolved in DMSO at a concentration of 10 mg/mL and then diluted with medium before addition. After incubation for 1 hr or 4 hr, the cells were washed three times with PBS and uptake of RFP was determined by counting radioactivity. In the case that unencapsulated RFP and LM-RFP were used, the uptake of RFP was determined by HPLC. The viability of these cells was confirmed to be more than 95% by trypan-blue exclusion test after 4 hr incubation.
To investigate in vitro uptake of LM and/or encapsulated drug in the LM, LM containing AdipoRed (LM-AdipoRed) were used, and the incorporation of these LM was assessed by flow cytometry (LSRII, Becton Dickinson, USA). The cell number used per assay was 20,000. An aliquot of the cells was used to make cytospin preparations on glass slides. Then fluorescent images were acquired by a confocal laser scanning microscope (Axiovert 200M, Carl Zeiss, Jana, Germany) and the dedicated software (30-mW LSM510 META;Carl Zeiss). The same amount of unencapsulated AdipoRed solution dissolved in PBS also was incubated with cells for comparison.
In Vivo Uptake of RFP
Alveolar macrophages were used for the in vivo uptake assay. Under anesthesia with pentobarbital (40 mg/kg), either unencapsulated RFP or LM-RFP (300 μL = 600 μg of RFP) was administered to rats via the intratracheal route. Administration was carried out by using a long needle coupled with a catheter (MEDIKIT Co., Tokyo, Japan). Bronchoalveolar cells were obtained by bronchoalveolar lavage fluid (BALF) using the standard technique (Ikegami et al. Citation2005) at 10 min after dosing. BALF contained a many macrophages.
RFP was determined by HPLC using a Shimadzu chromatography system (Kyoto, Japan) equipped with an ODS column (ODS-A 5 μm; 6 mm × 150 mm I.D. YMC Co., Ltd., Kyoto) with a mixture of methanol/10 mM sodium phosphate buffer (pH 2.6) containing 100 mM NaClO4 (3:1,v/v) as the mobile phase at a flow rate of 1.0 mL/min. Ten μL of the sample was injected, and the absorbance was monitored continuously at 330 nm with a ultraviolet spectrophotometer (SPD-6AV) and the column temperature was maintained at 40°C. Under these conditions, the retention time of RFP was ∼5.7 min.
To examine in vivo uptake of LM by alveolar macrophages, LM- AdipoRed was used. Flow cytometric analysis and fluorescent detection were carried out as described above.
Distribution Study
[3H] LM-RFP (2 mg/mL; 13,173,666 dpm/mL) were administered to male ICR mice via the intranasal or intravenous routes, while unencapsulated [3H]RFP (2 mg/mL; 9,666,666 dpm/mL) solution dissolved in physiological saline was given via the intranasal or oral routes. Intranasal administration (30 μL = 60 μg of RFP) was performed by using a tip coupled with a Pipetman™ under anesthesia with ketamine hydrochloride and xylazine. Then blood samples and organs were harvested followed by solubilization with Soluene® -350 (PerkinElmer Japan Co., Yokohama, Japan). An aliquot was used for determination of radioactivity (Takenaga et al. Citation1993b).
Statistical Analysis
Statistical analysis was performed by the Mann-Whitney U-test, and p < 0.05 was taken to indicate significance.
RESULTS
LM-RFP
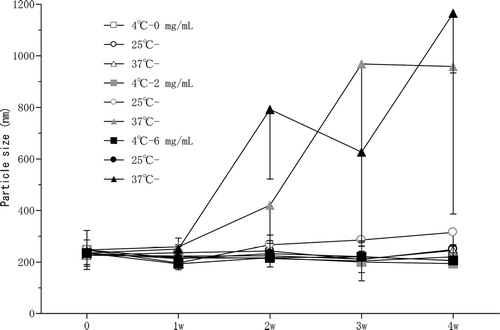
The LM formulation without RFP had particles measuring 226.9 ± 36.6 nm in size with zeta potential of −0.07 mV. The LM formulation containing RFP at 2 mg/mL (LM-RFP) had a particle size and zeta potential of 247.2 ± 75.7 nm and −0.35 mV, respectively, whereas 6 mg/mL LM-RFP had values of 234.7 ± 51.1 nm and −0.65 mV, respectively. There were no significant differences of particle size and zeta potential among these LM preparations. After storage at 4°C, 25°C, and 37°C, the size of the LM particles was determined (). Empty LM remained stable even at 37°C, but LM-RFP formulations were not stable at 37°C. However, LM-RFP (2 and 6 mg/mL) were stable at 4°C and 25°C for at least 4 weeks.
In Vitro Uptake of LM-RFP
To determine the in vitro uptake of RFP into macrophages, NR8383 cells were incubated with unencapsulated [3H]RFP or [3H]LM-RFP. The percentage(%) of RFP amounts to total applied unencapsulated [3H]RFP in the cells was 0.27 ± 0.024 after 1 hr incubation at 4°C (), while incubation at 37°C increased the value to 0.63 ± 0.034%. This indicates that 0.36% of the RFP dose was incorporated by cells under physiological conditions, since the specific uptake of RFP was defined to be the difference of radioactivity obtained from the incubation at 4°C and 37°C. Therefore, the cellular uptake of [3H]RFP was calculated to be 0.46% after 4 hr incubation.
FIG. 2 In vitro uptake of RFP by alveolar macrophages. NR8383 cells (1 × 106/mL) were incubated with 50 μL of [3H]RFP or [3H]LM-RFP (100 μg as RFP) at 4°C or 37°C for 1 hr or 4 hr. Then the amount of [3H]RFP incorporated was determined by counting the radioactivity. Values were expressed as the percentage (%) of cellular RFP amounts to applied total RFP. N = 4. Bars show the standard deviations.
![FIG. 2 In vitro uptake of RFP by alveolar macrophages. NR8383 cells (1 × 106/mL) were incubated with 50 μL of [3H]RFP or [3H]LM-RFP (100 μg as RFP) at 4°C or 37°C for 1 hr or 4 hr. Then the amount of [3H]RFP incorporated was determined by counting the radioactivity. Values were expressed as the percentage (%) of cellular RFP amounts to applied total RFP. N = 4. Bars show the standard deviations.](/cms/asset/5ff661ff-7973-4408-a890-3f4e63f69cd4/idrd_a_295419_uf0002_b.gif)
When [3H]LM-RFP were incubated with the cells, 1.005% (1 hr) and 1.929% (4 hr) of the drug dose was incorporated. When uncapsulated RFP or LM-RFP levels were determined by HPLC, similar results were obtained (data not shown).
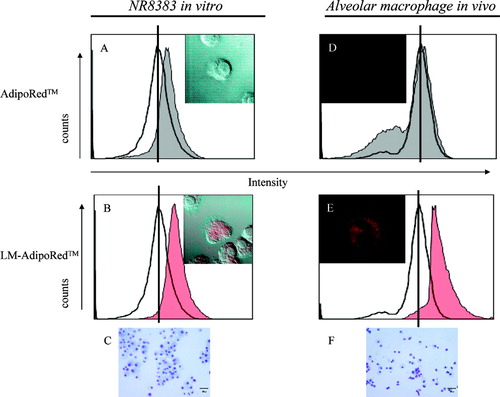
To examine the extent of LM uptake by macrophages, LM-AdipoRed was prepared. AdipoRed was used as a marker of LM and/or encapsulated drug, since it has a high affinity for triglyceride (soybean oil). The fluorescence intensity was much greater after incubation with LM-AdipoRed than after incubation with free AdipoRed ( and ). Representative photographs show that AdipoRed was markedly incorporated by the cells. Giemsa staining showed that these cells were macrophages ().
FIG. 3 In vitro and in vivo uptake of LM by alveolar macrophages. NR8383 cells (1 × 106/mL) were incubated with 50 μL of (A) AdipoRed solution or (B) LM-AdipoRed™ for 3 hr at 37°C. (C) Giemsa staining of NR8383. Under anesthesia, rats were given 200 μL of (D) AdipoRed solution or (E) LM-AdipoRed through intratracheal route. Bronchoalveolar cells were obtained at 10 min after dosing. (F) Giemsa staining of bronchoalveolar cells. Most cells were macrophages. Scale bar is 30 μm. Fluorescence intensity was determined by flow cytometry. As compared with cell alone (–), fluorescence intensity became higher with the increase cellular uptake of AdipoRed (grey) and LM- AdipoRed (red). Representative photographs show (A and D) AdipoRed-treated and (B and E) LM-AdipoRed-treated cells. AdipoRed is seen as the red-colored areas.
In Vivo Uptake of RFP
Next, we examined whether RFP was incorporated by alveolar macrophages after administration to rats. When LM-RFP (600 μg in 300 μL) was given intratracheally, 2.361 ± 0.47 (μg) (0.39% of the injected dose) was detected in the macrophages at 10 min after dosing (), which was over 30 times higher than that after administration of unencapsulated RFP.
TABLE 1 Uptake of Rifampicin (RFP) by alveolar pulmonary macrophages in vivo
To examine the extent of LM uptake by alveolar macrophages in vivo, LM-AdipoRed also were used. The fluorescence intensity was much greater after incubation with LM-AdipoRed than after incubation with free AdipoRed ( and ). Representative photographs show that AdipoRed in the LM was markedly incorporated into the cells. Giemsa staining showed that these cells were predominantly macrophages ().
Lung levels of LM-RFP
Intranasal administration of unencapsulated [3H]RFP resulted in rapid distribution to the blood and 2.62 ± 0.77%/mL of the injected dose(ID) of [3H]RFP was detected after 30 min (). Following intranasal administration of [3H]LM-RFP, only 0.77 ± 0.26%/mL of the ID of [3H]RFP was detected in the blood (p < 0.01 versus [3H]RFP group). The blood level of RFP decreased with time (), but it was 2.7-fold and 3.4-fold higher in the [3H]RFP group than in the [3H]LM-RFP group at 2 hr and 6 hr, respectively.
FIG. 4 Distribution of [3H]RFP following administration of [3H]RFP or [3H]LM-RFP to mice. Under anesthesia, normal male ICR mice were given 30 μL of [3H]RFP or [3H]LM-RFP via the intranasal route (in). At 30 min after dosing, the radioactivity of the blood and organs was determined. [3H]RFP also was given to mice via the oral route (po), while the LM formulation also was administered to mice intravenously (iv). Results are expressed as the percent (%) injected dose (ID) per g or mL. N = 3–6. Bars show the standard deviation. * p < 0.05; ** p < 0.01.
![FIG. 4 Distribution of [3H]RFP following administration of [3H]RFP or [3H]LM-RFP to mice. Under anesthesia, normal male ICR mice were given 30 μL of [3H]RFP or [3H]LM-RFP via the intranasal route (in). At 30 min after dosing, the radioactivity of the blood and organs was determined. [3H]RFP also was given to mice via the oral route (po), while the LM formulation also was administered to mice intravenously (iv). Results are expressed as the percent (%) injected dose (ID) per g or mL. N = 3–6. Bars show the standard deviation. * p < 0.05; ** p < 0.01.](/cms/asset/30f1760d-3c0a-45f5-8bda-83593b615212/idrd_a_295419_uf0004_b.gif)
TABLE 2 Distribution of [3H]RFP following intranasal administration of the formulations to mice (%/g or mL)
When [3H]LM-RFP was administered intranasally, [3H]RFP retention in the lungs was over 4-fold greater at 30 min after dosing compared with that after [3H]RFP administration (p < 0.05), and the lung/blood ratio was significantly higher. The level of radioactivity in the lungs also was significantly higher at 2 hr in the [3H]LM-RFP group(p < 0.01 versus the [3H]RFP group).
Hepatic levels of RFP were much higher in the [3H]RFP group than the [3H]LM-RFP group, with 8 to 12%/g of the ID being detected at 30 min, 2 hr, and 6 hr. Hepatic RFP levels were significantly lower in the [3H]LM-RFP group (p < 0.01). There was little difference in the spleen, but RFP levels were much higher in the kidneys and heart of the [3H]RFP group.
[3H]RFP also was given via the oral route, since RFP is administered orally in clinical practice. As a result, pulmonary levels were lower and hepatic levels were similar to those after intranasal administration. [3H]LM-RFP also were given to mice intravenously, since LM are administered intravenously administered in clinical practice. As a result, high levels were detected in the liver and blood. Although lower than after oral doing of [3H]RFP, the hepatic level was ∼10-fold higher than after intranasal administration. However, lung levels of RFP were lower than after intranasal administration.
DISCUSSION
TB/HIV patients are treated with RFP in combination with anti-HIV drugs (protease inhibitors) over the long term. Since it is a strong inducer of hepatic microsomal CYP3A4, oral RFP often makes anti-HIV drugs less effective by this interaction, in addition to not reaching the target site in the lungs. This study showed that administration of LM-RFP achieved higher RFP levels in the lungs when administered via the nasal route and delivered RFP efficiently to alveolar macrophages when administered intratracheally. All antituberculous drugs are designed for oral treatment at present. However, our findings indicate that LM could be a good carrier for an inhaled formulation of RFP, and LM-RFP could provide a new strategy for the treatment of pulmonary TB in the future.
We have already confirmed the LM was efficiently uptaken by macrophages following intravenous administration (Shoji et al. Citation1985). In the present study, flow cytometric analysis and confocal laser scanning microscopy revealed that AdipoRed, a marker of LM, and/or encapsulated agents in the LM was extensively taken up not only by NR8383 cells in vitro (), but also by alveolar macrophages following intranasal administration in vivo (). These findings indicate the possibility that LM could be efficiently taken up and deliver the RFP effectively to macrophages through respiratory tract.
In vitro uptake of RFP from the LM formulation was over 4 times higher than that of unencapsulated RFP (). It should also be noted that the incorporation of RFP from the LM formulation into alveolar macrophages was about 30-fold higher than that of unencapsulated RFP following intratracheal administration. Pulmonary delivery through a catheter can produce a significant damage with an inflammatory cell response and the migration of numerous phagocytic cells, even in normal animals. Also, the higher affinity of LM-RFP for inflammatory and phagocytic cells in the airways would have led to the present result. After the incorporation of LM-RFP by cells, RFP could be leaked from the LM and exert the pharmacological activity.
LM has been used clinically as a carrier for injectable preparations, such as LM formulations of PGE1 (Liple®, Palux®). RFP accumulated in the liver after systemic administration of LM-RFP, as shown in . When administered via the intranasal route, LM-RFP were retained in the lungs, and there was decreased RFP accumulation in the liver. Even when unencapsulated RFP was given via intranasal route, more of the drug was retained in the lungs than after oral administration.
These findings indicate that delivery via the respiratory tract could be feasible and that LM-RFP could achieve better drug uptake in the lungs than the administration of RFP alone. Higher level of RFP in the LM form (LM-RFP) in the lung would be included the efficient uptake by phagocytic cells such as alveolar macrophages, as demonstrated in LM-AdipoRed uptake in vivo study.
Our in vivo distribution study was performed by intranasal administration, but this route might not perfectly reflect the results obtained with aerosol inhalation and some RFP might have remained in the nasal mucosa. Pharmacological evaluation has already been done in TB-infected mice by the same dosing methods. It showed that intranasal LM-RFP therapy was superior to oral RFP therapy (under preparation for submission). These findings raise the possibility that LM-RFP are suitable for inhalation. However, it is still necessary to perform evaluation of LM-RFP inhalation in both normal mice and TB-infected mice.
Edwards et al. (Citation1997) already have reported that large porous particles were suitable for pulmonary delivery. Several researchers (Pillai1 et al. 1998; O'Hara and Hickey 2000; Makino et al. 2004; Yoshida et al. 2006) have shown that the efficient transfer of antituberculous agents to alveolar macrophages was achieved by PLGA microcapsules. As compared with PLGA microcapsules, the size of LM particles is smaller and has a narrow deviation. Our preliminary experiment showed that PLGA microcapsules containing RFP were efficiently taken up by NR8383 cells in vitro, but in vivo accumulation to macrophages has not been confirmed yet (data not shown). Further precise comparison study would be needed.
Stability of LM preparations is another important factor (Igarashi et al. Citation1996). If an encapsulated agent such as AdipoRed is stable in the LM preparation, its fate is the same as that of the LM particles. The size of LM particles was stable for at least 4 weeks during storage at 4°C and 25°C, but RFP affected the stability since the particle size increased at 37°C. The LM-RFP formulation was still stable for at least 1 week even when stored at 37°C. When LM-RFP was given via the intranasal route, RFP must have been rapidly taken up by alveolar macrophages. Subsequently, the drug would have leaked from residual LM in the nasal mucosa and its fate would have been the same as that of free intranasal RFP.
The particle size, phospholipids content, oil (triglyceride) content, and other factors can alter the characteristics of LM. If slower and more controlled release of RFP from the LM was obtained, along with more active targeting of alveolar macrophages, further improvement might be expected. In conclusion, this study showed the possibility of delivering LM-RFP via the respiratory tract for the treatment of pulmonary TB.
This study was supported by grants from the Human Science Foundation and from the Japanese Ministry of Health, Labor, and Welfare.
REFERENCES
- Deol P., Khuller G. K., Joshi K. Therapeutic efficacies of isoniazid and rifampin encapsulated in lung-specific stealth liposomes against Mycobacterium tuberculosis infection induced in mice. Antimicrob. Agents Chemother. 1997a; 41: 1211–1214
- Deol P., Khuller G. K. Lung specific stealth liposomes: stability, biodistribution and toxicity of liposomal antitubercular drugs in mice. Biochim. Biophys. Acta 1997b; 1334: 162–172
- Drobniewski F., Nikolayevsky V., Asmolov A., Bazhora Y., Servetsky S. Increasing trends in HIV and TB rates in Odessa and the Ukraine. Int. J. Std. AIDS 2005; 16: 374–378
- Dutt M., Khuller G. K. Chemotherapy of Mycobacterium tuberculosis infections in mice with a combination of isoniazid and rifampicin entrapped in poly (DL-lactide-co-glycolide) microparticles. J. Antimicrob. Chemother. 2001; 47: 829–835
- Edwards D. A., Hanes J., Caponetti G, Hrkach J, Ben-Jebria A, Eskew M. L., Mintzes J., Deaver D., Lotan N., Langer R. Large porous particles for pulmonary drug delivery. Science 1997; 276: 1868–1871
- Hoshi K., Mizushima Y., Kiyokawa S., Yanagawa A. Prostaglandin E1 incorporated in lipid microspheres in the treatment of peripheral vascular diseases and diabetic neuropathy. Drugs Exptl. Clin. Res. 1986; 12: 681–685
- Igarashi R., Takenaga M., Matsuda T. Distribution of a lipid microsphere preparation. Adv. Drug Del. Rev. 1996; 20: 147–154
- Ikegami M., Na C.-L., Korfhagen T. R., Whitsett J. A. Surfactant protein Dinfluences surfactant ultrastructure and uptake by alveolar type II cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2005; 288: L552–L561
- Justesen U. S., Andersen A. B., Klitgaard N. A., Brosen K., Gerstoft J., Pedersen C. Pharmacokinetic interaction between rifampin and the combination of indinavir and low-dose ritonavir in HIV-infected patients. Clin. Infect. Dis. 2004; 38: 426–429
- Justo O. R., Moraes A. M. Incorporation of antibiotics in liposomes designed for tuberculosis therapy by inhalation. Drug Del. 2003; 10: 201–207
- Kiyokawa S., Igarashi R., Iwayama T., Haramoto S., Matsuda T., Hoshi K., Mizushima Y. 99 mTc-labeled lipid microspheres (LM) would be useful for an imaging study of those diseases. Jpn. J. Inflam. 1987; 7: 551–557
- Makino K., Nakajima T., Shikamura M., Ito F., Ando S., Kochi C., Inagawa H., Soma G., Terada H. Efficient intracellular delivery of rifampicin to alveolar macrophages using rifampicin-loaded PLGA microspheres: effects of molecular weight and composition of PLGA on release of rifampicin. Colloids Surf. Biointerf. 2004; 36: 35–42
- Mizushima Y., Hoshi K., Aihara H., Kurachi M. Inhibition of bronchoconstriction by aerosol of a lipid emulsion containing prostaglandin E1. J. Pharm. Pharmacol. 1983; 35: 397
- Mizushima Y., Hamano T., Haramoto S., Kiyokawa A., Yanagawa A., Nakura K., Shintomi M., Watanabe M. Distribution of lipid microspheres incorporating prostaglandin E1 to vascular lesions. Prostagl. Leukotr. Essent. Fatty Acids 1990; 41: 269–272
- Niemi M., Backman J. T., Fromm M. F., Neuvonen P. J., Kivisto K. T. Pharmacokinetic interactions with rifampicin : clinical relevance. Clin. Pharmacokinet 2003; 42: 819–850
- O'Hara P., Hickey A. J. Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: manufacture and characterization. Pharmaceut. Res. 2000; 17: 955–956
- Pandey R., Sharma A., Zahoor A., Sharma S., Khuller G. K., Prasad B. Poly (DL-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. J. Antimicrob. Chemother. 2003; 52: 981–986
- Pandey R., Khuller G. K. Subcutaneous nanoparticle-based antitubercular chemotherapy in an experimental model. J. Antimicrob. Chemother. 2004; 54: 266–268
- Pillai R. S., Yeates D. B., Miller I. F., Hickey A. J. Controlled dissolution from wax-coated aerosol particles in canine lungs. J. Appl. Physiol. 1998; 84: 717–725
- Russell D. G. Mycobacterium tuberculosis: here today, and here tomorrow. Nat. Rev. Mol. Cell Biol. 2001; 2: 569–577
- Salem I. I., Flasher D. L., Duzgunes N. Liposome-encapsulated antibiotics. Meth. Enzymol. 2005; 391: 261–291
- Sharma A., Pandey R., Sharma S., Khuller G. K. Chemotherapeutic efficacy of poly (DL-lactide-co-glycolide) nanoparticle encapsulated antitubercular drugs at sub-therapeutic dose against experimental tuberculosis. Int. J. Antimicrob. Agents 2004; 24: 599–604
- Shoji Y., Mizushima Y., Yanagawa A., Yonaha T. Electron microscopic studies on tissue distribution of lipid microspheres used as drug delivery carriers. Drugs Exptl. Clin. Res. 1985; 11: 601–609
- Suarez S., O'Hara P., Kazantseva M., Newcomer C. E., Hopfer R., McMurray D. N., Hickey A. J. Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: screening in an infectious disease model. Pharm Res. 2001a; 18: 1315–1319
- Suarez S., O'Hara P., Kazantseva M., Newcomer C. E., Hopfer R., McMurray D. N., Hickey A. J. Airways delivery of rifampin. J. Antimicrob. Chemother. 2001b; 48: 431–434
- Tasdug S. A., Peerzada K., Koul S., Bhat R., Johri R. K. Biochemical manifestations of anti-tuberculosis drugs induced hepatotoxicity and the effect of silymarin. Hepatol. Res. 2005; 31: 132–135
- Takenaga M., Igarashi R., Tsuji H., Mizushima Y. Enhanced antitumor activity and reduced toxicity of 1,3-bis(2-chloroethyl)-1-nitrosourea administration in lipid microspheres to tumor-bearing mice. Jpn. J. Cancer Res. 1993a; 84: 1078–1085
- Takenaga M., Nakagawa T., Igarashi R., Mizushima Y. Application of lipid microspheres to prepare a thromboxane A2 receptor antagonist aerosol inhalation. J. Drug Targ. 1993b; 1: 293–301
- Takenaga M. Application of lipid microspheres for the treatment of cancer. Adv. Drug Del. Rev. 1996; 20: 209–219
- Takenaga M., Igarashi R., Matsumoto K., Takeuchi J., Mizushima N., Nakayama T., Morizawa Y., Mizushima Y. Lipid microsphere preparation of a lipophilic ceramide derivative suppresses colony formation in a murine experimental pulmonary metastasis model. J. Drug Targ. 1999; 7: 187–195
- Takenaga M., Igarashi R., Mizushima Y. Possibility of Lipo Y-24180 as an injectable drug and an inhaler for the treatment of bronchoconstriction. J. Allerg. Int. 2000; 14: 143–149
- Ul-Ain Q., Sharma S., Khuller G. K. Chemotherapeutic potential of orally administered poly(lactide-co-glycolide) microparticles containing isoniazid, rifampin, and pyrazinamide against experimental tuberculosis. Antimicrob. Agents Chemother. 2003; 47: 3005–3007
- Yoshida A., Matsumoto M., Hashizume H., Oba Y., Tomishige T., Inagawa H., Kohchi C., Hino M., Ito F., Tomoda K., Nakajima T., Makino K., Terada H., Hori H., Soma G. Selective delivery of rifampicin incorporated into poly(dl-lactic-co-glycolic) acid microspheres after phagocytotic uptake by alveolar macrophages, and the killing effect against intracellular Mycobacterium bovis Calmette-Guerin. Microbes. Infect. 2006; 8: 2484–2491