Abstract
In the present study, we addressed the factors modifying ciprofloxacin release from multiple coated beads. Beads were prepared by simple ionic cross-linking with sodium tripolyphoshate and coated with alginate and/or chitosan to prepare single, double, or multilayered beads. The water uptake capacity depended on the nature of beads (coated or uncoated) and pH of test medium. The number of coatings given to the beads influenced ciprofloxacin release rate. The coating significantly decreased the drug release from the beads in comparison to uncoated beads (p < 0.001). When the beads were given three coatings, viz., alginate, chitosan, and again alginate, the drug release appeared to follow the pattern exhibited by colon-targeted drug delivery systems with time dependent release behavior. The increase in coating formed a barrier for easy ingress of dissolution medium into the bead matrix, reducing the diffusion of drug.
In the past few years considerable research effort has been made in the development of solid oral sustained-release formulations. It is a more convenient form of drug administration considering the pain and possible infection caused by invasive modes of drug administration, thus leading to a higher patient compliance (Ko et al. Citation2002). Among the solid dosage forms, multiunit systems have been accepted to offer advantages over single unit systems (Mitrevej et al. Citation2000). Multiunit systems distribute the drug load more uniformly in the gastrointestinal tract with an aim to reduce local irritation (Bodmeier et al. Citation1991). Beads loaded with antibiotics are useful in treatment of gastric diseases like peptic ulcer (Shu and Zhu Citation2000; Orienti et al. Citation2002) and for ulcerative colitis, carcinomas, and infections in the intestine (Anal and Stevens Citation2005).
Most available drug delivery systems use natural polymers that are biodegradable, biocompatible, and nontoxic (Aiedeh et al. Citation1997) and are capable of rate- and/or time-controlled drug release (Anal and Stevens Citation2005). Chitosan [a (1 → 4) 2-amino-2-deoxy-β -D-glucon] is obtained by the alkaline deacetylation of chitin found in the exoskeleton of crustaceans, insects, and some fungi. The main commercial sources are the shell wastes of shrimp, lobster, krill, and crab (Struszczyk Citation1991; Roberts Citation1992). Chitosan being cationic, should interact with anionic polymers and form a water insoluble barrier that influences drug release (Mitrevej et al. Citation2000). The formation of complex between chitosan and alginate has been systematically studied (Becheran-Maron et al. Citation2004) and its application in drug delivery systems as a rate controlling membrane has been studied (Aral and Akbuga Citation1998; Sezer and Akbuga Citation1999; Riberio et al. Citation2005).
Ciprofloxacin is a widely used fluoroquinolone with high bactericidal activity against uropathogens and in the treatment of urinary tract infections with a biological half-life of 4 hr. It is advised in complicated intra-abdominal infections (in combination with metronidazole), infectious diarrhea, typhoid fever (enteric fever), and uncomplicated cervical and urethral gonorrhoea. It is beneficial in treatment of mild to moderate Crohn's disease and in maintenance of remission (Knutson et al. Citation2003). Ciprofloxacin is the first line of treatment in treating gastrointestinal anthrax caused by B. anthracis (Binkley et al. Citation2002) in which the death range is 25–60% (Pile et al. Citation1998). Extended-release formulation of ciprofloxacin provides systemic drug exposure comparable with that achieved with twice-daily administration of conventional, immediate-release ciprofloxacin, while also attaining higher maximum plasma concentrations with less inter patient variability (Blondeau Citation2004).
In various reports published previously, much of the attention in application of chitosan-alginate polyelectrolyte complex is limited to either chitosan coating of alginate beads (Pasparaski and Bouropoulos Citation2006; Xu et al. Citation2007) or chitosan as an additive in alginate bead (Murata et al. Citation2002; Lin et al. Citation2005) to achieve controlled release of drugs. The aim of the present work was to investigate applying multiple polymeric coating(s) on chitosan beads as an alternative to the use of chemical cross-linking agents to control drug release and to evaluate factors modifying the release rate of ciprofloxacin from these multilayered beads.
MATERIALS AND METHODS
Ciprofloxacin hydrochloride was a generous gift from M/s Dr. Reddy's Laboratories, (Hyderabad, India) Chitosan (mol. wt. 2 × 105, degree of deacetylation 82–88%) was obtained from M/s Kraeber Gmbh and Co. (Ellerbek, Germany). Sodium alginate (viscosity of 2% w/v dispersion, 250 cps at 25°C) was procured from Sigma (Chemicals, St. Louos, MO, USA). All other reagents were of analytical grade and used as received.
Preparation of Beads
Uncoated Beads
Chitosan solution (2% w/v) containing ciprofloxacin (1% w/v) was prepared in dilute acetic acid (2% v/v). The bubble-free polymeric-drug solution (pH 4–4.5) was added dropwise to a solution of sodium tripolyphosphate () using a syringe fitted with a blunt-end needle (23 G, inner diameter, 0.7 mm). After curing to a pre-identified time, the beads were filtered, washed with cold distilled water (thrice, 25 ml), and dried in an oven at 50°C for 4 hr and then at room temperature (25°C) for 12 hr.
TABLE 1 Composition and physical characteristics of the prepared multilayered chitosan beads
Single Layered Beads
Chitosan solution (2% w/v) containing ciprofloxacin (1% w/v) was prepared in dilute acetic acid (2% v/v). The bubble free polymeric-drug solution (pH 4–4.5) was added dropwise to a coagulation fluid (pH 5, ) using a syringe fitted with a blunt-end needle (23 G, inner diameter, 0.7 mm) to obtain single coated beads (Shiraishi et al. Citation1993). After 20 min of curing, the beads were filtered, washed with cold distilled water (thrice, 25 ml), and dried in an oven at 50°C for 4 hr and then at room temperature (25°C) for 12 hr.
Few batches of wet beads, as prepared above, were hardened for 30 min with calcium chloride solution (0.5 % w/v) or glutaraldehyde (0.5 % v/v), washed with sufficient distilled water until free from excess of cross-linking agents, and dried as above.
Multilayered Beads
Wet single layered beads, prepared as described, were transferred into a beaker containing chitosan solution (0.2–0.4 % w/v in 2% v/v acetic acid). The beads were allowed to cross-link for 1 hr, filtered, washed with cold water, and dried under the same condition as described for single layered beads. The dried beads had two layers of coating, alginate and chitosan layers, and thus designated as double layered beads. Triple layered beads were prepared by placing a few grams of the double layered beads in sodium alginate solution (1% w/v) and allowed to cross-link for a preselected time. After the cross-linking time, the beads were collected by filtration, washed with cold water, and dried in an oven at 50°C for 4 hr and later at room temperature (25°C) for 12 hr. All the dried beads were stored in airtight, dust free containers until further use.
For the purpose of easy identification, uncoated beads were designated as TP1; sodium alginate-treated beads (single layered/coated) as SA; single layered beads subsequently treated with chitosan (double layered/coated) as CT1 and CT2, and double layered beads finally treated with sodium alginate (triple layered) as CT3.
Evaluation of the Beads
Bead Size and Encapsulation Efficiency
The size of the prepared beads was measured with an optical micrometer fitted with a calibrated eye piece. The mean of 100 beads was noted as particle size. The sizes of both wet and dried beads were measured. All readings are average of three trials ±S.D
Fifty mg of beads were crushed in a glass mortar and digested in 0.1N hydrochloric acid (pH 1.2) for 24 hr in a graduated flask. The solution was filtered through a G-2 filter and an aliquot was used to assay for drug content spectrophotometrically (Jasco 7800, Japan) at 276 nm against a suitable blank. The encapsulation efficiency was calculated by expressing the percentage ratio of the actual drug entrapment to drug added. The values are average of three trials± S.D.
SEM Analysis
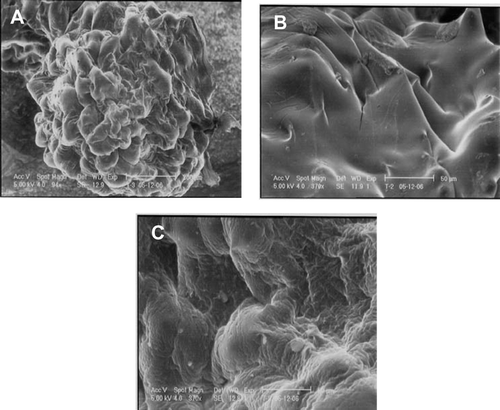
The surface morphology images were obtained by scanning electron microscope (Philips XL20, Holland) under vacuum. Beads were mounted on brass stubs using silver paste and scanned under vacuum at the required magnification at room temperature.
Water Uptake Studies
The water uptake capacity of the empty and ciprofloxacin loaded beads was determined in 0.1N HCl (pH 1.2) and pH 7.4. Beads (100 mg) were immersed in solutions of pH 1.2 and 7.4 and at regular intervals of time, the beads were reweighed after carefully wiping off excess of liquid with a tissue paper. The equilibrium water uptake was determined from the following equation;
where Wt and Wo are the weight of the beads at time “t” and under dry state, respectively.
In Vitro Drug Release Studies
In vitro release of ciprofloxacin from the beads was performed in USP XXIII dissolution apparatus II with a paddle speed of 50 rpm. The dissolution medium was 900 ml of simulated gastric fluid (SGF) without enzyme (0.1N HCl, pH 1.2) for the first 2 hr and subsequently rest of the release study was performed in simulated intestinal fluid (SIF; phosphate buffer, pH 7.4). At predetermined time intervals, 5 ml aliquot was withdrawn and replenished with an equal volume of fresh dissolution medium. The drug content in the aliquot was determined spectrophotometrically at 276 nm (Jasco 7800, Japan). A study was performed concurrently with placebo beads to record for any interference by the bead components.
Mechanism of Drug Release
In vitro release data were fitted to Higuchi's square root model Q = K√t (Higuchi Citation1963) to analyze the kinetics of drug release from the prepared beads. Where Q is the amount of drug released in time “t”, Qo is the initial amount of drug in dissolution medium, K is the release constant of respective equations, and t is the release time. Further, the data were fitted to Korsmeyer-Peppas' power law equation (Korsmeyer et al. Citation1983; Peppas Citation1985):
where Mt/M∞ is the fraction of drug released in time “t,” K is structural and geometric constant, and n is the release exponent.
Statistical Analysis
All the data were analyzed by Student's t-test and one-way ANOVA, wherever necessary, using Sigma Stat 2.0 (Jandel Scientific Corporation, USA) to determine the statistical difference in the results. A probability value p < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
On addition of drug containing chitosan solution to sodium-TPP solution, ionic cross-linking occurs between the protonated amine group on chitosan and phosphate ions of sodium-TPP (Bodmeier et al. Citation1989). Takahashi et al. (Citation1990) have previously studied formation of ionic interaction between amino group of chitosan and carboxyl groups of polyacrylate and alginate. When sodium alginate is included in the coagulation fluid, in addition to phosphate ions, the cross-linking proceeds between the free carboxylic group of alginates and protonated amino groups on chitosasn (Aral and Akbuga Citation1998) producing single layered beads. When these beads were subsequently placed in chitosan solution, cross-linking occurred between amino group on chitosan and carboxylic acid of alginate layer forming double layer over chitosan beads. Further treatment with alginate caused formation of the third layer over chitosan beads.
Bead Size and Morphology
The average size of wet uncoated bead was 2.23 ± 0.19 mm. On drying the size reduced drastically to 0.60 ± 0.09 mm. A fractional increase in the size of both wet and dried beads was observed on inclusion of alginate in the coagulation fluid (). Subsequent treatment with chitosan and later with alginate caused an increase in the size of beads markedly (p < 0.05). The weight of multilayered beads increased marginally in comparison to uncoated beads suggesting for increased coating. The surface morphology, as shown in , tends to alter with addition of alginate in the coagulation fluid.
Encapsulation Efficiency
As evident from , the encapsulation efficiency of the prepared beads increased with the addition of alginate in the coagulation fluid. The entrapment of ciprofloxacin in uncoated beads was 67.21 ± 1.95 %, whereas corresponding entrapment in the alginate-coated beads was 75.32 ± 2.67–78.08 ± 3.06%. This significant difference (p < 0.05) in the entrapment could be attributed to the formation of polyelectrolyte complex on the bead surface. In uncoated beads, the drug diffuses into the coagulation fluid due to the porous bead (Bodmeier et al. Citation1989). The formation of polyelectrolyte complex on the bead surface could have reduced the pore size by formation of tight junction between alginate and chitosan layers, resulting in better entrapment of ciprofloxacin.
Water Uptake of Beads
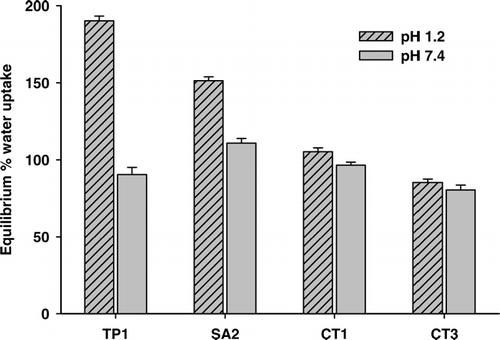
Both chitosan and sodium alginate exhibit pH dependent solubility. The former is more soluble in acidic environment and the latter polymer in alkaline condition. Therefore to simulate the possible effect of pH on drug release rate, a water uptake study was conducted in simulated gastric fluid (0.1N HCl, pH 1.2 without enzymes) and simulated intestinal fluid (pH 7.4). Uncoated beads (TP1) exhibited highest percentage of water uptake, 190.26 ± 3.04%, at pH 1.2 in comparison to 90.45 ± 4.58% at pH 7.4 (). This significant difference (p < 0.001) is best explained on the following lines. The pKa of chitosan is ∼6.3 (Yalpani and Hall Citation1984). Therefore in acidic pH, the hydrogen bond is broken and electrostatic repulsion arises between protonated amine groups on chitosan causing higher water uptake as in simulated gastric fluid. However, no such repulsion arises on placing the beads in simulated intestinal fluid. The percentage water uptake capacity of the beads was reduced significantly (p < 0.001) when the beads were treated with sodium alginate (single layered beads, SA2).
This observed reduction in the water uptake capacity is due to the formation of polyelectrolyte complex as a result of ionic interaction between carboxylic group of alginic acid and –NH+ on chitosan. With further coating (double coated-CT1 and triple coated-CT3), dense ionic cross-linking occurs on the bead, which acts as a barrier for the diffusion of the medium into the bead and decreases water uptake capacity.
Ciprofloxacin Release
Drug release behavior from alginate-reinforced chitosan beads was studied by pH change method. As the concentration of sodium alginate was increased, drug release decreased (). The cumulative amount of drug released from uncoated beads (TP1) and alginate-treated beads (SA1 and SA2) was statistically significant (p < 0.05). During bead formation, polyelectrolyte complexation occurs not only between chitosan and TPP, but also between chitosan and sodium alginate (Aral and Akbuga Citation1998). Carboxylate group of sodium alginate reacts with protonated amine group on chitosan by electrostatic interaction. Further as the complexation proceeds, hydrogen bonding occurs between chitosan and sodium alginate. This polyelectrolyte coating formation hinders the ingress of dissolution medium, hence decreased drug release. Increased alginate concentration leads to stronger interaction between the two polymers, which occupy the pores on the beads, resulting in slower drug release.
FIG. 3 Effect of polyelectrolyte coating and cross-linking on chitosan bead on the drug release rate: TP1 = (-•-), SA1 = (-ˆ-), SA2 = (-∇-), SA3 = (-▵-), SA4 = (-▪-) and SA5 = (-□-). (n = 3).
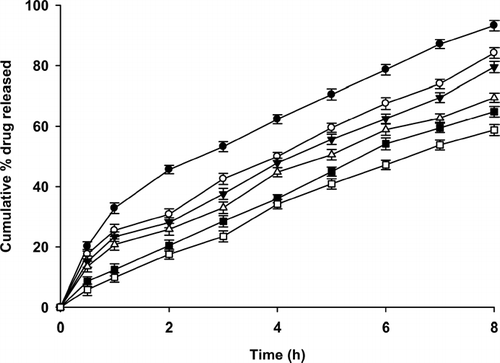
The drug release seems to follow a bimodal pattern with an instantaneous burst release and then sustained release corresponding to the instantaneous release of surface drug and later diffusion of drug through the matrix. At the end of 2 hr, the pH of dissolution medium was changed from 1.2 to 7.4. This change in pH causes slow disintegration of polyelectrolyte complex formed on the beads (Anal and Stevens Citation2005). However, at higher pH chitosan is insoluble and precipitates as a stronger bead and thus reduces the drug release rate.
A much more linear release profile was observed on cross-linking of sodium alginate coated beads with calcium ions (SA4) and glutaraldehyde (SA5). This was attributed to hardening of the polyelectrolyte complex, which made it more resistant to ingress of dissolution medium (). The increased water repellant as a result cross-linking of the beads reduced the significance of change in pH of dissolution medium on drug release rate. Among the cross-linking agents used, glutaraldehyde provided slower drug release than calcium chloride cross-linked beads.
The effect of chitosan coating on drug release from double-coated beads is shown in . Drug release from these beads was linear in comparison to that of uncoated beads. In comparison to single coated beads (SA1), the drug release from chitosan-coated beads decreased significantly (p < 0.05). Biphasic release pattern was observed from the chitosan-coated beads. Drug release in SGF was slower; however, as the dissolution medium was changed to SIF (pH 7.4), the drug release increased marginally. Drug release was much slower from triple coated beads, i.e., alginate-chitosan-alginate (CT3, ). At the end of 2 hr, 2.15 ± 1.48% of drug was released while after 8 hr, 26.54 ± 2.46% of drug was released. The observed release pattern was similar to those exhibited by the colon-targeted drug delivery systems that exhibit time dependent release pattern with maximum percentage of loaded drug released after a lag time of few hours.
FIG. 4 Drug release from multiple-coated beads. SA2 = (-•-), CT1 = (-ˆ-), CT2 = (-∇-), and CT3 = (-▵-),. (n = 3).
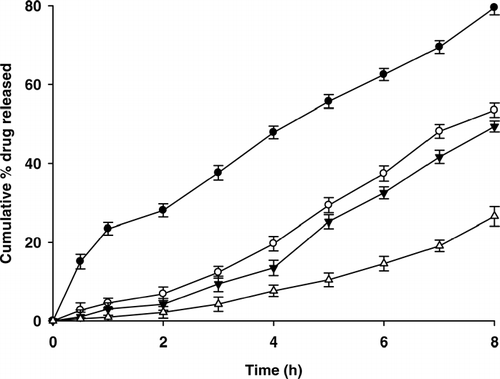
Drug release from these beads was governed by diffusion of dissolved drug through multiple polymeric complexes of chitosan and alginate. As the number of polymeric coatings was increased, drug release decreased significantly (p < 0.001) due to lesser diffusivity of dissolution medium into the bead matrix. Moreover, at the end of 2 hr, the pH of dissolution was changed to 7.4, where the ciprofloxacin (pKa 6.09) solubility is low. The change in the dissolution medium at the end of 2 hr, thus, alters the solubility of ciprofloxacin and reduces its diffusivity.
The release profiles from all the prepared batches correlate well with the water uptake capacity of the beads. Uncoated beads (TP1) exhibited higher swelling resulting in faster release of drug both in SGF (pH 1.2) and SIF (pH 7.4), in comparison to single and multiple coated beads. Swelling of the beads depended on the number of polyelectrolyte coatings and decreased with increase in the number of coatings.
Drug Release Kinetics
The drug release data fit linearly to Higuchi's square root kinetic equation (r2 = 0.9496–0.9803) except for multiple coated beads (batch CT1-CT3), which exhibited linearity to zero-order kinetics (r2 = 0.9329–0.9756). The deviation from zero-order kinetics of a few batches could be attributed to an initial burst release (∼10–15%) of ciprofloxacin from the formulations. This initial burst release was reduced with multiple coating, which caused the data to follow zero-order kinetics. The release exponent (n) was in the range of 0.40–1.2. For most of the batches the value was marginally higher than 0.5 but < 1, indicating that drug release from these systems was non-Fickian (anomalous) referring to a combination of diffusion and dissolution mechanism. When chitosan (CT1 and CT2) and alginate (CT3) were included in postcoagulation fluids (multiple coated beads), the release exponent was ∼1.0 indicating zero-order kinetics. Although higher molecular weight of ciprofloxacin (331.4) could be responsible for lower diffusivity leading to the observed deviation from Fickian mechanism of drug release (Sheu et al. Citation1992), the polymer (chitosan and sodium alginate) characteristics (solubility, pKa, tortuosity) and drug influence the mechanism of drug transport (Reza et al. Citation2003). In multilayered beads, the drug release is prominently dependent on the diffusion of solubilized drug through various layers of coating and the solubility/ swellability of the polymers in different dissolution medias.
As illustrated in , t50% of various batches corroborates with the drug release rate from various prepared batches. Uncoated and single coated beads exhibited lower t50%, but as the number of coatings was increased a substantial increase in t50% was observed. These results indicate that a sustained release multiparticulate system can be obtained by utilizing a general concept of polyelectrolyte complex formation between chitosan and alginate. By modifying a few variables it is possible to modulate drug release from these systems.
TABLE 2 Correlation coefficient, release exponent, and t50% of various batches
CONCLUSION
In this study, we evaluated several variables modifying the drug release. In general, the beads prepared by multiple coating (alginate, alginate-chitosan, or alginate-chitosan-alginate) provided sustained release of ciprofloxacin. Anal and Stevans (Citation2005) evaluated multilayered alginate beads for extended release. However, a considerable percentage of embedded drug was released at a faster rate from the beads. In our study, the release was slow and no untoward burst release was observed. The disintegration of chitosan in SGF was overcome by alginate coating, and in SIF chitosan is insoluble that makes the system more useful in targeting lower intestine. The discussed in vitro results forms the basis for our future work to evaluate in vivo and to optimize the formulations to achieve targeting to lower intestine.
One author, Srinatha, thanks the University Grants Commission, Goverment of India, for providing research fellowship for the study. The authors are grateful to Prof. O. N. Srivastava and Vijay Kumar, Department of Physics, Banaras Hindu University, for helping with SEM studies.
REFERENCES
- Aiedeh K., Giannsi E., Orienti I., Zecchi V. Chitosan microcapsules as controlled release systems for insulin. J. Microencaps. 1997; 14: 567–576
- Anal A. K., Stevens W. F. Chitosan-alginate multilayer beads for controlled release of ampicillin. Int. J. Pharm. 2005; 290: 45–54
- Aral C., Akbuga J. Alternative approach to the preparation of chitosan beads. Int. J. Pharm. 1998; 168: 9–17
- Becheran-Maron L., Peniche C., Arguelles-Monal W. Study of the inter-polyelectrolyte reaction between chitosan and alginate: influence of alginate composition and chitosan molecular weight. Int. J. Biol. Macromol. 2004; 34: 127–133
- Binkley C. E., Cinti S., Simeone D. M., Colletti L. M. Bacillus anthracis as an agent of bioterrorism: a review emphasizing surgical treatment. Ann. Surg. 2002; 236: 9–16
- Blondeau J. M. Current issues in the management of urinary tract infections: extended-release ciprofloxacin as a novel treatment option. Drugs 2004; 64: 611–628
- Bodmeier R., Chen H., Tyle P., Jarosz P. Pseudoephedrine HCl microspheres formulated into an oral suspension dosage form. J. Contr. Rel. 1991; 15: 65–77
- Bodmeier R., Oh K. H., Parmar Y. Preparation and evaluation of drug containing chitosan beads. Drug Dev. Ind. Pharm. 1989; 15: 1475–1494
- Higuchi T. Mechanism of sustained action medication: Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963; 52: 1145–1149
- Knutson D., Greenberg G., Cronau H. Management of Chron's disease—a practical approach. Am. Fam. Physician. 2003; 68: 707–714
- Ko J. A., Park H. J., Hwang S. J., Park J. B., Lee J. S. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int. J. Pharm. 2002; 249: 165–174
- Korsmeyer R. W., Gurny R., Doelker E. M., Buri P., Peppas N. A. Mechanism of solute release from porous hydrophilic matrices. Int. J. Pharm. 1983; 15: 25–35
- Lin Y. H., Liang H. F., Chung C. K., Chen M. C., Sung H. W. Physically crosslinked alginate/N,O-carboxymethyl chitosan hydrogels with calcium for oral delivery of protein drugs. Biomaterials 2005; 26: 2105–2113
- Mitrevej A., Sinchaipanid N., Rungvejahvuttivittaya Y., Kositchaiyong V. Multiunit controlled release diclofenac sodium capsules using complex of chitosan with sodium alginate or pectin. Pharm. Dev. Tech. 2000; 6: 385–392
- Murata Y., Kontani Y., Ohmae H., Kawashima S. Behaviour of alginate gel beads containing chitosan salt prepared with water-soluble vitamins. Eur. J. Pharm. Biopharm. 2002; 53: 249–251
- Orienti I., Cerchiara T., Luppi B., Bigucci F., Zuccari G., Zecchi V. Influence of different chitosan salts on the release of sodium diclofenac in colon-specific delivery. Int. J. Pharm. 2002; 238: 51–59
- Pasparaski G., Bouropoulos N. Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate-chitosan beads. Int. J. Pharm. 2006; 323: 34–42
- Peppas N. A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta. Helv. 1985; 60: 110–111
- Pile J. C., Malone D., Eitzen E. M., Friedlander A. M. Anthrax as a potential biological warfare agent. Arch. Int. Med. 1998; 158: 429–434
- Reza S., Quadir M. A., Haider S. S. Comparative evaluation of plastic, hydrophobic, and hydrophilic polymers as matrices for controlled release drug delivery. J. Pharm. Pharmceut. Sci. 2003; 6: 282–291
- Riberio A. J., Silva C., Ferreira D., Veiga F. Chitosan reinforced alginate microspheres obtained through the emulsification/internal gelation technique. Eur. J. Pharm. Sci. 2005; 25: 31–40
- Roberts G. A. F. Solubility and solution behavior of chitin and chitosan. Chitin chemistry, G. A. F. Roberts. MacMIllan, Houndmills 1992; 274–329
- Sezer A. D., Akbuga J. Release characteristics of chitosan treated alginate beads: II. Sustained release of a low molecular drug from chitosan treated alginate beads. J. Microencaps 1999; 16: 687–696
- Sheu M. T., Chou H. L., Kao C. C., Liu C. H., Sokolski T. D. Dissolution of diclofenac sodium from matrix tablet. Int. J. Pharm. 1992; 85: 57–63
- Shiraishi S., Imai T., Otagiri M. Controlled release of indomethacin by chitosan-polyelectrolyte complex: Optimization and in vivo/ in vitro evaluation. J. Contr. Rel. 1993; 25: 217–225
- Shu X. Z., Zhu K. J. A novel approach to prepare tripolyphosphate/chitosan complex beads for controlled release drug delivery. Int. J. Pharm. 2000; 201: 51–58
- Struszczyk H., Wawro D., Niekraszewicz A. Biodegradability of chitosan fibres. Advances in Chitin and Chitosan, C. J. Brine, P. A. Sandford, J. P. Zikakis. Elsevier Applied Sciences, London 1991; 580–585
- Takahashi T., Takayama T., Machida Y., Nagai T. Characteristics of polyion complexes of chitosan with sodium alginate and polyacrylate. Int. J. Pharm. 1990; 61: 35–41
- Xu Y., Zhan C., Fan L., Wang L., Zheng H. Preparation of dual crosslinked alginate-chitosan blend gel blend and in vitro release in oral site-specific drug delivery system. Int. J. Pharm. 2007; 336: 329–337
- Yalpani M., Hall L. D. Some chemical and analytical aspects of polysaccharide modification. 3. Formation of branched-chain, soluble chitosan derivatives. Macromolecules 1984; 17: 272–279