Abstract
This study was designed to investigate the possible histological effects of different intranasal (IN) formulations of indomethacin (IND) on nasal mucosa in sheep. For this purpose, oil-in-water (O/W) emulsion (E) and solution (S) formulations including 3 mg/mL of IND were prepared. Penetration enhancers such as polyvinylpyrolidone (PVP), citric acid (CA) and sodium taurocholate (NaT) were added to emulsion (1%) at the final step into the formulations. First, the effect of penetration enhancers on permeation of IND was evaluated by in vitro permeation studies in which sheep nasal mucosa was used. According to the permeation studies PVP showed the highest enhancing effect on the permeation rate of IND from sheep nasal mucosa. Furthermore, the IND permeation from E containing PVP (1.624 ± 0.045 mg) was significantly higher than that obtained from E (0.234 ± 0.012 mg) (p < 0.05).
For the histological studies, white Karaman sheep of approximately 20 ± 5 kg, aged 4 to 8 months were used. They were randomly divided into eight groups, each including three sheep. Five experimental groups received different formulations of IND emulsion without/ with penetration enhancers (E-PVP, E-CA, E-NaT, E) and IND solution (S), respectively. Parallel controls were composed of either untreated groups and were given blank emulsion or isotonic sodium chloride solution (0.31 mg/kg). 2 mL of each experimental formulation was applied to both nostrils of sheep, and 1/3 central and lower regions of the nose were dissected and prepared for light microscopy. Specimens stained with hematoxylin and eosin and Gomori's trichrome were examined by light microscopy. No signs of inflammation or erosion were noticed in the nasal mucosa of the control groups. Widened epithelial intercellular spaces were noticed in E-CA, E-NaT, and E-PVP groups as well with the E-PVP group showing the largest intraepithelial separations. E-CA and E-NaT groups showed significant decrease in the amount of goblet cells, while hypoplasia was considerably moderate in the E-PVP group.
Finally, intranasal administration of IND emulsion with PVP may be considered as an alternative to intravenous and per oral administrations of IND to overcome their adverse effects.
The inconvenience of application and poor patient compliance associated with parenteral drug administration have prompted recent investigation of alternative routes, such as oral, nasal, buccal, rectal, pulmonary, and transdermal (Gizurarson et al. Citation1993). The interest in and the importance of systemic effects of drugs administered via the nasal route have expended over the last decade. Nasal administration is considered to be a useful alternative when the parenteral route is inconvenient or oral administration results in unacceptably low bioavailability (Chien, Su, and Chang Citation1989). Prolongation of the duration in the nasal cavity is possible by increasing the viscosity of the vehicle, by using some bioadhesives, or by making other suitable formulation variations (i.e., emulsions, suspensions or liposomes) (Mitra et al. Citation2000; Aikawa et al. Citation2002; Li et al. Citation2005; Wingertzahn et al. Citation2005; Cui, Qian, and Yin Citation2006).
Emulsions generally provide the most suitable pharmaceutical formulations for dissolving lipophilic drugs, such as indomethacin, by raising the aqueous concentration of the drug. Additionally, the oil phase of an emulsion allows the hydrophobic drug to stay in the form of a solution. Therefore, emulsions can potentially enhance the bioavailability of drugs with poor solubility in water (Mitra et al. Citation2000; Illum et al. Citation2001; Tirucherai, Pezron, and Mitra Citation2002; Karasulu, Sanal, and Sözer Citation2004).
Morphological studies have shown that emulsions do not cause any significant effect on nasal mucosa (Mitra et al. Citation2000; Aikawa et al. Citation2002). The nasal mucosa consists of various differentiated cells with a total thickness of approximately 100 μ m. It is highly vascularized and has a large surface area. Proteolytic activity in the nasal mucosa is lower than in the gastrointestinal tract, and permeation is considerably higher. Although the nasal mucosa has a protective function against the penetration of foreign material to the submucosa, it has a relatively higher permeability to both hydro- and lipophilic compounds (Cui, Qian, and Yin Citation2006; Gavini et al. Citation2006).
Drugs cross the nasal mucosal membrane using two different pathways: transcellularly (across the cells) and paracellularly (between the cells). Lipophilic drugs, such as indomethacin (IND), are transported transcellularly by a concentration-dependent passive diffusion process, by facilitated diffusion using a receptor or carrier molecule, or by vesicular transport mechanisms (Illium Citation2002). It is possible to improve nasal absorption of polar drugs by administering them in combination with a penetration enhancer that promotes the transport of the drug across the nasal membrane (Illium Citation2002). Although the precise mechanism of action of the penetration enhancers is not known, it is speculated that these agents promote drug absorption by; i. increasing membrane fluidity, ii. expanding the dimension of paracellular pathway to solute transport, or iii creating transient pores by reverse micelle formation in the cell membrane. However, many penetration enhancers irritate to mucous membranes (Vermehren and Hansen Citation1998; Fang, Hwang, and Leu Citation2003; Wang et al. Citation2006) Because of the potential for structural damage to the mucosal membrane, the safety of any surfactant being considered for use as a nasal permeation enhancer must be carefully evaluated. The acceptance of a permeation enhancer is dependent not only on its ability to enhance absorption, but also on its overall safety profile with regard to both local and systemic effects (Sinswat and Tengamnuay Citation2003).
Indomethacin (IND), chosen as a lipophilic model substance, is a nonsteroidal anti-inflammatory drug (NSAID) used for its antipyretic and analgesic properties. It is not a simple analgesic and, because of its potential serious untoward effects, should not be used frivolously. It has been used effectively in the treatment of rheumatoid arthritis for more than a decade. The high incidence and severity of side-effects, which are dose-related and associated with long-term admininistration, have limited its use. It produces erosions and ulcers in the gastrointestinal tracts. An alternative route of administration was searched for IND that would allow to maintain the blood drug concentrations at the therapeutic range while at the same time would not cause any gastric irritation. This has led to the search for a new delivery system, which can overcome the side-effects by controlling the drug's release (Karasulu et al. Citation2003; Uzunkaya and Bergiadi Citation2003; Akhgari, Sadeghi, and Garekani Citation2006). One of the physicochemical properties of IND is its poorly soluble in water, although it is soluble in lipophilic medium (The Merck Index 1996). According to this concept, O/W emulsions have generally been preferred as the best pharmaceutical formulations for IND, by providing higher aqueous concentrations of the drug. Morphological studies have shown that emulsions do not cause any significant damage on nasal mucosa (Mitra et al. Citation2000; Aikawa et al. Citation2002). Thus, we suggest that, non-invasive IND administration may be considered as an alternative route to IND uses. Also, some penetration enhancers are included in the formulation to improve the nasal absorption of polar drugs. So, the IND administration may be useful for rapid onset of pharmacological effects.
The primary objective of this work was to develop an O/W emulsion loading with IND for nasal administration and to increase the IND permeation using suitable enhancers across sheep nasal mucosa. Our secondary objective was to investigate the potential histological effects of various intranasal formulations on the nasal mucosa of sheep following acute localized exposure.
MATERIALS AND METHODS
IND was supplied by Deva Pharmaceutical Fac. (Istanbul, Turkey). Emulsifying agents, polyoxyethylene 20 sorbitan monooleate (Tween 80) and sorbitan monooleat (Span 80), were purchased from E. Merck Co. (Schuchardt, Germany). The penetration enhancers, polyvinylpyrolidone (PVP), citric acid (CA), sodium taurocholate (NaT), and isopropyl myristate as an oily phase, were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All chemicals were used as analytical grade.
Preparation of Nasal Formulations
Oil-in-water (O/W) emulsion of isopropyl myristate was prepared using Tween 80 and Span 80 as emulgators. Sodium carbonate solution (2.5%) was used as the aqueous phase of emulsion. IND was incorporated into the oil phase of emulsion (3 mg/mL) and it was stirred with magnetic stirrer for 30 min at 40°C. Penetration enhancers such as polyvinylpyrolidon (PVP), citric acid (CA), and sodium taurocholate (NaT) were added to emulsion at the last stage (1%). All emulsions were stable and neutral pH during the time course of the experiment. Furthermore, IND was mixed with 2.5% sodium carbonate solution at a concentration of 3 mg/ml and pH of this solution (S-IND) including 3 mg/ml of IND was set at 7. The composition of different emulsions, coded E, E-PVP, E-CA, and E-NaT is shown in .
TABLE 1 The composition of emulsions (%) containing different penetration enhancers
Preparation of Nasal Mucosa Membranes
Nasal respiratory mucosa was isolated carefully from each nostril of the sheep. The mean weight of the white Karaman sheep, aged 4 to 8 months, was 35 ± 5 kg. The snouts were separated from the animals and opened to expose the conchae. The mucosa covering the conchae (cavity mucasa) was carefully removed using forceps and a scalpel, yielding two pieces (left and right) per snout. The study was approved by the Animal Ethical Committee in University of Ege, Faculty of Pharmacy, 35100 Bornova-Izmir, Turkey (01/121). Delipidized mucosa was prepared with 1 ml of chloroform-methanol (2:1, v/v) for 60 min in order to extract the lipids from the mucosa. The delipid process extracted the lipid content of the whole mucosa so no ultraviolet absorbance was observed. The delipidized sheep nasal mucosa was stored at −80°C. The mucosa was first hydrated for 24 hours in the buffer solution (pH 7.4 isotonic phosphate buffer) at 4°C and then permeation studies were carried out.
In Vitro Permeation Studies
In vitro permeation studies were performed using a vertical diffusion cell apparatus. Sheep nasal mucosa was mounted in the diffusion chambers with the mucosal and serosal sides facing the donor and receiver phases, respectively. The donor medium one mL of the emulsion or solution containing IND (3 mg/mL). The receptor medium was 10 ml of isotonic phosphate buffer at pH 7.4. The available diffusion area between cells was 1cm2. The stirring rate and temperature were kept at 600 rpm and 37°C, respectively. The aliquots withdrawn at various intervals for 8 hours were immediately analyzed for drug concentration spectrophotometrically (320 nm) directly and were refilled with the same volume of fresh solutions. Five replicates of each experiment were performed. In in vitro permeation studies, permeated emulsion without IND was used as blank to determine drug concentration.
Histological Examinations
Histological effects in sheep nasal mucosa were examined after exposure to intranasal formulations. White Karaman sheep (20 ± 5 kg), aged 4 to 8 months, were used for the experiments. The study was approved by the Animal Ethical Committee in University of Ege, Faculty of Pharmacy, 35100 Bornova-Izmir, Turkey (01/121). They were randomly divided into 8 groups each containing three sheeps as follows:
Group 1: 0.31 mg/kg IND in (O/W) emulsion containing PVP (E-PVP)
Group 2: 0.31 mg/kg IND in (O/W) emulsion containing CA (E-CA)
Group 3: 0.31 mg/kg IND in (O/W) emulsion containing NaT (E-NaT)
Group 4: 0.31 mg/kg IND in (O/W) emulsion (E)
Group 5: 0.31 mg/kg IND in 2.5% sodium carbonate solution (S-IND)
Group 6, 7, and 8 were comprised of parallel control groups either treated with O/W emulsion without IND (O/W) or saline solution (SF) and an untreated group (C).
Two ml of these formulations were applied intranasally into each nostril of sheep in the slaughterhouse. Sheep were sacrificed by decapitation 30 min later. One third central and lower regions of the nose were dissected and further processed for light microscopy. Each specimen was fixed in a 10% formalin solution for approximately 24 h, then washed with tap water, dehydrated through an increasing ethanol series, immersed in xylene, and finally embedded in paraffin wax at 56°C. Paraffin blocks were cut serially 5 μ m using a rotary microtome (RM 2145, Leica Co., Nussloch Germany). Sections were stained with hematoxylin and eosin and Gomori's Trichrome and examined by light microscope (Olympus BX-51, Tokyo, Japan). Three different microscopic areas were evaluated for each tissue sample from each treatment protocol and sections were scored according to the existence of intercellular spaces in the epithelium and leucocyte infiltration and the decrease in goblet cell number.
Data Treatment
The permeation of IND from nasal preparations was investigated. The cumulative amount over time profiles were plotted. A linear profile (steady state) was observed during 2- to 8-hour period and the slope of the linear portion of the curve was determined by linear regression. The effective permeability coefficients and flux values at steady state were calculated from the slope according to Eq. (1) and Eq. (2), respectively (Ferreira et al. Citation1995; Kissel and Werner Citation1998; Yetkin et al. Citation2001).
V: Volume of the receiver compartment (ml)
Co: Initial concentration in the donor compartments (mg/ml)
Peff: Effective permeability coefficient (cm/s)
J: Flux (mg/cm2s)
A: Permeation area (cm2) dc/dt:Pseudo steady-state change of concentration over time (mg/ml · sec)
Results are expressed as the mean ± SD from at least three measurements.
Statistical Analysis
Data were expressed as mean ± SD and the differences in the results of in vitro and ex vivo studies were evaluated using one-way analysis of variance (ANOVA) followed by Duncan or Dunnett C dependent to levene test result for homogeneity of variances. Differences were considered statistically significant when p < 0.05.
RESULTS AND DISCUSSION
Effect of Enhancers on In Vitro Permeation of Nasal Emulsion
The penetration of IND from from E (0.78 × 10− 4± 0.04 × 10− 4) was found significantly lower (p < 0.05) as compared with S-IND (3.65 × 10− 4 ± 0.3 × 1010 − 4) after 8 h. This finding can be explained with the inverse relation between viscosity and drug release property (Welin-Berner, Neelissen, and Bergenstahl Citation2001; Tas et al. Citation2006). However, it is known that, emulsions are viscose systems and increase the residence time of the drug in the nasal cavity (Kan et al. Citation1999; Mitra et al. Citation2000; Tirucherai, Pezron, and Mitra Citation2002). For this reason, they were preferred to the emulsion for nasal application because of retaining the drug at the site of action.
Therefore, the penetration enhancers were added to the E to increase the permeation rate of IND. It is known that permeation enhancers (usually polymers and bile salt derivatives) may increase the permeability by altering and /or damaging the tight junction of nasal epithelium (Longenecker et al. Citation1987; Illum et al. Citation2001; Yu et al. Citation2004). So, the effects of penetration enhancers on the permeation of IND from E across sheep nasal mucosa were investigated by comparing the permeation rate of IND in the presence vs absence of enhancers as in vitro. shows the permeation profiles of IND from S-IND and E emulsion formulations including penetration enhancers such as PVP, CA, and NaT. The permeation rate of IND from sheep nasal mucosa was greatly enhanced when the penetration enhancers were incorporated in the emulsion (p < 0.05).
FIG. 1 Comparison of the in vitro permeation profiles of E formulation with or without penetration enhancers and S-IND from sheep nasal mucosa. Each point shows the average of three determinations. Bars represent the SD of five experiments (n = 5).
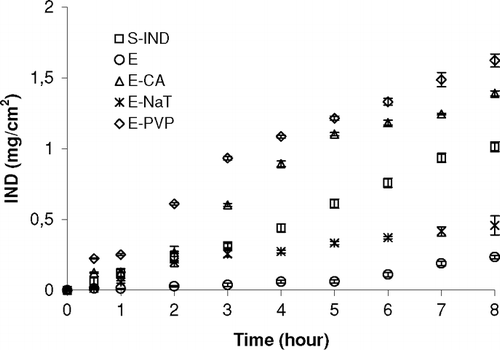
Permeability coefficients and flux values of IND across the sheep nasal mucosa in vitro were calculated using Eq. (1) and Eq. (2) (). When penetration enhancers were added into the emulsions, the amount of the IND across the mucosa increased consequently. In our study, after 8 hours, the permeation rates of IND from emulsions containing PVP, CA, or NaT were found to be 6.77, 6.62, and 1.99 times greater than that of the emulsion without enhancers, respectively. PVP showed the highest enhancing effect on the permeation rate of IND from sheep nasal mucosa when compared with NaT and CA. Furthermore, the IND permeation from E containing PVP (1.624 ± 0.045 mg) was significantly higher than that obtained from E (0.234 ± 0.012). The order of the enhancement effect was E-PVP > E-CA > S-IND > E-NaT > E (Flux values (mg/cm2s) = 5.2(± 0.2) × 10− 4 > 5.1 (± 0.4) ×10−4 > 3.6 (± 0.3) × 10− 4 > 1.5(± 0.1) × 10− 4 > 0.78 (±0.04) × 10− 4) for 8 h. There were significant differences in permeation coefficient (p < 0.05) compared with that without enhancers.
TABLE 2 Flux, Peff of IND across the nasal mucosa from emulsion with/without penetration enhancers and from S-IND
Effect of Enhancers on Histological Examinations of Nasal Emulsions
shows histological scoring of all experimental groups. No signs of inflammation or erosion were noticed in the nasal mucosa of the control groups (C, SF, O/W). Respiratory epithelium, ciliary morphology, mucosal structures, intercellular spaces, and junctions were completely normal, apart from a slight inflammation in the lamina propria under the epithelium of group O/W. Groups E and S-IND showed mild separations between epithelial cells, a slight decrease in the amount of goblet cells, indicating minimal hypoplasia, and a few leucocyte infiltration along with inflammation in the lamina propria. Widened epithelial intercellular spaces were noticed in E-CA, E-NaT, and E-PVP groups as well, with the E-PVP group showing the largest interepithelial separations. So the use of absorption enhancers can increase the residence time of drug in the nasal cavity, opening tight junctions between the epithelial cells and the results obtained here are in reasonable agreement with the results of other workers (Vermehren and Hansen Citation1998; Welin-Berner, Neelissen and Bergenstahl Citation2001; Fang, Hwang, and Leu Citation2003; Todo et al. Citation2004; Wang et al. Citation2006). E-CA and E-NaT groups showed significant decrease in the amount of goblet cells, while hypoplasia was considerably mild in the E-PVP group. Similarly, leucocyte infiltration with the signs of inflammation was intensely observed in the E-CA and E-NaT groups, but not in the E-PVP group.
TABLE 3 Histological scoring of nasal mucosa in all experimental groups (n = 3 in each group)
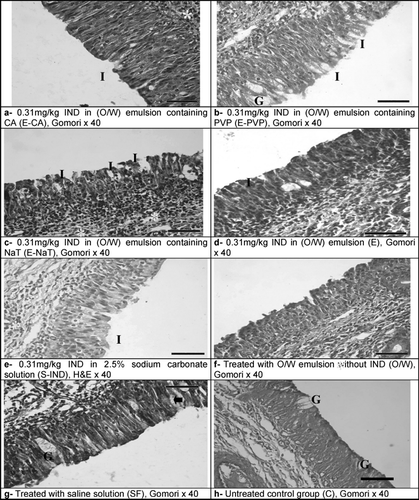
shows histological specimens of nasal mucosa surrounding administrations of various formulations. No signs of inflammation and erosions were seen at SF group and control groups (-f, g, and h, respectively). The morphology of the cilia was normal.
FIG. 2 Images from the histological evaluation of nasal mucosa. No signs of inflammation or erosion were noticed in the nasal mucosa of the control groups (f, g, h). Mild separations between epithelial cells, a slight decrease in the amount of goblet cells, indicating minimal hypoplasia, and a few leucocyte infiltration along with inflammation in the lamina propria (d, e); widened epithelial intercellular spaces (a, b, and c) and largest interepithelial separations (b); significant decrease in the amount of goblet cells and leucocyte infiltration with the signs of inflammation (a, c). Leukocyte infiltration (*), Intraepithelial spaces (I), goblet cells (G).
The nasal irritation by enhancers after a 0.5 h exposure was histologically investigated to explore the possible role of physicochemical responses of the mucosa to enhancers. Light microscopy indicated no observable damage to whole mucosa in the untreated group (data not shown).
PVP may be considered a biocompatible penetration enhancer, since PVP-containing emulsion did not induce significant damage to nasal mucosa (, -a). Furthermore, reasonably much larger intercellular spaces were noticed in the nasal epithelium exposed to PVP-containing emulsion compared to CA or NaT-containing emulsions (-b and c). Therefore, it may be concluded that PVP-containing emulsions serve as better solvents for the preparation of intranasal formulations of lipophilic drugs. For E-CA and E-NaT, available histological data indicate that they can have damaging effects. However, there are no histological data on the influence of E-PVP on the nasal epithelium. Furthermore, the most intercelluler spaces were seen at E-PVP.
Epithelial cells of sheep nasal mucosa that received emulsion application without IND was normal. However, moderate inflammation was detected in lamina propria. On the contrary, intercelluler spaces of sheep nasal mucosa, which received E and S-IND (-d and e), were wide and more inflammation was seen. The nasal cavity was to a major extent lined by ciliated columnar epithelium. Squamous epithelium was seen on the conchae.
The results from histological examinations indicate that E-PVP can be considered to be biocompatible and do not induce serious histological changes in the nasal mucosa. Further experiments are required to study the influence of these particular permeation enhancers in vivo focusing on the production of proinflammatory cytokines and enzymes indicative of cell damage.
CONCLUSION
In the investigation of Huang and colleagues (Citation1995), the pharmacokinetics of IND solution at a dosage of 3 g.L− 1 was studied after oral (po), intravenous (iv), and intranasal (ins) administrations. The area under the curve (AUC) after po, iv, and ins administrations were calculated as 71.9 ± 3.1, 84.9 ± 3.6, and 67.4 ± 3.1, respectively. They have shown that AUC after ins was close to that obtained after po dosing (p > 0.05). It was suggested that IND easily passes through both gastrointestinal tract and nasal mucosa to the systemic circulation because of its high lipophylic property. They also showed that the time to peak (tmax) of intranasal IND solution was 0.08 h, approached that after intravenous route. So the incorporation of IND intranasal emulsion may result in a considerable increase in nasal permeation. For this purpose, O/W emulsion containing IND were prepared for nasal application and permeation rate of IND from sheep nasal mucosa was examined using vertical diffusion cell apparatus. Furthermore, in this study, the effect of the penetration enhancers on permeation of IND from E-O/W was also studied. After in vitro permeation studies, permeation rate of IND increased with enhancers and PVP was an excellent candidate to be used as an enhancer of IND permeation across the sheep nasal mucosa (). There is no doubt that the emulsion containing PVP as enhancer has been shown to be more effective in increasing the permeation from sheep nasal mucosa compared with S-IND, E, E-NaT, E-CA. It is known that, emulsions due to their viscosity are preferred over solutions for nasal application. In addition both hydrophilic and lipophilic drugs can also incorporate in the emulsion phases as solution form. Permeation of IND from E-PVP was higher than the permeation of S-IND (p < 0.05). As a result of the histological studies, after administration of E-PVP to sheep nose, an increase in the intercellular spaces was noted and as a result of this indomethacin penetration was increase. For this reason, E-PVP, can be suggested for nasal application.
As a conclusion, according to the above information, if this formulation was administered in vivo, we suppose that, because viscosity of the formulations, residence time of emulsions in nasal cavity and absorption of drug will increase. These results are considered noteworthy in that, intranasal administration of IND may be considered as an alternative to intravenous administration of IND to overcome its disadvantages. Finally, it was concluded that the emulsion prepared was suitable with respect to the in vitro characteristics for in vivo studies.
The authors thank Pınar Integrated Meat and Flour Inc. for kindly providing us with sheep and Erdinç YILMAZ from Ege University, School of Medicine, Department of Histology and Embriology for his technical assistance.
REFERENCES
- Aikawa K., Matsumoto K., Mitsutake N., Uda H., Tanaka S., Shimamura H., Aramaki Y., Tsuchiya S. Drug release from pH-response polymer to nasal delivery. S. T. P. Pharm Sci. 2002; 12: 69–74
- Akhgari A., Sadeghi F., Garekani H. A. Combination of time-dependent and pH-dependent polymethacrylates as a single coating formulation for colonic delivery of indomethacin pellets. Int. J. Pharm. 2006; 320: 137–142
- Chien Y. W., Su S. E., Chang S. F. Nasal Systemic Drug Delivery, Vol. 39. Ed. Marcel Dekker, New York 1989
- Cui F., Qian F., Yin C. Preparation and characterization of mucoadhesive polymer-coated nanoparticles. Int. J. Pharm. 2006; 316: 154–161
- Fang J. Y., Hwang T. L., Leu Y. L. Effect of enhancers and retarders on percutaneous absorption of flurbiprofen from hydrogels. Int. J. Pharm. 2003; 250: 313–325
- Ferreira L. A. M., Seiller M., Grossiord J. L., Marty J. P., Wepierre J. Vehicle influence on in vitro release of glucose: w/o, w/o/w and o/w systems compared. J.Control. Release 1995; 33: 349–356
- Gavini E., Hegge A. B., Rassu G., Sanna V., Testa C., Pirisino G., Karlsen J., Giunchedi P. Nasal administration of Carbamazepine using chitosan microspheres: In vitro/in vivo studies. Int. J. Pharm. 2006; 307: 9–15
- Gizurarson S. The relevance of nasal physiology to the design of drug absorption studies. Adv. Drug Del. Rew. 1993; 11: 329–347
- Huang Z. L., Kagoshima M., Kagawa E., Shimada H. Absorption of indomethacin from nasal cavity in rats. Acta Pharma. Sin. 1995; 16: 117–120
- Illium L. Nasal drug delivery: New developments and strategies. DDT. 2002; 7: 1184–1189
- Illum L., Fisher A. N., Jabbal-Gill I., Davis S. S. Bioadhesive starch microspheres and absorption enhancing agents act synergistically to enhance the nasal absorption of polypeptides. Int. J. Pharm. 2001; 222: 109–119
- Kan P., Chen Z. B., Kung R. Y., Lee C. J., Chu I. M. Study on the formulation of O/W emulsion as carriers for lipophilic drugs. Colloids and Surfaces B Biointerfaces. 1999; 15: 117–125
- Karasulu E., Karasulu H. Y., Ertan G., Kırılmaz L., Güneri T. Extended release lipophilic indomethacin microspheres: formulation factors and mathematical equations fitted drug release raes. E. J. Pharm. Sci. 2003; 19: 99–104
- Karasulu H. Y., ™anal Z. E., Sözer S. Potent anti-inflammatory activity of intranasal indomethacin emulsion in rats. European Conference on Drug Delivery and Pharmaceutical Technology. 2004, (Sevilla-2004). 77
- Kissel T., Werner U. Nasal delivery of peptides: an in vitro cell culture model for the investigation of transport and metabolism in human nasal epithelium. J.Control. Release 1998; 53: 95–203
- Li Y., Jiang H. L., Zhu K. J., Liu J. H., Hao Y. L. Preparation characterization and nasal delivery of α -cobrotoxin-loaded poly(lactide-co-glycolide)/polyanhydride microspheres. J. Control. Release 2005; 108: 10–20
- Longenecker J. P., Moses A. C., Flier J. S., Silver R. D., Carey M. C., Dubovi E. J. Effects of sodium taurodihydrofusidate on nasal absorption of insulin in sheep. J. Pharm. Sci. 1987; 76: 351–355
- The Merck Index, 24th ed. Merck Research Laboratories Division of Merck and Co., Inc., Whitehouse Station, NJUSA 1996, Ed: Susan Budavari, USA
- Mitra R., Pezron I., Chu W. A., Mitra A. K. Lipid emulsions as vehicles for enhanced nasal delivery of insulin. Int. J. Pharm. 2000; 205: 127–134
- Sinswat P., Tengamnuay P. Enhancing effect of chitosan on nasal absorption of salmon calcitonin in rats: comparison with hydroxypropyl- and dimethyl-β -cyclodextrins. Int. J. Pharm. 2003; 257: 15–22
- Tas C., Ozkan C. K., Savaser A., Ozkan Y., Tasdemir U., Altunay H. Nasal absorption of metoclopramide from different Carbopop 981 Based Formulations: In vitro, ex vivo and in vivo evaluation. Eur. J. Pharm. Biopharm. 2006; 64: 246–254
- Tirucherai G. S., Pezron I., Mitra A. K. Novel approaches to nasal delivery of peptides and proteins. STP Pharm. Sci. 2002; 12: 3–12
- Todo H., Okamoto H., Iida K., Danjo K. Improvement of stability and absorbability of dry insulin powder for inhalation by powder-combination technique. Int. J. Pharm. 2004; 271: 41–52
- Uzunkaya G., Bergiadi N. In vitro drug liberation and kinetics of sustained release indomethacin suppository. Il Farmaco 2003; 58: 509–512
- Vermehren C., Hansen H. S. Shape changes in the erythrocyte membrane induced by the absorption enhancer didecanoylphosphatidylcholine. Int. J. Pharm. 1998; 174: 1–8
- Wang J., Lu W.-L., Liang G-W., Wu K-C., Zhang C-G., Zhang X., Wang J-C., Zhang H., Wang X-O., Zhang O. Pharmacokinetics, toxicity of nasal cilia and immunomodulating effects in Sprague–Dawley rats following intranasal delivery of thymopentin with or without absorption enhancers. Peptides 2006; 27: 826–835
- Welin-Berner K., Neelissen J. A. M., Bergenstahl B. The effect of reological behaviour of a topical anasthetic formulation on the release and permeation rates of the active compound. Eur. J. Pharm. Sci. 2001; 13: 309–318
- Wingertzahn M. A., Sato H., Nave R., Nonaka T., Mochizuki T., Takahama S., Kondo S. Comparison of nasal tissue concentrations in rabbits following administration of hypotonic and isotonic ciclesonide suspensions. J.Allergy. Clin. Immunol. 2005; 115: 126
- Yetkin G., Celebi N., Ozogul C., Demiryurek A. T. Enhancement of nasal absorption of salmon calcitonin in rabbits using absorption enhancer. STP Pharm. Sci. 2001; 11: 187–191
- Yu S., Zhao Y., Wu F., Zhang X., Lü W., Zhang H., Zhang O. Nasal insulin delivery in the chitosan solution: in vitro and in vivo studies. Int. J. Pharm. 2004; 281: 11–23