Abstract
Biomedical applications of antioxidants have increased dramatically since the link between human diseases and oxidative stress was established. This paper focuses on α -tocopherol and on the possibility of employing molecularly imprinted polymers as a controlled release device for α -tocopherol in gastrointestinal simulating fluids. Polymers were synthesized using methacrylic acid as functional monomer and ethylene glycol dimethacrylate as cross-linker. Considerable differences in recognition characteristics between imprinted and non-imprinted polymers, both in organic and in aqueous media, were observed. Imprinted polymers bound much more α -tocopherol and showed a controlled/sustained drug release capacity in gastrointestinal simulating fluids.
INTRODUCTION
In the last decade, many epidemiological, biological and clinical studies have proven that free-radical-induced oxidative damage to cell membranes, DNA, and proteins might play a causative role in several degenerative diseases, such as cancer, atherosclerosis, and other chronic diseases (CitationBarnham et al. 20004; Cooke et al. Citation2003; Finkel et al. Citation2000; Fernandez-Robredo et al. Citation2005; Tian et al. Citation2005). Reactive oxygen and nitrogen species (ROS/RNS) are essential to energy supply, detoxification, chemical signalling, and immune function (Young et al. Citation2001). These species are continuously produced in the human body, and their concentrations are controlled by endogenous enzymes (superoxide dismutase, glutathione peroxidase, and catalase). When there is an over-production of ROS and RNS, an exposure to external oxidant substances, or a failure in the defence mechanisms, biomolecular damage may occur (Iemma et al. Citation2007). Some compounds, such as0 α -tocopherol (Kalogeropouloset al. 2007), L-ascorbic acid (Jayathilakan et al. Citation2007), and β -carotene (Bairati et al. Citation2006), generally named antioxidants, might have beneficial effects in protecting human tissue against such damage.
Our interest focussed on α -tocopherol (α -TP), a representative oil-soluble antioxidant of the vitamin E type (Qian et al. Citation2005). The physiological relevance of α -TP, and the severe pathological consequences of its deficiency, impose a major challenge to the living organisms for sustaining an adequate supply of this compound to different tissues, particularly those highly sensitive to α -TP deficiency such as the brain and gonads (Mardones et al. Citation2004). α -TP deficiency could be overcome by dietary supplementation—several studies have described the beneficial effect of oral supplementation with vitamin E on the prevention and treatment of cardiovascular diseases and cancer (Morris et al. Citation2005).
The goal of this work, preliminarily presented at a 2006 conference on molecularly imprinted polymers (MIPs) (Cirillo et al., Citation2006), is the preparation of a new controlled drug delivery device for α -TP oral supplementation.
A controlled drug delivery system would make it possible to maximize drug efficacy and safety and to provide a suitable rate of delivery of the therapeutic dose, at the most appropriate site in the body. This would prolong the duration of the drug's pharmacological activity, to reduce side-effects, and minimize administration frequency, thus enhancing patient compliance (Cunliffe et al. Citation2005; Alvarez-Lorenzo et al. Citation2004).
Our approach for the realization of α -TP delivery system uses MIPs. Molecular imprinting is a technique that produces synthetic materials containing highly specific receptor regions with an affinity for a target molecule (Ye et al. Citation2001). MIPs can mimic the recognition and binding properties of natural biomolecules such as antibodies and enzymes (Mayes et al. Citation2005).
The simplicity of creating tailored recognition sites in synthetic materials by molecular imprinting, as compared with that of complicated multi-step organic synthesis (Vlatakis et al. Citation1993), is very attractive from an application viewpoint, and when compared with biomolecules, the main advantages of MIPs are their relatively high stability over a wide range of conditions (temperature, pressure, organic solvents, acidic or basic solutes, etc) and their low cost.
MIPs were used for several different applications, such as chromatographic stationary phases (Hishiya et al. Citation2003), enantiomeric separation (Adbo et al. Citation2001), solid-phase extraction (SPE) (Puoci et al. Citation2008), and catalysis (Anderson et al. Citation2005); MIPs were also used as receptors (Haupt et al. Citation2003), antibodies (Svitel et al. Citation2001), enzyme mimics (Nicholls et al. Citation1996), and affinity and sensing materials (Syu et al. Citation2006). In addition, in recent years, MIPs have been reported to be suitable as drug delivery systems (DDS) (Puoci et al. Citation2007a), and as base excipients for controlled release devices of several drugs (Spivak Citation2005).
To produce MIPs, we used the non-covalent approach (Caro et al. 2006), which involves the preorganization of functional monomers around a template molecule to form a pre-polymerization complex by hydrogen bonding, ionic, or hydrophobic interactions. The formed complex is subsequently radically copolymerized in a solution containing a high ratio of a suitable cross-linker to form, after the subsequent removal of the template, macroporous matrices having microcavities that retain specific orientation of functional groups in a three-dimensional structure complementary to that of the template about which they were formed (Caro et al. 2006).
Based on these considerations, we describe the preparation of molecularly imprinted polymers able to rebind selectively and to release α -TP in gastrointestinal simulating fluids. These MIPs was synthesized using methacrylic acid (MAA) as functional monomers and ethylene glycol dimethacrylate (EGDMA) as a cross-linking agent (Puoci et al. Citation2007b). The recognition properties of the synthesized materials were tested both in organic (i.e., acetonitrile) and in aqueous media (i.e., an ethanol/water 6/4 v/v mixture). Considerable differences in the recognition characteristics between imprinted and non-imprinted polymers have been observed. The selectivity of the polymeric device was also evaluated using a molecule structurally similar to the template, in particular 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (HMCA) was employed (). Finally the release profile of α -TP in gastrointestinal simulating fluids was tested.
MATERIALS AND METHODS
Materials
Ethylene glycol dimethacrylate (EGDMA), methacrylic acid (MAA), 2,2′-azoisobutyronitrile (AIBN), α -tocopherol (α -TP), and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (HMCA) were obtained from Sigma Chemical Co. (St. Louis, MO). All solvents were reagent grade or HPLC-grade, were used without further purification, and were provided by Fluka Chemie (Buchs, Switzerland).
HPLC Analysis
The liquid chromatography consisted of an Jasco BIP-I pump and Jasco UVDEC-100-V detector set at 292 nm. A 250 × 4 mm C-18 Hibar® column, particle size 10 mm (Merck, Darmstadt, Germany) was used. The mobile phase was methanol and the flow rate was 1.0 ml/min.
Synthesis of α -Tocopherol Imprinted Polymers
α−TP imprinted polymers (MIPs) were prepared using MAA as functional monomers. Briefly, 0.430 g (1 mmol) of template α -tocopherol, 1.38 g (16 mmol) of MAA were dissolved in 5.25 ml of chloroform in a thick-walled glass tube, and then 4.95 g (25 mmol) of EGDMA and 0.100 g (0.60 mmol) of AIBN were added. The tube was sparged with nitrogen, sonicated for 10 min, and then photo-polymerized for 24 h with 360 nm light at 4°C. After the photolysis, the tubes were incubated at 60°C for 24 h. The resultant bulk rigid polymers were crushed, ground into powder, and strained through a 63 nm stainless steel sieve. The sieved MIP materials were collected, and the very fine powder, suspended in the supernatant solution (acetone), was discarded. The resultant MIP materials were placed in a Soxhlet extracted with 200 ml of a tetrahydrofuran:acetic acid (8:2 v/v) mixture for at least 48 h, followed by 200 ml of tetrahydrofuran for others 48 h. The extracted MIP materials were dried in an oven at 60°C overnight. The washed MIP materials were checked to be free of α -tocopherol and any other compound by high-performance liquid chromatography (HPLC). Blank polymers (to act as a control) were also prepared when polymerization was carried out in the absence of α -tocopherol.
Binding Experiments
The binding efficiency of polymeric matrices toward α -TP was evaluated in the rebinding experiments, which were performed both in acetonitrile and in an ethanol:water mixture (6:4 v/v). Briefly, 50 mg of polymer particles were mixed with 1 ml α -TP solution (0.2 mM) in a 1 ml eppendorf and sealed. The eppendorf were oscillated by a wrist action shaker (Burrell Scientific) in a water bath at 37 ± 0.5°C for 24 h. Then the mixture was centrifuged for 10 min (10000 rpm) in an ALC®microcentrifugette® 4214 and the α -TP concentration in the liquid phase was measured by HPLC. The amount of α -TP bound to the polymer was obtained by comparing its concentration in the MIPs samples to the NIPs samples.
The same experiments were performed using HMCA solutions. Experiments were repeated five times, and results were expressed as means (± SEM)
Drug Loading by the Soaking Procedure
Two grams of polymeric matrix were immersed in 20 ml of a α -TP solution (5.5 mM) in acetonitrile and soaked for 3 days at room temperature. During this time, the mixture was continuously stirred, and then the solvent was removed. Finally, the powder was dried under vacuum overnight at 40°C.
In Vitro Release Studies
Release studies were done using the dissolution method described in the USP XXIV (apparatus 1-basket stirring element). To mimic the pH in the digestive tract simulated, 0.1 N HCl (pH 1.0) was used as a stimulated gastric fluid, and after 2 h, disodium hydrogen phosphate (0.4 M) was added to adjust the pH value to 6.8 to simulate a intestinal fluid. To improve the solubility of released α -TP in the simulated fluid, each testing sample contained 0.1% of sodium dodecylsulfate (SDS).
The experiments were performed as follows: 30 mg of MIP and NIP particles loaded with α -TP were dispersed in flasks containing 10 ml of 0.1 N HCl and maintained at 37 ± 0.5°C in a water bath for 2 h under magnetic stirring (50 rpm). Disodium hydrogen phosphate (0.4 M, 5 ml) was then added to the samples. These conditions were maintained throughout the experiment. To characterize the drug release, 2 ml of samples were drawn from the dissolution medium at designated time intervals, and the same volume of simulated fluid was supplemented. α -TP was determined by HPLC analysis, and the amount of α -TP released from five samples of each formulation was used to characterize drug release. The percentage of α -TP released was calculated considering 100% the α -TP content in polymeric samples after drying procedure (Pitarresi et al. Citation2004). Experiments were repeated five times results and were expressed as means (± SEM)
RESULTS AND DISCUSSION
Synthesis of α -Tocopherol Imprinted Polymers
For the preparation of molecularly imprinted polymers, generally three different approaches have been developed to date: the covalent, the semi-covalent, and the non-covalent approach.
The first, pioneered by Wulff's group (Wulff Citation2002), involves the formation of complex between functional monomers and template molecules via reversible covalent bond (such as boronate ester, ketal and acetal, or Schiff base) both prior to polymerization and in the rebinding experiments.
In the semi-covalent approach, covalent interactions are involved in MIP synthesis, while the subsequent rebinding phase is based on non-covalent interactions.
The non-covalent approach, pioneered and extensively developed by Mosbach and coworkers (Zhang et al. Citation2006), instead, involves only non-covalent interactions (such as hydrogen bonds, ionic interactions, hydrophobic interactions, and metal-ion chelating interactions) for both the molecular imprinting process and the subsequent rebinding. This approach is the most flexible because of the absence of complicated synthetic processes and the subsequent possibility of using a far greater variety of functional monomers.
For these reasons, we chose the non-covalent imprinting method for the preparation of bulk imprinted polymers for the sustained/controlled release of α -tocopherol. MAA was used as functional monomers and EGDMA as cross-linker.
The whole of the reaction conditions have to maximize the interactions between the template and the functional monomer and consequently to ensure strong and selective binding of the substrate to the polymeric matrices. Two main parameters must be considered. The first is the polymerization temperature. The formation of the complex is a dynamic process and, when a template with few functional groups able to create hydrogen bond is used, a low temperature is needed to reduce the kinetic energy of the system. In this case, indeed, a high temperature could drive the equilibrium away from the template-functional monomer complex toward the unassociated species, resulting in a decrease in the number of imprinted cavities and thus in the recognition properties of the final materials. Better results were obtained using photopolymerization processes. Furthermore, in these conditions, a lesser degree of polymerization was observed, so the performance of photopolymerized materials must be improved after high-temperature treatment of the initially formed polymer (Cheong et al. 1997). Based on these consideration, MIPs were synthesized under UV irradiation at 4°C for 24 h and then with thermal stabilization at 60°C for another 24 h.
The second parameter to be considered is the nature of the porogenic agent. The inert solvent used in the polymerization mixture may play a major role in determining the properties (surface area, internal pore volume, etc.) of the resulting polymer. Moreover, since polar solvents are more able to solvate polar molecules, this leads to the disruption of H-bonds between the template and the functional monomer (Whitcombe et al. Citation1995; Cheong et al. 1997). Thus, our general procedure employed to improve the recognition properties of molecularly imprinted polymers was the choice of the least polar solvent in which the reagents dissolves. In our case, we chose chloroform.
Recognition Properties of Polymers
Evaluation of the capacity of the matrices to recognize and bind the template was performed in acetonitrile (organic medium), and also in ethanol/water mixture (6/4 v/v).
Polymeric particles were incubated with an α -TP solutions 0.2 mM for 24 hours. shows the percentage of α -TP bound by imprinted and non-imprinted polymers.
It should be noted that the synthesized materials have good recognition properties. The imprinted polymers, indeed, bind much more template than do their respective non-imprinted counterparts, confirming the presence of α -TP specific cavities. The higher amounts of bound α -TP in aqueous medium, compared with that in acetonitrile, are ascribable to the presence of more hydrophobically driven bonds, the extent of which depends on the hydrophobicity of the template and the surface of the material. But also in this case, MIPs samples bind much more α -TP than NIPs ones.
Well imprinted polymers are asked to interact selectively with the template around which they are formed, compared with some structurally analogous molecules. The selectivity of the imprinted polymers was tested by performing the same binding experiments using 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (HMCA) instead of α -TP (). The chemical difference between the two analytes drives the interactions with the polymeric matrices. The carboxylic group of HMCA makes this molecule much more hydrophilic than α -TP.
TABLE 1 Percentage of bound α -TP and HMCA by MIP and NIP in organic (CH3CN) and aqueous (ETOH/H2O 6/4 v/v mixture) media
It should be noted that the polymers practically do not interact with HMCA in organic media, whereas in water solution the binding percentage of the analog is higher but considerably lower than that of the original template. Furthermore, in all the environments tested, the amounts of HMCA bound by the imprinted and the non-imprinted polymers are practically the same, and this clearly shows the non specific nature of these interactions.
In Vitro α -TP Releasing Properties
The possibility of employing the synthesized MIPs as devices for the controlled release of α-TP in gastrointestinal simulated fluids was investigated. In vitro release studies were performed by immersing aliquots of the microparticles loaded with α -TP at pH 1.0 (simulated gastric fluid) for 2 hours and then at pH 6.8 (simulated intestinal fluid) using the pH change method. To improve the solubility of α -TP in aqueous media, SDS was added to the solutions.
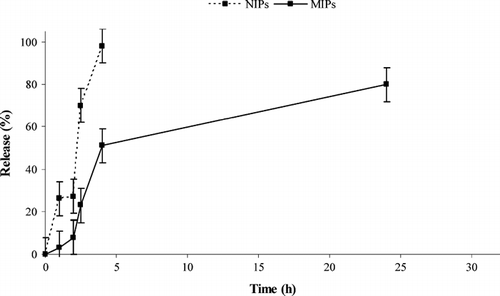
Our hypothesis was that α -TP imprinted polymers have a better ability to control drug (α -TP) release in compared with non-imprinted polymers due to the presence of specific binding sites in the polymeric network that are able to release the drug much more slowly. The experimental data confirm this hypothesis; the drug release from NIPs was indeed remarkably faster than that observed from MIPs. In particular, while in the first case the drug is completely released within 4 hours, only 50% of α -TP was released from MIP samples during the same period ().
After pH changing, in intestinal simulating fluids, α -TP release from MIPs continues, and in 24 hours the percentage of α -TP released was about 80% (100% within 40 h). These remarkable differences depend on the different recognition properties of the two polymeric matrices. The non-imprinted polymers, indeed, do not have specific binding cavities for the drug, while the MIP samples, because of their specific structure, strongly bound α -TP by non-covalent interactions in the cavities formed during the polymerization procedure in the presence of the analyte.
This observation supports a model of retention mechanism, which assumes that the acid groups of the selective sites have stronger interaction with the drug than the non-selective sites. At low pH (1.0) values, the carboxylic groups are not ionized and there is a good interaction with the template. These results might help us to understand the behavior of these matrices when the pH increases. Under these conditions, that simulate the intestinal fluid, in the non-imprinted polymers the antioxidant is bound with non-covalent interactions on the surface of the matrices. At pH 6.8, the diffusion rate of the buffer on the polymer surface is fast, the carboxylic groups are ionized, and the drug is rapidly released. Instead, in the MIP case, the diffusion rate of the buffer into specific cavities of imprinted polymers is slower, and the functional groups are ionized more slowly, resulted in well controlled release.
For these reason, the rate of the release was considerably different, and MIPs represent a very useful polymeric device for the selective and controlled release on the antioxidant agent in gastrointestinal fluids.
CONCLUSIONS
The aim of this work was the preparation of a specific oral delivery system for α -TP. To this end, we used molecular imprinting. The polymerization conditions involves the use of MAA and EGDMA as functional monomers and a cross-linking agent, respectively. To improve the recognition properties of the synthesized materials, a photopolymerization procedure was employed. Before evaluating of the α -TP release profile, the recognition and selectivity properties of the polymeric devices, both in organic and in aqueous media, were studied when performing rebinding experiments. The percentage of α -TP bounded by the molecularly imprinted polymers was remarkably higher in both the environments tested compared with the non-imprinted ones, and MIPs also showed a high selectivity.
In vitro release tests were performed in gastrointestinal simulating fluids. MIPs samples was found to be able to release slowly the drug compared with NIPs, thus the synthesized polymeric matrices represent a very useful novel controlled drug dosage device.
ACKNOWLEDGMENT
This work was financially supported by MIUR (Programma di ricerca di rilevante interesse nazionale 2005) and the University funds.
REFERENCES
- Adbo K., Nicholls I. A. Enantioselective solid-phase extraction using Troger's base molecularly imprinted polymers. Anal.Chim. Acta. 2001; 435: 115–120
- Alvarez-Lorenzo C., Concheiro A. Molecularly imprinted polymers for drug delivery. J. Chromatogr. B 2004; 804: 231–245
- Anderson C. D., Shea K. J., Rychnovsky S. D. Strategies for the generation of molecularly imprinted polymeric nitroxide catalysts. Org. Lett. 2005; 7: 4879–4882
- Bairati I., Meyer F., Jobin E., Gelinas M., Fortin A., Nabid A., Brochet F., Tetù B. Antioxidant vitamins supplementation and mortality: A randomized trial in head and neck cancer patients. Int. J. Cancer 2006; 119: 2221–2224
- Barnham K. J., Masters C. L., Bush A. I. Neurodegenerative diseases and oxidatives stress. Nature Rev. Drug Discov. 2004; 3: 205–214
- Caro E., Marce R. M., Cormack P. A. G., Sherrington D. C., Borrull F. Novel enrofloxacin imprinted polymer applied to the solid-phase extraction of fluorinated quinolones from urine and tissue samples. Anal. Chim. Acta. 2006a; 562: 145–151
- Caro E., Marce R. M., Cormack P. A. G., Sherrington D. C., Borrull F. Direct determination of ciprofloxacin by mass spectrometry after a two-step solid-phase extraction using a molecularly imprinted polymer. J. Sep. Sci. 2006b; 29: 1230–1236
- Cheong S. H., McNiven S., Rachkov A., Levi R., Yano K., Karube I. Optical detection of chloramphenicol using molecularly imprinted polymers. Anal. Chem. 1997a; 69: 2017–2021
- Cheong S. H., McNiven S., Rachkov A., Levi R., Yano K., Karube I. Testosterone receptor binding mimic constructed using molecular imprinting. Macromolecules. 1997b; 30: 1317–1322
- Cirillo G., Curcio M., Puoci F., Spizzirri U. G., Iemma F., Picci N. Molecularly imprinted polymers for α -tocopherol oral delivery. 4th International Workshop on Molecular Imprinting, Cardiff (UK), September, 10th—14th, 2006
- Cooke M. S., Evans M. D., Dizdaroglu M., Lunec J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003; 17: 1195–1214
- Cunliffe D., Kirby A., Alexander C. Molecularly imprinted drug delivery systems. Adv. Drug Del. Rev. 2005; 57: 1836–1853
- Fernandez-Robredo P., Moya D., Rodriguez J. A., Garcia-Layana A. Vitamins C and E reduce retinal oxidative stress and nitric oxide metabolites and prevent ultrastructural alterations in porcine hypercholesterolemia. IOVS. 2005; 46: 1140–1146
- Finkel T., Holbrook N. J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000; 408: 239–247
- Haupt K. Imprinted polymers—tailor-made mimics of antibodies and receptors. Chem. Commun. 2003; 2: 171–177
- Hishiya T., Asanuma H., Komiyama M. Molecularly imprinted cyclodextrin polymers as stationary phases of high performance liquid chromatography. Polym. J. 2003; 35: 440–445
- Iemma F., Cirillo G., Puoci F., Trombino S., Castiglione M., Picci N. Iron (III) chelation and antioxidant properties of myo-inositol phosphorilated polymeric microspheres. J. Pharm. Pharmacol. 2007; 59: 597–601
- Jayathilakan K., Sharma G. K., Radhakrishna K., Bawa A. S. Effect of natural antioxidants on the lipid stability of fluidised bed-dried mutton. Food. Chem. 2007; 100: 662–668
- Kalogeropoulos N., Chiou A., Mylona A., Ioannou M. S., Andrikopoulos N. K. Recovery and distribution of natural antioxidants (α -tocopherol, polyphenols and terpenic acids) after pan-frying of Mediterranean finfish in virgin olive oil. Food Chem. 2007; 100: 509–517
- Mardones P., Rigotti A. Cellular mechanisms of vitamin E uptake: relevance in α -tocopherol metabolism and potential implications for disease. J. Nutr. Biochem. 2004; 15: 252–26
- Mayes A. G., Whitcombe M. J. Synthetic strategies for the generation of molecularly imprinted organic polymers. Adv. Drug. Del. Rev. 2005; 57: 1742–1778
- Morris M. C., Evans D. A., Tangney C. C., Bieinias J. L., Wilson R. S., Aggarwal N. T., Scherr P. A. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am. J. Clin. Nutr. 2005; 81: 508–514
- Nicholls I. A., Matsui J., Krook M., Mosbach K. Some recent developments in the preparation of novel recognition systems: A recognition site for the selective catalysis of an aldol condensation using molecular imprinting and specific affinity motifs for α -chymotrypsin using a phage display peptide library. J. Mol. Recognit. 1996; 9: 652–657
- Pitarresi G., Pierro P., Giammona G., Iemma F., Muzzalupo R., Picci N. Drug release from α,β -poly(N-2-hydroxyethyl)-dl-aspartamide-based microparticles. Biomaterials 2004; 25: 4333–4343
- Puoci F., Curcio M., Cirillo G., Iemma F., Spizzirri U. G., Picci N. Molecularly imprinted solid-phase extraction for cholesterol determination in cheese products. Food Chemistry 2008; 106: 836–842
- Puoci F., Iemma F., Cirillo G., Picci N., Matricardi P., Alhaique F. Molecularly imprinted polymers for 5-fluorouracil release in biological fluids. Molecules 2007a; 12: 805–814
- Puoci F., Cirillo G., Curcio M., Iemma F., Spizzirri U. G., Picci N. Molecularly imprinted solid phase extraction for the selective HPLC determination of α -tocopherol in bay leaves. Anal. Chim. Acta. 2007b; 593: 164–170
- Qian J., Morley S., Wilson K., Nava P., Atkinson J., Manor D. Intracellular trafficking of vitamin E in hepatocytes: The role of tocopherol transfer protein. J. Lipid Res. 2005; 46: 2072–2082
- Spivak D. A. Optimization, evaluation and characterization of molecularly imprinted polymers. Adv. Drug Del. Rev. 2005; 57: 1779–1794
- Svitel J., Surugiu I., Dzgoev A., Ramanathan K., Danielsson B. Functionalized surfaces for optical biosensors: Applications to in vitro pesticide residual analysis. J. Mater. Sci.- Mater. M. 2001; 12: 1075–1078
- Syu M. J., Chiu T. C., Lai C. Y., Chang Y. S. Amperometric detection of bilirubin from a micro-sensing electrode with a synthetic bilirubin imprinted poly(MAA-co-EGDMA) film. Biosens. Bioeletron. 2006; 22: 550–557
- Tian N., Thrasher K. D., Gundy P. D., Hughson M. D., Manning R. D., Jr. Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension. 2005; 45: 934–939
- Vlatakis G., Andersson L. I., Muller R., Mosbach K. Drug assay using antibody mimics made by molecular imprinting. Nature. 1993; 361: 645–647
- Whitcombe M. J., Rodriguez M. E., Villar P., Vulfson E. N. A new method for the introduction of recognition site functionality into polymers prepared by molecular imprinting: Synthesis and characterization of polymeric receptors for cholesterol. J. Am. Chem. Soc. 1995; 117: 7105–7111
- Wulff G. Enzyme-like catalysis by molecularly imprinted polymers. Chem. Rev. 2002; 102: 1–27
- Ye L., Yu Y., Mosbach K. Towards the development of molecularly imprinted artificial receptors for the screening of estrogenic chemicals. Analyst 2001; 126: 760–765
- Young I. S., Woodside J. V. Antioxidants in health and disease. J. Clin. Pathol. 2001; 53: 176–186
- Zhang H., Ye L., Mosbach K. Non-covalent molecular imprinting with emphasis on its application in separation and drug development. J. Mol. Recognit. 2006; 19: 248–259