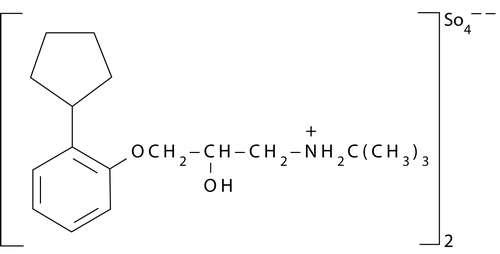Abstract
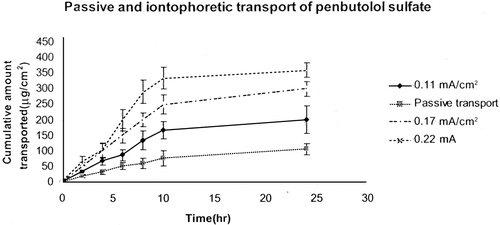
Iontophoretic transport of penbutolol sulfate across porcine ear skin was studied. Passive transdermal flux of the drug in phosphate-buffered saline was 7.65 μg/cm2 hr. There was statistically significant flux enhancement when direct current iontophoresis was applied. Iontophoresis (0.11 mA/cm2, 0.17 mA/cm2, and 0.22 mA/cm2) for 6 hr, resulted in net transport of 87.36 μg/cm2, 137.51 μg/cm2, and 201.12 μg/cm2 of penbutolol sulfate, respectively. After 24 hr, cumulative amount of penbutolol transported were 201.63, 300.76, and 359.98 μg/cm2, respectively. There was a 2.20- (0.11 mA/cm2), 3.26- (0.17 m/Acm2), and 4.28-fold (0.22 mA/cm2) enhancement in transcutaneous steady-state flux values compared to passive delivery. Steady-state fluxes of penbutolol sulfate also increased proportionally to current density. Steady-state fluxes calculated from the linear portion of the cumulative amount versus time curves for penbutolol sulfate were 16.68, 24.97, and 32.76 μg/cm2/hr at current densities of 0.11, 0.17, and 0.22 mA/cm2. This study provides initial evidence for the potential use of iontophoresis for enhanced transdermal delivery of penbutolol sulfate.
Introduction
Penbutolol [1-t-butylamino-3-(2-cyclopentylphenoxy) pro- pan-2-ol] () is a noncardioselective β-adrenoreceptor blocking agent and used for the treatment of hypertension (CitationSchlanz and Thomas 1990; Aqil, Sultana, and Ali Citation2006). Penbutolol is mainly metabolized to 4-hydroxypenbutolol in humans (Funato et al. Citation2006). The conventional dosage form for penbutolol is tablet, which has limitations including hepatic first-pass metabolism, high incidence of adverse effects due to variable absorption profile, and poor patient compliance.
Transdermal delivery offers an alternative mode of drug administration (CitationPrasad et al. 2007). A stable plasma drug concentration can be maintained over a prolonged period (CitationLi et al. 2003). However, few drugs can penetrate the excellent barrier provided by the stratum corneum. Over the past few decades, iontophoresis has attracted considerable interest as a method of enhancing transdermal drug delivery. Iontophoresis involves the use of a low-level electric current to drive ionized drug molecules into or through the skin (Banga, Citation1998; CitationKalia et al. 2004; Dixit et al. Citation2007). Typically, the current may be about 0.2 mA/sq cm and is imperceptible at this level. By using an electrode having the same charge as that of the drug, it is possible to increase drug flux through electrorepulsion. Iontophoresis is useful for polar and charged compounds (Patel et al. Citation2007). However, neutral drugs may also be delivered through electroosmosis, which is the convective flow of solvent on application of electric field (Femenia-Font et al. Citation2006). The major advantage of iontophoresis is that trancutaneous drug permeation can be controlled by varying the externally applied current (Meidan et al. Citation2003).
The aim of the present research was to investigate transdermal iontophoretic delivery of penbutolol sulfate across porcine ear skin in vitro.
Materials and methods
Chemicals and materials
Penbutolol sulfate was kindly donated by Schwarz Pharma (Mequon, WI 53092, USA). The high performance liquid chromatography (HPLC) grade acetonitrile and water were purchased from Sigma (St Louis, MO). Silver wire (99.99%, 0.5 mm diameter) and phosphate-buffered saline were purchased from Sigma-Aldrich and silver/silver chloride electrodes from in vivo Metrics (Healdsburg, CA). Sunfire (C18 5 μm 4.6 × 100 mm) reversed-phase column was obtained from Waters (Milford, MA). Trifluoroacetic acid was purchased from Sigma-Aldrich (St Louis, MO).
High-performance chromatography
A high-performance liquid chromatographic technique was developed for the assay of penbutolol sulfate using Waters HPLC system equipped with ultraviolet (Waters 2487 dual absorbance) and fluorescence (Waters 2475 multiwavelength) detectors, autosampler (Waters 717), binary pump (Waters 1525), and a reversed-phase (Sunfire, Waters C18 5 μm 4.6 × 100 mm) column. Penbutolol was assayed at 271 nm. The mobile phase comprising 30% acetonitrile and 70% water with 0.025% trifluoroacetic acid was delivered at a flow rate of 1 ml/ml. The injection volume was 10 μl and the limit of detection was 10 ng/ml.
Skin preparation
The experiments were carried out under the approval of the Institutional Animal Care and Use Committee at Touro University, Vallejo, California. Porcine ear skin was obtained from a local slaughterhouse. After cleaning under water, the whole skin was removed from the outer region of the ear and separated from the underlying cartilage with a scalpel. Skin pieces were then maintained at −20°C for no longer than 2 months before use (CitationSchuetz et al. 2005).
In vitro Iontophoretic studies
In vitro permeation of penbutolol sulfate by ionto phoresis was conducted using vertical Franz diffusion cells (Permegear, Bethlehem, PA) and pig ear skin as a membrane. The receptor chamber was filled with phosphate-buffered saline and temperature was maintained at 37°C. Skin was mounted between the donor and receptor chambers. Donor chamber area was 1.77 cm2 whereas receptor compartment volume was 15 ml. The donor chamber was filled with 1 ml of 17 mg/ml penbutolol sulfate in phosphate-buffered saline.
For iontophoresis, Ag (Sigma-Aldrich Mo,USA) and Ag/AgCl (in vivo Metrics, CA) electrodes were used as anode and cathode, respectively. The Ag electrode was placed in the donor chamber such that the electrode was immersed in the solution approximately 5 mm from the skin surface (Chaturvedula et al. Citation2005). Anodal iontophoresis was used because penbutolol is positively charged. The AgCl electrode was placed in the sampling port of the receptor chamber. A constant current power source (Phoresor II Model PM 850, Iomed, Salt lake City, UT, USA) was connected to the electrodes and current (0.1, 0.2, or 0.3 mA) applied. Samples were collected at 2, 4, 6, 8, 10, and 24 hr, and analyzed by HPLC. Control experiments were also carried out by placing drug solutions into donor compartments without applying iontophoresis.
Permeation data were plotted as the cumulative amount of the drug collected in the receiver compartment as a function of time. Penbutolol fluxes were calculated from the slope of the linear portion of the cumulative amount of the drug transported versus time using linear regression analysis.
Effect of current density was evaluated using a one-way analysis of variance with significance levels set at p < .05.
Results
The present investigation was carried out to assess transdermal permeation of penbutolol sulfate across porcine ear skin. An HPLC method was developed for penbutolol assay. Transdermal flux of penbutolol sulfate across porcine ear skin was studied using vertical Franz diffusion cells.
Passive and iontophoretic transport of penbutolol sulfate across porcine ear skin are shown in . Iontophoresis (0.11 mA/cm2, 0.17 mA/cm2, and 0.22 mA/cm2) for 6 hr resulted in a net transport of 87.36 μg/cm2, 150.51 μg/cm2, and 201.13 μg/cm2, respectively. Iontophoresis significantly (p < .05) increased the permeability of penbutolol sulfate across pig ear skin in comparison with passive delivery. There was a 2.20- (0.11 mA/cm2), 3.26- (0.17 mA/cm2), and 4.28-fold (0.22 mA/cm2) enhancement in transcutaneous flux values.
Discussion
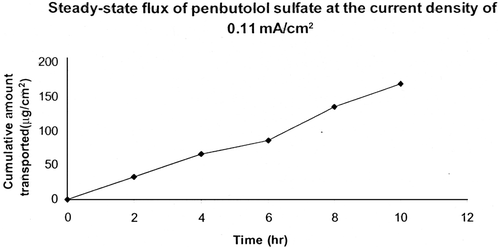
The present study demonstrated that whereas passive penetration of penbutolol sulfte across porcine ear skin was negligible, iontophoresis significantly increased transdermal delivery. At the current density of 0.11 mA/cm2, penbutolol flux was 16.68 μg/cm2 whereas at 0.17 mA and 0.22 mA, the corresponding flux values were 24.97 μg/cm2 and 32.76 μg/cm2, respectively. Iontophoretic flux was calculated by linear regression of the permeation plot from 2 to 6 hr. Postiontophoretic flux was also calculated from the slope of the linear portion of the plot from 6 to 24 hr. Enhanced transdermal delivery of the drug did not stop after discontinuation of current. However, postiontophoretic flux was lower than iontophoretic flux. It is possible that a penbutolol depot was formed in the skin (CitationHirsch, Upasani, and Banga 2005).
Iontophoretic flux of a charged species, X, at steady state can be considered as the sum of two separate transport mechanisms, electromigration JEMX and electroosmosis JEOX, assuming that passive permeability is negligible (CitationKalia et al. 2004):
Electromigration refers to the ordered movement of ions in the presence of an applied electric field. Electromigratory flux of an ion, X, is related to current flow, iX, by Faraday’s constant, F,
where A represents the cross-sectional area for transport across the skin and zx is the charge.
Ionic current flow is related applied current, I, by a proportionality constant, tx, the transport number of X (0 < tx < 1), which describes the fraction of the total current transferred by X (CitationKalia et al. 2004).
Electroosmosis can be defined either as a flow process or as a solvent velocity, V. Hence,
The existence of this second mechanism of transport means that cations may benefit from a second driving force in addition to electromigration (CitationKalia et al. 2004).
The inference from the foregoing equations is that iontophoretic flux may increase with increase in current density. In this study, anodal iontophoresis was used to deliver penbutolol sulfate. The results seem to suggest that electromigration is the major contributory mechanism toward iontophoretic transport of penbutolol sulfate. Transdermal delivery of penbutolol sulfate has not been previously investigated (Aqil, Sultana, and Ali Citation2006). However, this route of delivery has been evaluated for propranolol which is also an aryloxypropranomaline. Chesnoy et al. (1999) recently studied iontophoretic delivery of propranolol hydrochloride across human epidermal membrane. The authors reported a 1.6-fold increase in flux after direct current iontophoresis. In contrast, iontophoretic flux of the drug decreased after pretreatment with hexadecyl trimethylammonium bromide.
CitationKanikkannan et al. (2001) also studied the effect of iontophoresis on transdermal transport of timolol maleate across the skin of rat, rabbit, mouse, guinea pig, and human. Iontophoretic transport after 2 hr was highest across human skin (77.83 ± 3.74 μg/cm2). Steady-state flux was 10.14 ± 2.91 μg/cm2/hr. The corresponding passive transport value was 0.264 ± 0.0069. In that study, the current density was 0.375 mA/cm2.
In our study, the cumulative amount of penbutolol transported after 24 hr of iontophoresis was 201.63 (0.11 mA/cm2), 300.76 (0.17 mA/cm2), and 359.98 μg/cm2 (0.22 mA/cm2), respectively. There was a 2.20- (0.11 mA/cm2), 3.26- (0.17 m/Acm2), and 4.28-fold (0.22 mA/cm2) enhancement in transcutaneous steady-state flux values. Steady-state flux of penbutolol sulfate at the current density of 0.11 cm2 is shown in .
There was a statistically significant increase in transdermal steady-state flux enhancement of penbutolol sulfate after iontophoretic application compared to passive delivery (7.65 μg/cm2). The results presented here serve as initial evidence for the potential of iontophoresis to enhance transcutaneous delivery of penbutolol sulfate.
Acknowledgment
Declaration of interest: The authors report no conflicts of interest.
References
- Aquil M, Sultana Y, Ali A 2006. Transdermal delivery of beta-blockers. Expert. Opin. Drug. Deliv. 3, 1–14.
- Banga AK 1998. Electrically Assisted Transdermal and Topical Drug Delivery. London, UK, Taylor and Francis.
- Chaturvedula A, Joshi DP, Anderson C, Morris R, Sembrowich WL 2005. Banga Dermal AK subdermal and systemic concentrations of granisetron by iontophoretic delivery. Pharm. Res. 22, 1313–1319.
- Chesnoy S, Durand D, Doucet J, Couarraze G. 1999. Structural parameters involved in the permeation of propranolol HC1 by iontophoresis and enhancers. J. Contrd. Release. 58: 163–175.
- Dixit N, Bali V, Baboota S, Ahuja A, Ali J 2007. Iontophoresis—an approach for controlled drug delivery: a review. Curr. Drug. Deliv. 4, 1–10.
- Femenía-Font A, Balaguer-Fernāndez C, Merino V, LŌpez-Castellano A. (2006. Combination strategies for enhancing transdermal absorption of sumatriptan through skin in J. Pharm. 323:125–130.
- Funato K, Imai T, Nakashima K, Otagiri M 2006. High-performance liquid chromatography with chemiluminescence detection of penbutolol and its hydroxylated metabolite in rat plasma. J. Chromatogr. B. Biomed. Sci. Appl. 757, 229–235.
- Hirsch AC, Upasani RS, Banga AK 2005. Factorial design approach to evaluate interactions between electrically assisted enhancement and skin stripping for delivery of tacrime. J. Contrd. Release. 103, 113–121.
- Kalia YN, Naik A, Garrison J, Guy RH 2004. Iontophoretic drug delivery. Adv. Drug. Deliv. Rev. 56, 619–658.
- Kanikkannan N, Singh J, Ramarao P. 2001. In vitro transdermal iontophoretic transport of timolol maleate: effect of age and species . J. Contrld. Release. 71:99–105.
- Li GL, Grossklaus A, Danhof M, Bouwstra JA 2003. Iontophoretic R-apomorphine delivery in combination with surfactant pretreatment: in vitro validation studies. Int. J. Pharm. 266, 61–68.
- Meidan VM, Al-Khalili M, Michniak BB 2003. Enhanced iontophoretic delivery of buspirone hydrochloride across human skin using chemical enhancers. Int. J. Pharm. 264: 73–83.
- Patel SR, Zhong H, Sharma A, Kalia YN 2007. In vitro and in vivo evaluation of the transdermal iontophoretic delivery of sumatriptan succinate. Eur. J. Pharm. Biopharm. 66, 296–301.
- Prasad R, Koul V, Anand S, Khar RK 2007. Effect of DC/mDC iontophoresis and terpenes on transdermal permeation of methotrexate: in vitro study. Int. J. Pharm. 333, 70–78.
- Schlanz KD, Thomas RL 1990. Penbutolol: a new beta-adrenergic blocking agent. Ann. Pharmacother. 24, 403–408.
- Schuetz YB, Naik A, Guy RH, Vuaridel E, Kaua YN. 2005. Transdermal iontophoretic delivery of triptorelin . J. Pharm. Sci. 94: 2175–2182.