Abstract
Corticosteroids have been used for treatment of posterior segment eye diseases, but the delivery of drug to the posterior segments is still a problem to resolve. In our study, we explore the feasibility of Sub-tenon’s Controllable Continuous Drug Delivery to ocular posterior segment. Controllable continuous sub-tenon drug delivery (CCSDD) system, intravenous injections (IV) and sub-conjunctival injections (SC) were used to deliver dexamethasone disodium phosphate (DEXP) in rabbits, the dexamethasone concentration was measured in the ocular posterior segment tissue by Shimadzu LC-MS 2010 system at different time points in 24 h after first dose injection. Levels of dexamethasone were significantly higher at 12, 24 h in CCSDD than two other approaches, and at 3, 6 h in CCSDD than IV in vitreous body (p < 0.01); at 6, 12, 24 h in CCSDD than two other approaches, and at 1, 3 h in CCSDD than IV in retinal/choroidal compound (p < 0.01); at 3, 6, 12, 24 h in CCSDD than two other approaches, and at 1 h in CCSDD than IV in sclera (p < 0.05). The AUC0–24 in CCSDD group is higher than two other groups in all ocular posterior segment tissue. Our results demonstrated that dexamethasone concentration could be sustained moderately higher in the posterior segment by CCSDD than SC and IV, indicating that CCSDD might be a therapeutic alternative to treat a variety of intractable posterior segment diseases.
Introduction
Corticosteroids have been used for treatment of posterior segment eye diseases (Lowder et al., Citation2011; Chennamaneni et al., Citation2013; Calvo et al., Citation2015; Tsang et al., Citation2016) such as uveitis, diabetic retinopathy and macular degeneration which can lead to irreversible visual impairments (Hettinga et al., Citation2014; Borooah et al., Citation2015; Zatic et al., Citation2015). Inflammation of ocular posterior segment is often difficult to treat because of poor tissue permeability (Bourges et al., Citation2003; Bansal et al., Citation2016; Lajunen et al., Citation2016). Delivery of drug to the posterior segments is of great concern but also challenging due to the anatomical and physiological barriers of the eye and short drug duration.
Steroids used to treat posterior segment diseases are typically administered in four ways such as topical, systemic, intravitreal (IVT) and periocular routes (Kang-Mieler et al., Citation2014). The topical route is well tolerated (Hennessy et al., Citation2010; Quek et al., Citation2011), but inefficient in delivering therapeutic drug levels (Chen et al., Citation2012; Liu et al., Citation2016; Thakur et al., Citation2016), owing to rapid drainage through the nasolacrimal ducts, systemic absorption, low permeability of the corneal (Abdul et al., Citation2016) and conjunctival epithelium, and the blood–aqueous barrier. Systemic administrations of corticosteroid show low bioavailability (Thakur et al., Citation2016) and significant systemic side effects, it require large dosage for the effective therapeutic concentration in the eye (Andonova, Citation2016), nonspecific accumulation of drugs in other organs, the blood–retina barrier, and not well tolerated by all patients (Peptu et al., Citation2015). Intravitreal injections or implants can deliver drugs to the posterior segments effectively and it can reach the targeted tissue directly with minimized systemic side effects. But it is the most invasive, and can lead to numerous significant local complications such as retinal drug toxicity, retinal detachment, vitreous and retinal hemorrhage, floaters, endophthalmitis, lens injury, and elevated intraocular pressure, which will affect the vision of patients (Huang et al., Citation2016). Consequently, the ideal routes of drug delivery to the posterior segment are from the eye of periocular (Hsu, Citation2007), which is considered to be minimal painful and direct penetration pathway (Nagai et al., Citation2014; Huang et al., Citation2016). It included the retrobulbar, peribulbar, sub-tenon and subconjunctival (SC) routes. Both the SC and the sub-tenon routes are widely used in research of transscleral drug delivery as their neighboring layer is the sclera. With SC injection, the formulation is placed beneath the conjunctival membrane that covers the sclera. This enables the drugs to bypass the conjunctiva–cornea barrier, giving direct access to the transscleral route. Sub-tenon injection involves the placement of a formulation between the sclera and Tenon’s capsule, an avascular membrane. Therefore, the contact time between the administered drug and the sclera is prolonged. Consequently, the sub-tenon route is considered to be one of the most promising routes for targeting the posterior segment (Ghate et al., Citation2007). But it is also restricted for the short duration in most of the drugs currently used. Sub-tenon drug delivery system is a better choice for ophthalmologists, because it is not only for the greater bioavailability but also the higher processing safety. The sub-tenon’s route is regarded as the most effective one for it can lay the agents proximal to the sclera (Thrimawithana et al., Citation2011).
Researches showed that sub-tenon capsule sustained release implants can release drugs and keep certain vitreous, retinal, and choroidal drug concentration, but it is in a lower level due to the limited total drug dosage. The aim of our study was to evaluate the feasibility of controllable continuous sub-tenon drug delivery (CCSDD) to ocular posterior segment in rabbit. To investigate whether the CCSDD system can release drugs such as the dexamethasone disodium phosphate (DEXP) and keep a higher level of corticosteroid in the posterior segment in vivo, and evaluate its distribution in the ocular posterior tissues.
Methods and materials
Animals
All experiments were carried out in accordance with the statement of the Association for Research in Vision and Ophthalmology (ARVO) for the Use of Animals in Ophthalmic and Vision Research. The studies were approved by the Animal Ethics Committee of the Third Xiangya Hospital of Central South University. Male and female New Zealand albino rabbits (about four months old and weighing 2.0–2.5 kg) were acquired from the Third Xiangya Hospital of Central South University (Changsha, China). All rabbits were examined thoroughly, and no ocular disease, then were randomly allocated to different groups for subsequent ocular PK studies. Animals were anesthetized by intramuscularly injecting Xylazine Hydrochloride Injection (2 ml:0.2 g, Huamu Animal Health Products Co., Jilin, 0.1–0.2 ml/kg) and dropped Oxybuprocaine Hydrochloride Eye Drops (20 ml:80 mg, Sa) three times, interval 2 min per time.
Drug administration and sample collection
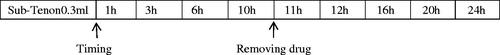
Rabbits were randomly divided into three groups and six rabbits for each group per time point. Intravenous injection (IV, 1 mg/kg dexamethasone), SC injection (5 mg/ml dexamethasone, 0.3 ml) and CCSDD system (CCSDD 0.3 ml initial doses, 5 mg/ml dexamethasone, 0.1 ml/h sustained pump) were performed. After the last instillation in IV group (1 h, 3 h, 6 h, 12 h, 24 h) and SC injection group (1 h, 2 h, 3 h, 4 h, 6 h, 8 h, 12 h, 24 h), respectively, the ocular tissue were collected. In the CCSDD group, after placing embedded tubes in sub-tenon (), 0.3 ml 5 mg/ml dexamethasone trickled into it and starting from that time, the pump drugs at the rate of 0.1 ml/h to the scleral surface for 10 h (), and collects plasma and ocular tissue in 1 h, 3 h, 6 h, 10 h, 11 h, 12 h, 16 h, 20 h, 24 h. Animals were euthanized by 3 ml lidocaine (3 ml/rabbit) and 3 ml air intravenously, immediately after death, ocular tissues were collected. Vitreous, choroid/retina and sclera were dissected and weighted as the solid tissues, the samples were stored at −20 °C and then processed for analysis.
Drug assay
We determined the levels of dexamethasone in the rabbit samples by Shimadzu LC-MS 2010 system. Frozen rabbit samples were thawed at ambient temperature, the solid samples were fixed with 400 ml 0.9% normal saline (NS) and homogenized. A 100 μl aliquot of Triamcinolone diacetate (I.S. 200 ng/ml) standard solution was added to 100 μl of each fluid sample and 400 μl 0.9% NS or homogenized samples. After a thorough vortex mixing for 30 s, the mixture was extracted with 1 ml n-hexane and acetate ester (1:1, v/v) for 60 min, the mixture was centrifuged at 14 000 rpm for 5 min. The organic layer was removed and evaporated under a gentle stream nitrogen gas at 45 °C until it was completely dry. The dried residue was dissolved with 50 μl mobile phase. After centrifugation, 5 μl of the clear supernatant was injected into the LC–MS system: LC-10AD VP low pressure gradient pump, CTO-10A VP column temperature oven, SCL-10AD VP system controller, and LC-MS chemstation. Separation was achieved on a Thermo Hypersil-Hypurity C18 column (150 mm × 2.1 mm, i.d., 5 μm, USA) at 40 °C. Compounds were eluted up to a total retention time of 4.5 min using an isocratic mobile phase consisting of 5 mM ammonium formate (pH 4.0)–methanol acetonitrile (30:5:65, v/v/v) at 0.22 ml/min, and the injection volume was 5 μl. The operating parameters of ESI–MS (electrospray ionization–mass spectrometry) were as follows: capillary voltage was 4.5 kV; nebulizer nitrogen gas flow-rate was 1.5 l/min; drying N2 flow was 10 l/min; drying gas temperature was 250 °C, the gas used was of high purity, and system control and data evaluation were carried out using LC-MS chemstation (Japan). The mass selective detector (MSD) was operated in the positive ionization mode with selected-ion monitoring (SIM) at 393.6 for dexamethasone (m/z) and m/z 479.6 for Triamcinolone diacetate. Quantitation was performed by a linear regression analysis of peak areas ratio from a standard curve containing seven standard points.
Pharmacokinetic and statistical analysis
Dexamethasone concentrations were analyzed for pharmacokinetics in different tissues respectively, which are calculated using kinetica 5.1 (kinetica 5.1, Innaphase, Philadelphia, PA) software by a model Non Compartmental of Extravascular analysis. The following pharmacokinetic parameters were obtained: peak concentration (Cmax), time to peak concentration (Tmax), elimination half-life (T1/2), area under the concentration–time curve between 0 and 24 h (AUC0–24).
Statistical analyses were performed by SPSS 18.0 (SPSS Inc., Chicago, IL). Data of dexamethasone concentrations were expressed as mean ± standard deviation. Tissue concentrations were analyzed using one-way analysis of variance (ANOVA). If there were differences in three groups, we used Dunnett T3 to analyze two groups. All other results were descriptive measures. Statistical significance was accepted at a level of p < 0.05.
Results
Concentration of dexamethasone
The mean levels of dexamethasone concentrations in the posterior segment tissue at different time intervals following IV, SC and CCSDD administration are shown in , and the results are presented in .
Figure 2. The mean levels of dexamethasone concentrations in the posterior segment tissue at different times in vitreous (a), choroid/retina compound (b), and sclera (c).
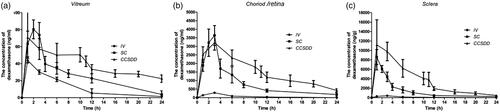
Table 1. The dexamethasone concentrations in the posterior segment tissue following CCSDD, IV, SC.
Levels of dexamethasone concentration in the vitreous () humor were significantly higher at 12 and 24 h (n = 6 per time per group, p < 0.05) and negligible at 1, 3, 6 h between CCSDD and SC (n = 6 per time per group, p = 0.917 at 1 h, p = 0.164 at 3 h, p = 0.105 at 6 h); and significantly higher at 3, 6, 12 and 24 h (n = 6 per time per group, p < 0.01) and negligible at 1 h between CCSDD and IV (p = 0.191).
Figure 3. Levels of dexamethasone concentration in vitreous (a), choroid/retina compound (b), sclera (c). (*denotes that compared with CCSDD group, the difference was statistically significant).
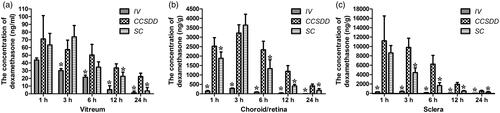
Levels of dexamethasone concentration in the choroid/retinal compound () were significantly higher at 1, 6, 12 and 24 h (n = 6 per time per group, p < 0.05) and negligible at 3 h between CCSDD and SC (n = 6 per time per group, p = 0.586); and significantly higher at 1, 3, 6, 12 and 24 h between CCSDD and IV (p < 0.01).
Levels of dexamethasone concentration in the sclera () were significantly higher at 3, 6, 12 and 24 h (p < 0.05) and negligible at 1 h between CCSDD and SC (n = 6 per time per group, p = 0.612); and significantly higher at 1, 3, 6, 12 and 24 h between CCSDD and IV (p < 0.01).
Pharmacokinetics
The pharmacokinetic parameters of dexamethasone in the rabbits' tissue are shown in .
Table 2. The pharmacokinetic parameters of dexamethasone in the rabbits' posterior segment tissue following CCSDD, IV, SC.
Following the IV route, the maximum concentrations (Cmax) were 43.78 ng/ml, 284.82 ng/g and 402.99 ng/g in vitreous body, choroid/retinal compound and sclera, respectively. The time of maximum concentration (Tmax) was 1 h, 3 h, 3 h, respectively. The AUC0–24 were 285.23 ng·h/ml, 1571.71 ng·h/g and 2858.71 ng·h/g, respectively. The T1/2 were 4.52 h, 3.85 h, 6.88 h, respectively.
Following an SC administration, the peak average dexamethasone levels (Cmax) were 80.45 ng/ml, 3638.80 ng/g and 8604.95 ng/g, respectively, in vitreous body, choroid/retinal compound and sclera, and the Tmax were 2 h, 3 h, 1 h, respectively. The AUC0–24 were 628.84 ng·h/ml, 22411.70 ng·h/g and 35582.30 ng·h/g, respectively. The T1/2 were 5.51 h, 8.29 h and 6.26 h, respectively.
Following an CCSDD administration, the Cmax were 71.05 ng/ml, 3219.35 ng/g and 11192.20 ng/g in vitreous body, choroid/retinal compound and sclera, respectively, and the Tmax were 1 h, 3 h, 1 h, respectively. The AUC0–24 were 1411.65 ng·h/ml, 41400.70 ng·h/g and 93577.80 ng·h/g, respectively. The T1/2 were 14.32 h, 7.65 h, 4.89 h, respectively.
In IV and SC, it was the maximum dosage, and we observed that the AUC0–24 in CCSDD group is higher and the concentration of dexamethasone levels were not less than two other groups in all ocular posterior segment tissues.
Discussion
Effectively administrating drugs to the posterior segment of the eye is very important for the treatment of the retinal, choroidal and vitreous diseases (Edelhauser et al., Citation2010). The aim of the present study was to develop a way of drug delivery that could release dexamethasone in a sustained and controllable manner at a given period and could be applied locally to the outer part of the sclera. For this purpose, the pump was selected to sustain release drug. Typical release profiles from pump was characterized by an initial push drug following the tube suturing the scleral surface, then by a controllable continuous release of dexamethasone at the rate of 0.1 ml/h in concentration of 5 mg/ml. In this report, we presented data showing the feasibility of delivering dexamethasone in a controllable and continuous mode by pump.
The sclera, a highly porous sponge-like tissue consisting of 68% water, is permeable to a series of hydrophilic compounds with various molecular weight compounds from 4 to 150 kDa (Janoria et al., Citation2007; Pescina et al., Citation2015). Iatrogenic perforation of the sclera at the injection site did not result in increased intraocular delivery, which indicated that lateral surface diffusion did not play a significant role in transscleral entry (Ambati et al., Citation2000), it demonstrates that sclera is porous indirectly. Human sclera facilitates diffusion due to its hypocellularity and large surface area, and its remarkable tolerance of foreign bodies overlying its surface makes it a long-term transscleral delivery device to be clinically feasible. The transscleral intraocular tissue distribution of corticosteroids was primarily driven by the drug solubility (Thakur et al., Citation2011). Scleral permeability depends on the molecular radius rather than the molecular lipophilicity (Ambati et al., Citation2000) and molecules of up to 70 000 Da can readily penetrate the sclera (Ambati et al., Citation2000). Subconjunctival injections increase the absorptive capacity of the sclera and lead to systemic absorption (Weijtens et al., Citation1997), the gradual delivery used in these experiments may permit more complete scleral absorption because the drug sclera contact time is greater than the lag time to steady state flux. Sub-tenon drug delivery deposited the therapeutic agents against the external surface of the sclera, which can avoid the risk of the intraocular drug delivery, make it safe and efficient to deliver the agents to posterior segment. So we selected the sub-tenon way to implant controllable continuous drug delivery tube.
An important aspect of continuous controllable-delivery system is that they provide continuous release, high concentrations of drug to achieve the desired pharmacological response. We sutured the tube to the sclera surface to make it close proximity to the sclera, increase drug sclera contact, thus improving scleral absorption, this can increase the bioavailability of the CCSDD, and the release time and rate can be adjusted according to the need. DEXP, a water soluble phosphate of dexamethasone, of which the molecular mass is 516.41 Da, can leak out from the tube and easily pass the sclera to the choroids. Dexamethasone has been used frequently in ocular posterior-segment treatments. The high potency and relatively acceptable toxicity of this compound, combined with its multifunctional roles as an anti-inflammatory, antiangiogenic and antipermeability agent, confirm that it is a candidate for various ocular drug-delivery platforms. Overall, the high concentrations of the dexamethasone in the posterior segments and the characteristic of adjustability of drug release rate and time, ensure that the CCSDD can act as an alternative choice to the posterior drug delivery.
We set the SC and IV of DEXP as control group for SC as it is the easiest and most popular method among periocular injection routes applied by the ophthalmologists (Hosseini et al., Citation2008; Hamdan et al., Citation2015; Liu et al., Citation2015) and IV is the basic method for most refractory ocular posterior segment disease. But we did not compare CCSDD and IVT, because IVT is invasive, and can lead to numerous significant local complications (Smith et al., Citation2014), meanwhile it cannot be repeated everyday. We observed that the level of dexamethasone in CCSDD group was greater than or equal to SC and IV at any time in all ocular posterior segment tissue. In CCSDD group, the levels of dexamethasone in posterior segment tissue at 24 h were roughly equal to the concentration levels in SC group at 12 h. Meanwhile, the levels of dexamethasone in posterior segment tissue at 24 h were almost disappearing in SC and IV. This demonstrated that the administration of CCSDD can act as an option for the refractory posterior segment diseases. AUC0–24 in CCSDD group is higher than other two groups. The dexamethasone exposure to vitreous humor in CCSDD group (AUC0–24=1411.65 ng·h/ml) was about five times as much as the AUC0–24 in IV group, 2.24 times in SC group. In choroid/retinal component (AUC0–24=41400.70 ng·h/g), it was about 26.34 times as much as in IV group, 1.85 times in SC group. In sclera (AUC0–24=93577.80 ng·h/g), it was 32.74 times as much as in IV group, 2.63 times in SC group.
We propose a novel sub-tenon approach for drug delivery via a sutured tube connecting to the scleral surfaces with a pump, we hypothesize that tube can infuse solutions containing soluble molecules, nanoparticles, and microparticles into the sclera in a minimally invasive manner. This would enable delivery of free drug or drug encapsulated within nanoparticles or microparticles for controlled release over time on the scleral surface. Drug could then diffuse from the sclera to neighboring choroidal and retinal tissues to treated the posterior segment diseases.
In SC group, the Cmax and Tmax in vitreous body were similar to those in previous study. When a depot corticosteroid preparation is injected subconjunctivally and side effect occurs, the remainder of the depot can be surgically removed (Huang et al., Citation2016), it is similar in CCSDD. Intrascleral drug delivery to the eye using hollow microneedles can infuse solutions into the sclera for minimally invasive delivery of soluble molecules, nanoparticles and microparticles (Jiang et al., Citation2009). But it is only experimented in vitro, and it may have the defect of perforated eye. Compared to the most popular IVT dexamethasone implant (Miserocchi et al., Citation2016), the CCSDD of the tube allowing the replenishment of the drug, facilitates for the need for a long time therapy. More importantly, although the CCSDD needs surgical implantation to the sub-Tenon’s sac, it does not mechanically break the normal structure of the retina, choroids and can be easily removed if severe complication occurs. As the IVT implantation needs surgical penetration of the sclera, choroids and retina, it can wander in the vitreous cavity and cause vitreous traction. What is more, if severe complication occurs, removal of the drug was not so easy for the ophthalmologists. Compared with controlled release formulations (Meng et al., Citation2014; Huang et al., Citation2016), CCSDD can reach a higher drug level in designed time and can control the rate of drug release.
At present, there are ultrasound-mediated transscleral deliveries (Suen et al., Citation2013), across the conjunctiva using iontophoresis (Eljarrat-Binstock & Domb, Citation2006) and transscleral iontophoresis administration (Eljarrat-Binstock et al., Citation2005,Citation2008). While avoiding the complications of intraocular injection, these transscleral methods nonetheless involve hypodermic injection or implantation or the use of sophisticated electronic devices applied to the ocular surface. Though there was result suggesting that repeated transscleral iontophoresis drugs may be safe for treatment of ocular disorders (Patane et al., Citation2013), there was still not enough evidence to prove that iontophoresis and ultrasound may not cause any damage to the eye tissue. Meanwhile CCSDD delivers drug only through physical penetration, without any damage to the eye tissue, which can release drugs continuously at a controllable speed. However, there are also some limitations in our study. First, our data were observed only based on rabbits not humans, both species are different even similar. Rabbits’ eyes are smaller and have a thinner sclera, which facilitates drug penetrations into the choroid. They have a higher ocular flow rate and blood circulation rate, which may lead to a shorter half-life time of drug in rabbit ocular tissue. They have a much smaller body weight than humans. All of them can affect the ocular drug pharmacokinetic progress. Furthermore, this article only considered healthy rabbit’s eyes. Future work should investigate in diseased eyes (uveitis). Finally, it is only the preliminary study, the CCSDD system should be designed more simple and practical.
Conclusions
In conclusion, the CCSDD system in this study could provide sustained release of DEXP to the posterior segment of the eye and it showed good bioavailability. It seems that the CCSDD can act as an alternative choice for the transscleral drug delivery to the posterior segment of the eye. It can release drugs precisely controlled. However, the CCSDD system is only the preliminary study, and should improve the comfort in the future for clinical using.
Declaration of interest
All authors declare no potential conflicts of interest.
The study was funded by Aier Eye Hospital Group Project and Changsha Science and Technology Planning Project.
Acknowledgements
The authors thank all staff in Aier School of Ophthalmology of Central South University and the Animal Center of Third Xiangya Hospital of Central South University for their support and generous help in animal work. The study was funded by Aier Eye Hospital Group Project and Changsha Science and Technology Planning Project.
References
- Abdul NN, Agarwal P, Agarwal R, et al. (2016). Intraocular distribution of topically applied hydrophilic and lipophilic substances in rat eyes. Drug Deliv 23:2765–71
- Ambati J, Canakis CS, Miller JW, et al. (2000). Diffusion of high molecular weight compounds through sclera. Invest Ophthalmol Vis Sci 41:1181–5
- Ambati J, Gragoudas ES, Miller JW, et al. (2000). Transscleral delivery of bioactive protein to the choroid and retina. Invest Ophthalmol Vis Sci 41:1186–91
- Andonova VY. (2016). A new direction in ophthalmic development: nanoparticle drug delivery systems. Curr Pharm Des. [Epub ahead of print]
- Bansal P, Garg S, Sharma Y, Venkatesh P. (2016). Posterior segment drug delivery devices: current and novel therapies in development. J Ocul Pharmacol Ther 32:135–44
- Borooah S, Jeganathan VS, Ambrecht AM, et al. (2015). Long-term visual outcomes of intravitreal ranibizumab treatment for wet age-related macular degeneration and effect on blindness rates in south-east Scotland. Eye (Lond) 29:1156–61
- Bourges JL, Gautier SE, Delie F, et al. (2003). Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest Ophthalmol Vis Sci 44:3562–9
- Calvo P, Ferreras A, Al AF, et al. (2015). Dexamethasone intravitreal implant as adjunct therapy for patients with wet age-related macular degeneration with incomplete response to ranibizumab. Br J Ophthalmol 99:723–6
- Chen Q, Zheng Y, Li Y, et al. (2012). The effect of deacetylated gellan gum on aesculin distribution in the posterior segment of the eye after topical administration. Drug Deliv 19:194–201
- Chennamaneni SR, Mamalis C, Archer B, et al. (2013). Development of a novel bioerodible dexamethasone implant for uveitis and postoperative cataract inflammation. J Control Release 167:53–9
- Edelhauser HF, Rowe-Rendleman CL, Robinson MR, et al. (2010). Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci 51:5403–20
- Eljarrat-Binstock E, Domb AJ. (2006). Iontophoresis: a non-invasive ocular drug delivery. J Control Release 110:479–89
- Eljarrat-Binstock E, Orucov F, Aldouby Y, et al. (2008). Charged nanoparticles delivery to the eye using hydrogel iontophoresis. J Control Release 126:156–61
- Eljarrat-Binstock E, Raiskup F, Frucht-Pery J, Domb AJ. (2005). Transcorneal and transscleral iontophoresis of dexamethasone phosphate using drug loaded hydrogel. J Control Release 106:386–90
- Ghate D, Brooks W, McCarey BE, Edelhauser HF. (2007). Pharmacokinetics of intraocular drug delivery by periocular injections using ocular fluorophotometry. Invest Ophthalmol Vis Sci 48:2230–7
- Hamdan J, Boulze M, Aziz A, et al. (2015). Corneal neovascularisation treatments compared: subconjunctival bevacizumab injections and/or photodynamic therapy. J Fr Ophtalmol 38:924–33
- Hennessy AL, Katz J, Covert D, et al. (2010). Videotaped evaluation of eyedrop instillation in glaucoma patients with visual impairment or moderate to severe visual field loss. Ophthalmology 117:2345–52
- Hettinga YM, Verhagen FH, van Genderen M, de Boer JH. (2014). Characteristics of childhood uveitis leading to visual impairment and blindness in the Netherlands. Acta Ophthalmol 92:798–804
- Hosseini K, Matsushima D, Johnson J, et al. (2008). Pharmacokinetic study of dexamethasone disodium phosphate using intravitreal, subconjunctival, and intravenous delivery routes in rabbits. J Ocul Pharmacol Ther 24:301–8
- Hsu J. (2007). Drug delivery methods for posterior segment disease. Curr Opin Ophthalmol 18:235–9
- Huang Z, Yang W, Zong Y, et al. (2016). A study of the dexamethasone sodium phosphate release properties from a periocular capsular drug delivery system. Drug Deliv 23:849–57
- Janoria KG, Gunda S, Boddu SH, Mitra AK. (2007). Novel approaches to retinal drug delivery. Expert Opin Drug Deliv 4:371–88
- Jiang J, Moore JS, Edelhauser HF, Prausnitz MR. (2009). Intrascleral drug delivery to the eye using hollow microneedles. Pharm Res 26:395–403
- Kang-Mieler JJ, Osswald CR, Mieler WF. (2014). Advances in ocular drug delivery: emphasis on the posterior segment. Expert Opin Drug Deliv 11:1647–60
- Lajunen T, Nurmi R, Kontturi L, et al. (2016). Light activated liposomes: functionality and prospects in ocular drug delivery. J Control Release. [Epub ahead of print]
- Liu S, Dozois MD, Chang CN, et al. (2016). Prolonged ocular retention of mucoadhesive nanoparticle eye drop formulation enables treatment of eye diseases using significantly reduced dosage. Mol Pharm 13:2897–905
- Liu X, Wang M, Zhao C, et al. (2015). The efficacy and safety of subconjunctival triamcinolone acetonide injections in treatment of uveitic macular edema. Zhonghua Yan Ke Za Zhi 51:734–8
- Lowder C, Belfort RJ, Lightman S, et al. (2011). Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol 129:545–53
- Meng Y, Sun S, Li J, et al. (2014). Sustained release of triamcinolone acetonide from an episcleral plaque of multilayered poly-ɛ-caprolactone matrix. Acta Biomater 10:126–33
- Miserocchi E, Berchicci L, Iuliano L, et al. (2016). Dexamethasone intravitreal implant in serpiginous choroiditis. Br J Ophthalmol. [Epub ahead of print]
- Nagai N, Kaji H, Onami H, et al. (2014). A polymeric device for controlled transscleral multi-drug delivery to the posterior segment of the eye. Acta Biomater 10:680–7
- Patane MA, Schubert W, Sanford T, et al. (2013). Evaluation of ocular and general safety following repeated dosing of dexamethasone phosphate delivered by transscleral iontophoresis in rabbits. J Ocul Pharmacol Ther 29:760–9
- Peptu CA, Popa M, Savin C, et al. (2015). Modern drug delivery systems for targeting the posterior segment of the eye. Curr Pharm Des 21:6055–69. 2015-01-20
- Pescina S, Govoni P, Antopolsky M, et al. (2015). Permeation of proteins, oligonucleotide and dextrans across ocular tissues: experimental studies and a literature update. J Pharm Sci 104:2190–202
- Quek DT, Ong GT, Perera SA, et al. (2011). Persistence of patients receiving topical glaucoma monotherapy in an Asian population. Arch Ophthalmol 129:643–8
- Smith SJ, Smith BD, Mohney BG. (2014). Ocular side effects following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol 98:292–7
- Suen WL, Wong HS, Yu Y, et al. (2013). Ultrasound-mediated transscleral delivery of macromolecules to the posterior segment of rabbit eye in vivo. Invest Ophthalmol Vis Sci 54:4358–65
- Thakur A, Kadam RS, Kompella UB. (2011). Influence of drug solubility and lipophilicity on transscleral retinal delivery of six corticosteroids. Drug Metab Dispos 39:771–81
- Thakur SR, Tekko I, McAvoy K, et al. (2016). Minimally invasive microneedles for ocular drug delivery. Expert Opin Drug Deliv. [Epub ahead of print]
- Thrimawithana TR, Young S, Bunt CR, et al. (2011). Drug delivery to the posterior segment of the eye. Drug Discov Today 16:270–7
- Tsang AC, Virgili G, Abtahi M, Gottlieb CC. (2016). Intravitreal dexamethasone implant for the treatment of macular edema in chronic non-infectious uveitis. Ocul Immunol Inflamm. [Epub ahead of print]
- Weijtens O, van der Sluijs FA, Schoemaker RC, et al. (1997). Peribulbar corticosteroid injection: vitreal and serum concentrations after dexamethasone disodium phosphate injection. Am J Ophthalmol 123:358–63
- Zatic T, Bendelic E, Paduca A, et al. (2015). Rapid assessment of avoidable blindness and diabetic retinopathy in Republic of Moldova. Br J Ophthalmol 99:832–6