Abstract
Leukotriene B4, as a kind of 5-lipoxygenase metabolite of arachidonic acid, is known to influence osteoclast formation and bone resorption. In order to determine whether Leukotriene B4 could directly stimulate human osteoclast differentiation and activation independent of RANKL (ODF), three different concentrations of Leukotriene B4 (10−9 M, 10−8 M, 10−7 M) were added to the culture of CD14+ monocyte fraction of peripheral blood mononuclear cell (PBMC) in the presence of macrophage colony-stimulating factor (M-CSF). Under these conditions, Leukotriene B4 could induce multinucleated cells, which were positive for Tartrate-resistant acidic phosphatase (TRAP) staining and capable of bone resorption. Addition of osteoprotegerin (OPG) to PBMC cultures does not abrogate osteoclast formation induced by LTB4. Osteoclastogenesis induced by Leukotriene B4 were dose-dependently increased and weaker than that of RANKL. These results indicated that Leukotriene B4, elevated in many inflammatory diseases, is directly capable of inducing osteoclast formation by a RANKL-independent mechanism.
INTRODUCTION
Osteoclasts are highly specialized multinucleated cells uniquely responsible for bone resorption both in physiological bone remodeling and in pathological conditions, such as Rheumatoid arthritis, periodontal disease, metastatic cancer et al., which are derived from hematopoietic progenitor cells [Citation[1-3]]. Mononuclear osteoclast precursors are bone marrow-derived cells, which circulate in the monocyte fraction of peripheral blood and differentiate into functional osteoclast at or near the bone surface [Citation[4]].
Recent breakthroughs in understanding of osteoclast differentiation and activation come from the analysis of a family of biologically related tumor necrosis factor (TNF) receptor (TNFR)/TNF-like proteins: osteoprotegerin (OPG), receptor activator of nuclear factor(NF)-κ B(RANK) and RANK ligand (RANKL), which together regulate osteoclast function [Citation[5]]. Osteoclast formation requires the presence of macrophage colony-stimulating factor (M-CSF) and involves an interaction between RANK, expressed on osteoclast precursors, and RANKL, which is expressed by several cell types, including osteoblast, RA synovial fibroblast and activated T lymphocyte [Citation[6-8]]. Several cytokine factors have been shown to stimulate osteoclast formation through increasing RANKL expression of osteoblast, bone marrow stromal cell and bone lining cell [Citation[9]]. It has also recently been shown that tumor necrosis factor-α (TNF-α) can directly stimulate osteoclast differentiation and activation by a RANKL-independent mechanism [Citation[10], Citation[11]], and Interleukin-1 can directly induce bone-resorbing activity of osteoclast independent of RANKL mechanism [Citation[12]].
Leukotriene B4 is a kind of inflammatory mediator formed by the oxygenation of arachidonic acid by the enzyme 5-lipooxygenase, which is elevated in many diseases, such as asthma, arthritis, and inflammatory periodontal disease. Leukotriene B4 has been shown to stimulate bone resorption both in vitro and in vivo [Citation[13]]. Leukotiene B4 has shown that avian osteoclast can be directly stimulated to resorb calcified matrices [Citation[14]], and was shown to stimulate resorption pit formation by isolated rat osteoclasts within a 20-hours incubation time frame [Citation[13]]. Leukotriene B4 was also shown to increase osteoclast formation in murine marrow cultures [Citation[13]]. In this study, we have sought to determine whether Leukotriene B4 could directly induce human osteoclast formation from human peripheral blood mononuclear cell independent of the RANKL/RANK signaling system.
MATERIALS AND METHODS
1. Reagents
For all incubations, Dulbecco's Modified Eagle medium (DMEM, Gibco, USA) was supplemented with 100 IU/mL penicillin, 100 µg/mL streptomycin sulphate. Recombinant human M-CSF, human sRANKL and human OPG were purchased from Cytolab/Peprotech Ltd (Rehovot, Israel). Leukotriene B4 was purchased from Cayman Chemical Company (Michigan, USA) and Sigma-Aldrich Inc. (Saint Louis, Missouri, USA). Ficoll-Paque Plus solution was purchased from Amersham Bioscience Corporation (Piscataway, USA). Acid Phosphatase Leukocyte Kit for TRAP staining was purchased from Sigma Diagnostics Inc. (Saint Louis, Missouri, USA). Toluidine blue was purchased from Fluka Inc. (Buchs, Switzerland). Fetal bovine serum was purchased from Hyclone Laboratory Inc. (South Logan, Utah, USA). All incubations were carried out at 37°C in 5% CO2.
2. Preparation of Human Peripheral Blood Monocyte
Human monocytes were isolated by Ficoll-paque solution discontinuous density gradient sedimentation and adherence onto poly-lysine coated glass coverslips or bovine cortical slices from the peripheral blood of normal healthy subjects. Peripheral blood was diluted with an equal volume of PBS, then layered over Ficoll-Paque Plus solution (the ratio of diluted peripheral blood and Ficoll-Paque Plus solution was 4:3), and then centrifuged at 400 g for 30 minutes. Peripheral blood mononuclear cell (PBMC) at the interface were collected and washed twice with PBS through centrifugation at 100 g for 10 minutes, and the pellet was resuspended in DMEM with 10% fetal bovine serum (FBS). The number of cells in the cell suspension was finally counted in a hemocytometer. PBMCs in DMEM/FBS were added onto poly-lysine coated glass coverslips or bovine cortical slices in 16-mm-diameter wells of 24-wells tissue culture plate and incubated for 2 hours at 37°C in 5% CO2, after which time the glass coverslips or bovine cortical slices were vigorously rinsed in PBS solution to remove nonadherent cells before being transferred back to 24-wells tissue culture plate, so human monocytes adhere to the glass coverslips or bovine cortical slices as osteoclast precursor.
3. Osteoclast Formation From Human Monocyte
Human peripheral blood mononuclear cells (PBMCs; 7 × 106 cells/well) were added to 16-mm–diameter wells of a 24-wells tissue culture plate containing either 10 × 10 mm glass coverslip or bovine cortical slice. After 2 hours incubation at 37°C in 5% CO2, the glass coverslips or bovine cortical slices were removed from the wells, rinsed vigorously in PBS solution to remove nonadherent cells (lymphocytes), and transferred back to corresponding well in 24-wells tissue culture plate containing 2.8 ml DMEM/FBS. These cultures were incubated for up to 16 days on glass coverslips and up to 21 days on bovine cortical slice. The negative control group was incubated in the presence of 25 ng/ml M-CSF. The positive control group was incubated in the presence of 25 ng/ml M-CSF and 30 ng/ml sRANKL. There were four experimental groups, which were respectively incubated in the presence of 25 ng/ml M-CSF, and 10−7 M LTB4 or 10−8 M LTB4 or 10−9 M LTB4, and another experimental group was incubated in the presence of 25 ng/ml M-CSF, 10−7 M LTB4 and 100 ng/ml OPG, which acts to block RANKL binding to its receptor RANK. The corresponding cytokines and culture medium were completely changed every three days.
4. Characterization of Cell Cultures for Osteoclast Phenotypic Marker
The glass coverslips were removed from the wells after 16 days culture. The cells on the glass coverslips were stained cytochemically for the expression of tartrate-resistant acid phosphatase (TRAP), an osteoclast-associated marker [Citation[15]]. Using a commercially available kit, the cells were fixed in citare/acetone solution and stained for acid phosphatase, using naphthol AS-BI as a substrate, in the presence or absence of 1.0 mol/L tartrate; the product was reacted with Fast-Garnet GBC salt. The cell preparations were counterstained with hematoxylin staining. The multinucleated TRAP staining (+) cells were called osteoclast-like cells. The number of osteoclast-like cells on three 10 × 10 mm glass coverslips of each group were counted as the differentiation effect and compared between each group.
5. Functional Evidence of Osteoclast Activation
The bovine cortical slices were removed from the wells after 21 days culture, rinsed with distilled water, fixed with 2.5% glutaraldehyde for 7 minutes, and then rinsed through ultrasonication in 0.25% ammonium hydroxide for three times (5 minutes each time) to remove the adherent cells. After dehydration with 70%, 95%, 100% ethanol in turn, the bovine cortical slices were stained with 1% (v/v) toluidine blue prior to examination by light microscopy. The surface of each bovine cortical slice was examined for evidence of lacunar bone resorption (Howship pit). The number of resorption pits on three 10 × 10 mm bovine cortical slices of each group were counted as the activation effect and compared between each group.
6. Preparation of Cultures on Bovine Cortical Slices for Examination by SEM
The toluidine-stained bovine cortical slices were rinsed with distilled water for three times through ultrasonication (5 minutes each time). The bovine cortical slices were then passed through graded alcohols to absolute (70%, 95%, 100% ethanol in turn), and then allowed to air dry before being sputter coated with gold and examined in a JEDL JSM-6100 scanning electron microscope.
7. Statistical Significance
There were three wells in every group of each experiment. Each experiment was carried out in triplicates. Experimental data were expressed as mean number +/− standard deviation. Statistical analysis was performed using Student's t test and P values less than 5% were considered significant.
RESULTS
1. LTB4 Induced Osteoclast Differentiation from Human Monocyte
After 2 hours incubation on glass coverslips, monocytes adhered to the glass coverslips which showed negative for the osteoclast marker TRAP.
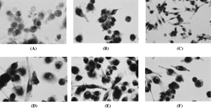
In the presence of 25 ng/ml M-CSF alone (negative control group), there was few multinucleated TRAP staining (+) cells (osteoclast-like cells/OCLs) (284.6 +/− 197.2). Mononuclear or multinucleated TRAP staining (+) cells were seen after 16 days incubation of monocytes in the presence of M-CSF and sRANKL or M-CSF and Leukotriene B4 ().
Figure 1 Human monocyte cultures incubated on glass coverslips in the presence of (A) 25 ng/ml M-CSF, (B) 25 ng/ml M-CSF and 30 ng/ml sRANKL, (C) 25 ng/ml M-CSF and 10−9 M LTB4, (D) 25 ng/ml M-CSF and 10−8 M LTB4, (E) 25 ng/ml M-CSF and 10−7 M LTB4, (F) 25 ng/ml M-CSF, 10−7 M LTB4 and 100 ng/ml OPG for 16 days. After TRAP staining and counterstained with hematoxylin, showing that (A) no TRAP-positive multinucleated cell (original magnification ×400) and (B) (C) (D) (E) (F) numerous TRAP-positive mononuclear and multinucleated cells (original magnification ×200 in C group and ×400 in B, D, E, F group).
The mean number of TRAP staining (+) osteoclast-like cells formed in response to 10−7 M LTB4 (M-CSF)(5122.6 +/− 903.5) or 10−8 M LTB4(+ M-CSF) (3870.4 +/− 197.2) or 10−9 M LTB4(+M-CSF) (2618.2 +/− 429.7) was lower than that observed in cultures incubated with sRANKL and M-CSF (7285.3 + 457.9). There were significantly more TRAP staining (+) osteoclast-like cells in all three LTB4-stimulating experimental groups and positive control group compared with the negative control group (P < 0.05). The mean number of TRAP staining (+) osteoclast-like cells in three LTB4-stimulating experimental groups was dose-dependently increased (P < 0.05). The mean number of TRAP staining (+) osteoclast-like cells in response to 10−7 M LTB4 (+M-CSF) and 100 ng/ml OPG (5066.525 +/− 860.258) was similar to that without 100 ng/ml OPG (P > 0.05) ().
Figure 2 The number of multinucleated TRAP staining (+) cells in 16-day cultures of monocyte in the presence of (A) 25 ng/ml M-CSF + 10−7 M LTB4 + 100 ng/ml OPG.(experimental group), (B) 25 ng/ml M-CSF + 10−7 M LTB4 (experimental group), (C) 25 ng/ml M-CSF + 10−8 M LTB4 (experimental group), (D) 25 ng/ml M-CSF + 10−9 M LTB4 (experimental group), (E) 25 ng/mlM-CSF + 30 ng/ml sRANKL (positive control group), (F) 25 ng/ml M-CSF alone (negative control group). The results are expressed as the mean number of osteoclast-like cells +/− standard error of the mean. *p < 0.05 compared to monocyte cultures treated with M-CSF alone.

2. Lacunar Resorption By LTB4-induced Osteoclast
After 2 hours incubation on poly-lysine coated bovine cortical slices, monocytes adhered to the bovine cortical slices, which showed no lacunar resorption.
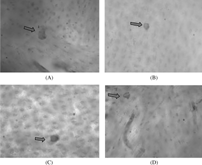
Twenty-four hours cultures of monocyte on bovine cortical slices showed no evidence of lacunar resorption. Evidence of resorption pit formation in cultures of monocytes was seen when these cells were cultured on bovine cortical slices in the presence of M-CSF and sRANKL or M-CSF and LTB4 for 21 days ().
Figure 3 Cultures (21 days) of monocytes incubated on bovine cortical slices in the presence of (A) 25 ng/ml M-CSF and 30 ng/ml sRANKL, (B) 25 ng/ml M-CSF and 10−7 M LTB4, (C) 25 ng/ml M-CSF and 10−8 M LTB4, (D) 25 ng/mlM-CSF and 10−9 M LTB4. After removing adherent osteoclasts, the bovine cortical slices were stained with toluidine blue showing resorption pits (original magnification ×200).
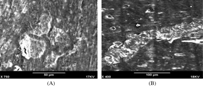
The mean number of resorption pits in monocyte cultures on bovine cortical slices incubated with 25 ng/ml M-CSF and 30 ng/ml sRANKL (positive control group) was 63.3 +/− 17.6. Compared with positive control group, significantly fewer resorption pits were observed in monocyte cultures on bovine cortical slices incubated with 10−7 M LTB4 (21.3 +/− 1.53, P < 0.05) or 10−8 M LTB4 (16.7 +/− 1.53, P < 0.05) or 10−9 M LTB4 (11.3 +/− 1.53, P < 0.05) in the presence of 25 ng/ml M-CSF. In the experimental groups, the number of resorption pits in monocytes cultures on bovine cortical slices incubated with different concentrations LTB4 was dose-dependently increased (P < 0.05). (). In contrast to large resorption pits seen in RANKL-treated monocytes cultures, numerous small, round or ovoid resorption pits were formed in LTB4-treated cultures. The bone resorption pits were verified through examination of scanning electron microscope, shown as .
Figure 4 The mean number of resorption pits on 10 × 10 mm bovine cortical slices in 21-day cultures of monocytes incubated in the presence of (A) 25 ng/mlM-CSF + 30 ng/ml sRANKL, (B) 25 ng/ml M-CSF + 10−7 M LTB4, (C) 25 ng/ml M-CSF + 10−8 M LTB4, (D) 25 ng/ml M-CSF + 10−9 M LTB4. The results are expressed as the mean number of resorption pits +/− standard error of the mean. *P < 0.05 compared to monocytes cultures treated with 25 ng/ml M-CSF + 30 ng/ml sRANKL.

Figure 5 The SEM image of bovine cortical slices on which monocytes were cultured for 21 days in the presence of (A) 25 ng/ml M-CSF and 10−7 M LTB4, (B) 25 ng/ml M-CSF and 30 ng/ml sRANKL, showing that numerous small round or ovoid resorption pits in LTB4-treated monocytes cultures and large confluent resorption pits in sRANKL-treated monocytes cultures.
DISCUSSION
LTB4 was previously known as a kind of pro-inflammatory mediator synthesized in myeloid cells from arachinoid acid, which had a pathogenetic role in chronic neutrophil-mediated inflammation [Citation[16]]. Leukotrienes are more potently harmful than PGE2 for the inflammatory process, because the former are potent chemotactic agents and can increase microvascular permeability [Citation[17-19]].
Recently, LTB4 has been shown to stimulate bone resorption both in vitro and in vivo, to stimulate osteoclast formation in murine marrow culture and resorption pit formation by isolated rat osteoclasts [Citation[13]] and avian osteoclasts [Citation[14]]. That is to say, LTB4 is also a kind of osteotropic agent and involved in bone loss, which occurs when chronic inflammatory tissues lie adjacent to bone surfaces. LTB4 has been reported to be elevated in many kinds of human diseases with bone erosion phenomenon, such as RA [Citation[20]].
Osteoclast formation involves two-stages: differentiation to osteoclast-like cells from hematopoietic precursor cells and activation of osteoclast-like cells [Citation[21]]. The combination of M-CSF and RANKL is essential and sufficient for human osteoclast formation [Citation[22]]. M-CSF, which is imperative for macrophage maturation, binds to its receptor, c-Fms, on early osteoclast precursors, thereby providing signals required for their survival and proliferation [Citation[23]]. The major role of RANKL in bone is the stimulation of osteoclast differentiation, activation/activity [Citation[6]]. In previous studies, several pro-inflammatory cytokines, such as TNF-alpha and IL-6/11, provide an alterative (i.e., RANKL-independent) pathway of osteoclast formation in the presence of M-CSF [Citation[10], Citation[11], Citation[24]].
We utilize healthy human peripheral blood monocytes as osteoclast precursors, which can differentiate into mature functional osteoclast [Citation[4], Citation[25]]. Through the incubation of PBMC on glass coverslips or bovine cortical slices for 2 hours at 37°C in 5% CO2, more than 95% adherent cells were monocytes, which expressed CD68, CD11b, and CD14 macrophage-associated antigens [Citation[22]]. In our present study we have shown that, in the presence of M-CSF, LTB4 can directly stimulate human osteoclast differentiation and activation from PBMC by a RANKL-independent mechanism. We found that LTB4 induced osteoclast formation in cultures of CD14 + monocytes, which do not contain RANKL-expression cells [Citation[9]]. And our results show that the addition of OPG to PBMC cultures does not abrogate osteoclast formation induced by LTB4.
The differentiation effect was assessed through the enumeration of TRAP-positive multinucleate osteoclast-like cells [Citation[15], Citation[26]]. According to our experimental results, the number of osteoclast-like cells in monocytes cultures treated with LTB4 in the presence of M-CSF was dose-dependently increased and significantly more than that of M-CSF alone group, which shows that LTB4 can directly stimulate human osteoclast differentiation independent of RANKL and has stronger differentiation effect with the increase of LTB4 concentration (10−9 M, 10−8 M, 10−7 M). As the number of osteoclast-like cells of LTB4 experimental group was significantly lower than that seen in M-CSF + sRANKL positive control group, it shows that LTB4 has weaker human osteoclast differentiation effect than that of RANKL.
The activation effect was assessed through the enumeration of resorption pits [Citation[13]]. The number of resorption pits was dose-dependently increased (10−9 M, 10−8 M, 10−7 M) in LTB4 experimental group and significantly lower than that of M-CSF + sRANKL positive control group, which shows that LTB4 can directly stimulate human osteoclast activation independent of RANKL and has weaker activation effect than that of RANKL.
In conclusion, LTB4 can directly stimulate osteoclast formation, which is capable of bone-resorption. Because LTB4 is elevated in inflammatory conditions, so it is possible that LTB4 induce a “salvage” pathway of osteoclast formation, which is not operative to any great extent in normal bone reconstruction, but may be clinically important in pathological conditions of bone and joint.
Two G-protein-coupled-receptors for LTB4, BLT1 and BLT2, have been isolated [Citation[27], Citation[28]], and shown to be a high- and low-affinity receptor, respectively. The optimal range of LTB4 concentrations is 10−9 M to 10−7 M for BLT1, and 10−7 M to 10−5M for BLT2. These two receptors are co-expressed in various subsets of human PBMCs in different quantities. BLT1 expression is highest in CD 14 + monocytes [Citation[29]]. In our experiment, we utilized 10−9 M to 10−7 M LTB4, which was optimal LTB4 concentrations for BLT1 receptor, to treat monocytes culture. So we speculated that the human osteoclast formation effect of LTB4 from monocytes was mainly mediated through binding to BLT1 receptor. Garcia et al. reported that avian osteoclast can be directly stimulated to resorb bone in 10−6 M to 10−10 M, which was wider than LTB4 concentration in our study, because these avian osteoclasts were found to have both high and low affinity binding sites [Citation[14]]. The direct stimulation effect for human osteoclast formation from monocytes of larger range LTB4 concentration than our study is for further study in future.
Our study shows that LTB4 can directly stimulate human osteoclast formation from monocytes, but this does not rule out a potential indirect effect of LTB4 on RANKL-expressing cells to stimulate human osteoclast formation through increasing the expression of RANKL, which is the focus for future study.
The study was supported by National Natural Science Foundation of China (3007064).
We thank Mr. Wen-ding Zhang (Chinese Academy of Science) for his help in preparation of bovine cortical slices. We are also gateful for the technical assistance of Mr. Jian-qiang Dong (Central Lab of Peking University People's Hospital).
REFERENCES
- Suda, T., Takahashi, N., Martin, T.J. (1992). Modulation of osteoclast differentiation. Endocr. Rev. 13(1): 66–80. [PUBMED], [INFOTRIEVE], [CSA]
- Suda, T., Udagawa, N., Takahashi, N. (1996). Osteoclast generation, in Principles of Bone Biology, 2nd ed., J.P. Bilizikian, L.G. Raisz, G.A. Rodan, Eds., Academic Press: San Diego, CA, pp. 87–102.
- Athanasou, N.A. (1996). Cellular biology of bone-resorbing cells. J Bone Joint Surg Am. 78(7): 1096–1122. [PUBMED], [INFOTRIEVE], [CSA]
- Fujikawa, Y., Quinn, J.M.W., Sabokbar, A., McGee, J.O.D., Athanasou, N.A. (1996). The human osteoclast precursor circulates in the monocyte fraction. Endocrinology 137(9): 4058–4060. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Khosla, S. (2001). Minireview: the OPG/RANKL/RANK system. Endocrinology 142(12): 5050–5055. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lacey, D.L., Timms, E., Tan, H.L., Kelley, M.J., Dunstan, C.R., Burgess, T., Elliot, R., Colombero, A., Elliott, G., Scully, S., Hsu, H., Sullivan, J., Hawkins, N., Davy, E., Capparelli, C., Eli, A., Qian, YX., Kaufman, S., Sarosi, I., Shalhoub, V., Senaldi, G., Guo, J., Delaney, J., Boyle, W.J. (1998). Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93(2): 165–176. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Yasuda, H., Shima, N., Nakagawa, N., Mochizuki, S.I., Yano, K., Fujise, N., Sato, Y., Goto, M., Yamuguchi, K., Kuiyama, M., Kanno, T., Murakami, A., Tsuda, E., Morinaga, T., Higashio, K. (1998). Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): A mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139(3): 1329–1337. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gravallese, E.M., Manning, C., Tsay, A., Naito, A., Pan, C., Amento, E., Goldring, S.R. (2000). Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis & Rheumatism 43(2): 250–258. [INFOTRIEVE], [CSA], [CROSSREF]
- Hofbauer, L.C., Khosla, D., Dunston, C.R., Lacey, D.L., Boyle, W.J., Riggs, B.L. (2000). The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Mineral Res. 15(1): 2–12. [CSA]
- Kobayashi, K., Takahashi, N., Jimi, E., Udagawa, N., Takami, M., Kotake, S., Nakagawa, N., Kinosaki, M., Yamaguchi, K., Shima, N., Yasuda, H., Morinaga, T., Higashinio, K., Martin, TJ., Suda, T. (2000). Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of ODF/RANKL-RANK interaction. J Exp Med. 191(2): 275–286. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Fuller, K., Murphy, C., Kirstein, B., Fox, S.W., Chambers, T.J. (2002). TNF-α potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology 143(3): 1108–118. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jimi, E., Nakamura, I., Duong, L.T., Ikebe, T., Takahashi, N., Rodan, G.A., Suda, T. (1999). Interleukin-1 induces multinucleation and bone-resorbing activity of osteoclasts in the absence of osteoblast/stromal cells. Exp Cell Res. 247(1): 84–93. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Garcia, C., Boyce, B.F., Gilles, J., Dallas, M., Qiao, M., Mundy, G.R., Bonewald L.F. (1996). Leukotriene B4 stimulates osteoclastic bone resorption both in vitro and in vivo. J Bone Miner Res. 11(11): 1619–1627. [PUBMED], [INFOTRIEVE], [CSA]
- Flynn, M.A., Qiao, M., Garcia, C., Dallas, M., Bonewald, L.F. (1999). Avian osteoclast cells are stimulated to resorb calcified matrices by and pocess receptors for leukotriene B4. Calcif Tissue Int. 64(2): 154–159. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mikin, C. (1982). Bone acid phosphates: Tartrate-resistant acid phosphates as a marker of osteoclast function. Calcif Tissue Int. 34(3): 285–290. [CSA]
- Crooks, S.W., Stockley, R.A. Molecules in focus: Leukotriene B4. (1998). Int J Biochem Cell Biol. 30(2): 173–178. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Penrose, J.F., Austen, K.F. (1999). The biochemical, molecular, and genomic aspects of leukotriene C4 synthase. Proc Assoc Am Physicians 111(6): 537–546. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Los, M., Schenk, H., Hexel, K., Baeuerle, P.A., Droge, W., Schulze-Osthoff, K. (1995). Il-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipooxygenase. EMBO J 14(15): 3731–3740. [PUBMED], [INFOTRIEVE], [CSA]
- Gok, S., Ulker, S., Huseyinov, A., Hatip, F.B., Cinar, M.G., Evinc, A. (2000). Role of leukotrienes on coronary vasoconstriction in isolated hearts of arthritis rats: effect of in vivo treatment with CI-986, a dual inhibitor of cyclooxygenase and lipoxygenase. Pharmacology 60(1): 41–46. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ahmadzadeh, N., Shingu, M., Nobunaga, M., Tawara, T. (1991). Relationship between leukotriene B4 and immunological parameters in rheumatoid synovial fluids. Inflammation 15(6): 497–503. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Roodman, G.D. (1999). Cell biology of the osteoclast. Exp Hematol. 27(8): 1229–1241. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Quinn, J.M., Elliot, J., Gillespie, M.T., Martin, T.J. (1998). A combination of osteoclast differentiation factor and macrophage-colony stimulating factor is sufficient for both human and osteoclast formation. Endocrinology 139(10): 4424–4427. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Udagawa, N., Takahashi, N., Akatsu, T., Tanaka, H., Sasaki, T., Nishihara, T., Koga, T., Suda, T. (1990). Origin of osteoclasts: Mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenviroment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U.S.A. 87(18): 7260–7264. [PUBMED], [INFOTRIEVE], [CSA]
- Kudo, O., Sabokbar, A., Pocock, A., Itonaga, I., Fujikawa, Y., Athanasou, NA. (2003). Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone 32(1): 1–7. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Quinn, J.M.W., Neale, S., Fujikawa, Y., McGee, J.O.D., Athanasou, N.A. (1998). Human osteoclast formation from blood monocytes, peritoneal macrophages, and bone marrow cells. Calcif Tissue Int. 62(6): 527–531. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ibbotson, K.J., Roodman, G.D., McManus, L.M., Mundy, G.R. (1984). Identification and characterization of osteoclast-like cells and their progenitors in culture of feline marrow mononuclear cells. J Cell Biol. 99(2): 474. [CSA], [CROSSREF]
- Yokomizo, T., Izumi, T., Chang, K., Takuwa, Y., Shimizu, T. (1997). A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 387(6633): 620–624. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Yokomizo, T., Kato, K., Terawaki, K., Izumi, T., Shimizu, T. (2000). A second leukotriene B4 receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med. 192(3): 421–432. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Yokomizo, T., Izumi, T., Shimizu, T. (2001). Co-expression of two LTB4 receptors in human mononuclear cells. Life Sci. 68(19–20): 2207–2212. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]