Abstract
A laboratory-scale study of bioconversion of local lignocellulosic material, oil palm biomass (OPB) was conducted by evaluating the enzyme production through microbial treatment in solid state bioconversion (SSB). OPB in the form of empty fruit bunches (EFB) was used as a solid substrate and treated with the white-rot fungus, Phanerochaete chrysosporium, to produce ligninase. The results showed that the highest ligninase activity of 400.27 U/liter was obtained at day 12 of fermentation. While the optimum study indicated the enzyme production of 1472.8 U/liter with moisture content of 50%, 578.7 U/literwith 10% v/w of inoculum size, and 721.8 U/liter with co-substrate concentration of 1% (w/w) at days 9, 9 and 12 of fungal treatment, respectively. The parameters glucosamine and reducing sugar were observed to evaluate the growth and substrate utilization in the experiment.
INTRODUCTION
The oil palm industries in Malaysia are producing a huge potential of exploitation of non-oil palm biomass that are about 90 million tonnes of lignocellulosic biomass each year, of which about 40 million tonnes are in the form of empty fruit bunches (EFB), oil palm trunks (OPT) and oil palm fronds (OPF) [Citation[1]]. They are residue left after the replantation activities and mills operation and oil palm residues continue to accumulate. At present, the biomass is either left at plantation to provide organic nutrients to oil palm trees, burned illegally or used as solid fuel in the boiler to generate steam or electricity at the mills [Citation[2]].
The presence of lignin in the cell wall of lignocellulosic materials offers a way to produce ligninase enzyme by microbial treatment. Lignin is found in the cell walls in a complex form with cellulose and hemicellulose, providing protection to these carbohydrates from biological degradation [Citation[3]]. The white rot fungi, i.e. Phanerochaete chrysosporium, is the most recognized fungus to biodegrade lignin and other components in the cell wall by secreting the extracellular enzyme, ligninase (LiP) and manganese dependent peroxidase (MnP) [Citation[4], Citation[5]]. The ligninocellulosic components are converted to reducing sugar, which is utilized as rich carbon source to enhance the enzyme production in solid state bioconversion (SSB) process. The attraction of SSB comes from its simplicity and its closeness to the natural way of life for many microorganisms, low capital costs for equipment, high volumetric productivity, decreased operational costs, and as an alternative in preventing environmental pollution [Citation[6]].
Very limited research has been undertaken in order to provide a better utilization of oil palm wastes for value-added product applications [Citation[7-9]]. Based on these current trends of treating the waste, therefore, this study was inspired to develop indigenous environmentally friendly processes for the production of ligninase enzyme by microbial treatment of OPB in solid state bioconversion. This study offers a smart alternative for a better utilization of oil palm wastes for value added products besides achieving zero waste strategies at plants. In fact, ligninase enzymes possess a good promise of application in the pulp and paper industry, biodegradation of toxic chemicals, specialty chemical synthesis and textile industry.
MATERIALS AND METHODS
Substrate and Co-substrate
The substrate used for this work was empty fruit bunches (EFB), as the oil palm biomass is cheap and a readily available source of lignocellulosics. The EFB was collected from oil palm industry (Seri Ulu Langat Palm Oil Mill Sdn. Bhd., Dengkil, Selangor, Malaysia). The sample was collected as crushed EFB with a few centimeters of fibrous length, and stored into autoclavable plastic begs in a cold room at 4°C. Meanwhile, wheat flour was used as the co-substrate and additional carbon source. The previous study reported it enhances biodegradation and the enzyme production [Citation[10]].
Fungal Strain and Inoculum Preparation
Culture of Phanerochaete chrysosporium (PC-9) obtained from the lab stocks was maintained on potato dextrose agar (PDA) plates and incubated at 37°C for seven days. After that, the cultured plates (4 plates) were washed with 100 ml of sterile distilled water. The surface was gently rubbed with a sterilized glass rod and the mycelial suspension was directly transferred into sterilized 250 ml of conical flask for the use of final inoculum after measuring its concentration [Citation[11]]. The inoculum was kept in the refrigerator at 4°C for further studies.
Mineral Solution
Mineral solutions were made with the following quantities of nutrient salts (NS) per liter: 2.0 g NH4H2PO4; 0.86 g urea; 0.6 g KH2PO4; 0.4 g K2HPO4; 0.5 g MgSO4·7H2O; 74.0 mg CaCl2·2H2O; 12.0 mg ferric acid citrate; 6.6 mg ZnSO4·7H2O; 5.0 mg MnSO4; 1.0 mg CoCl2·6H2O; 1.0 mg CuSO4·5H2O; and 0.1 mg thiamine hydrochloride [Citation[12]].
Treatment Procedures
The EFB were thoroughly washed to make them dust–free and then dried [Citation[13]]. After that, these were cut into 5–10 mm size. Treatment was carried out in duplicate by using 500 ml Erlenmeyer flask incorporating the fermentation medium contained 28% (w/w) of EFB, 2% (w/w) of wheat flour, 5% (v/w) of inoculum, 35% (v/w) of distilled water and 30% (v/w) of mineral solution. The samples were incubated at 37°C for 15 days. The sampling was done at an interval of 3, 6, 9, 12 and 15 days of treatment. The fermented sample for each sampling was mixed with 100 ml distilled water by shaking in a rotary shaker for 2 hours at room temperature (30±2°C) to extract the products, and the extracted mixture was filtered and collected for analysis such as enzyme activity assay, reducing sugar and glucosamine estimation.
Data Analysis
Ligninase assay was carried out by the method suggested by Tien and Kirk [Citation[14]]. The standard activity of ligninase was measured on the basis of the oxidation reaction of Veratryl alcohol in the presence of hydrogen peroxide. A 600 µL Veratryl alcohol solution (10 mM) was mixed with 1.50 ml of distilled water, 50 µL of LiP sample solution, and 600 µL of pH 2.5 tartrate buffer (0.25 M). The absorbance of the product, veratraldehyde, at 310 nm was measured for 1 min after the addition of 240 µL of 5 mM H2O2. The molar extinction coefficient of veratraldehyde is 9300 M−1 cm−1. One unit of enzyme activity is defined as the amount of enzyme generating 1 µmol of veratraldehyde per minute under the described reaction conditions. Determination of glucosamine as the growth indicator was based on the method suggested in Zheng and Shetty [Citation[15]]. Reducing sugar was measured according to dinitrosalicylic acid (DNS) method suggested by Miller [Citation[16]]. The data were the average of three replications.
Optimization Study
The traditional method of optimization “one-factor-at-a-time” technique was used in this study. This method is determined by varying one factor while keeping the other factors at a constant level. The effect of inoculum size, co-substrate dosage and total moisture content were studied for optimum enzyme production.
RESULTS AND DISCUSSION
Production of Ligninase by Solid State Bioconversion
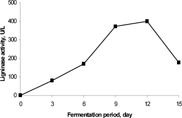
The production of ligninase was carried out in a solid state bioconversion system using oil palm empty fruit bunches (EFB) as solid substrate. shows the ligninase activity (LiP) was successfully detected. The ligninase activity was observed as increased with the fermentation time, reached the maximum at day 12 and decreased at the end of the fermentation. The highest ligninase activity of 400.27 U/liter appeared at day 12 of fermentation. Fujian [Citation[17]] had studied the highest Ligninase activity, which appeared at 365.12 U/liter for 5 days fermentation using steam-exploded straw as substrate.
Figure 1 Production of ligninase (LiP) from oil palm empty fruit bunches (EFB) by P. chrysosporium in solid state bioconversion.
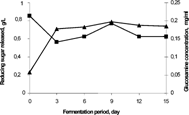
shows glucosamine concentration and reducing sugar released during 15 days of fermentation. Glucosamine indicated the fungal growth in the bioconversion process as increasing trend (batch growth curve) while reducing sugar showed substrate conversion to simple sugar as the decreasing trend. This result was supported with the result of glucosamine (biomass indicator) and reducing sugar released, which is proportional with the enzyme activity. At day 0, it was found that the reducing sugar was higher due to the presence of wheat flour in the fermentation medium that had not been fully utilized. After that, reducing sugar was decreased up to 3 days of treatment as the co-substrate was utilized by microbe. After 3 days of treatment, it was increased due to the production of ligninase enzyme by the P. chrysosporium which might enhance the biodegradation of lignocellulosic components into simple sugar i.e. reducing sugar. Finally the microbe utilized the simple sugar to enhance the growth as well as production and sugar was decreased again in the process. From the previous study, the presence of inhibiting factor (phenols) that retarded the ligninase activity in the culture extracts had been identified [Citation[18]]. According to Del Pilar Castillo [Citation[18]], during fermentation these phenols (inhibiting factor) might be consumed by the fungus, since the inhibition of ligninase activity decreases progressively, and finally disappears in the process. In correlation with the result obtained in this study, the highest ligninase activity at day 12 might be obtained after the inhibiting factors had been consumed by the fungus, which reached the maximum growth at day 9.
Effect of Inoculum Size
The effect of inoculum sizes 5-25% (v/w) on enzyme production is shown in . In general, ligninase activity increased with fermentation time and decreased at the end of fermentation, but in some cases, the ligninase activity was observed increased back at day 15 for 10% and 25% inoculum (). The highest ligninase activity of 578.71 U/liter was obtained from 10% inoculum at day 9 while 25% inoculum showed a very poor ligninase activity for same fermentation days. It indicates increase of inoculum size, which does not mean the enzyme activity will increase too [Citation[19]]. With the increase in mycelial mass, the production of enzyme declined due to the exhaustion of nutrients in the fermentation medium.
Effect of Co-substrate (Wheat Flour)
It is noted that lignin in wood cannot be degraded by white rot fungi unless a more easily metabolizable carbon source is used. In fact, the presence of additional carbon sources such as wheat flour in fermentation medium can enhance the enzyme production [Citation[10]]. shows the effect of co-substrate on the ligninase activity. Fermentation medium containing 1% wheat flour had shown the highest ligninase activity of 721.78 U/liter obtained at day 12. In fact, for the whole fermentation time, except at day 3 and 6, ligninase activity of 1% wheat flour appeared higher than other fermentation mediums. Meanwhile, 5% wheat flour had shown a very poor ligninase activity for the whole fermentation period. The previous study showed that addition of carbon source at concentration above 1% w/w level has lead to a significant reduction in enzyme synthesis [Citation[10]].
Effect of Moisture
As the moisture content of the medium has a critical importance to SSB, the effect of moisture content in the culture on the production enzyme was studied. shows the effect of total moisture content on ligninase activity for 50%, 60%, 70% and 80% moisture. The result indicated that 50% moisture gave the higher ligninase activity during fermentation compared to other treatments. The maximum value of ligninase activity of 1472.5 U/liter was obtained from 50% moisture at day 15. The results from the previous study stated that the ideal moisture content was 70% and the reduction in enzyme yield could occur with low moisture content [Citation[20]].
Product Yield from Biomass
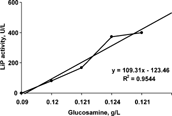
The product yield from biomass was measured to evaluate the treatment performance through biomass production by P. chrysosporium in solid state bioconversion. shows the product yield (ligninase) from biomass, which is considered as the glucosamine concentrations. The results showed the enzyme production to be 109.3 U/g, indicating that 1 gram (g) of glucosamine (biomass) is required to produce 109.3 U of lignin peroxidase (ligninase enzyme).
CONCLUSION
The study showed the ligninase activity of 400 U/liter appeared at day 12 of fermentation period in the solid state bioconversion process. The higher ligninase production were obtained with the optimum process condition at inoculum size of 10%, co-substrate (wheat flour) concentration of 1% and total moisture content of 50%. Overall, the study proves that the lignocellulosic waste EFB has a good potential to be used as solid substrate in SSB system for production of ligninase (LiP) using the white rot fungus P. chrysosporium.
The authors are grateful to the Research Center (RC), International Islamic University Malaysia (IIUM), for their support by approving a research grant (LT 33)
REFERENCES
- Malaysian Palm Oil Board (MPOB). (2003). Latest development and commercialization of oil palm biomass. In MPOB-FMM Seminar on Business Opportunity in Oil Palm Biomass, 21 August, Selangor, Malaysia.
- Jaafar, M., Sukaimi, J. (2001). The future of palm oil in the new millennium in Malaysia. Burotrop Bulletin No. 16.
- Higuchi, T. (1985). Biosynthesis of lignin, in Biosynthesis and Biodegradation of Wood Components, T. Higuchi, Ed., Academic Press: Orlando, Florida, pp. 141–160.
- Cruz-Cordova, T., Roldan-Carrillo, D., Diaz-Cervantes, D., Ortega-Lopez, J., Saucedo-Castanedia, G., Tosmasini-Campocosia, A., Rodriguez-Vazquez, R. (1999). CO2 evolution and ligninolytic and proteolytic activities of Phanerochaete chrysosporium grown in solid state fermentation. Resources, Conservation and Recycling 27: 3–7. [CSA], [CROSSREF]
- Dorado, J., Almendros, G., Camarero, S., Martinez, A.T., Vares, T., Hatakka, A. (1999). Transformation of wheat straw in the course of solid-state fermentation by four ligninolytic basidiomycetes. Enzyme Microb. Technol. 25: 605–612. [CSA], [CROSSREF]
- Pandey, A. (1994). Solid State Fermentation: An overview, in Solid State Fermentation, Wiley Eastern: , New Delhi.
- Deraman, M. (1993). Carbon pellets prepared from fibers of oil palm empty fruit bunches: A quantitative X-ray diffraction analysis, PORIM Bull., Palm Oil Res. Inst., Malaysia, No. 26.
- Umikalsom, M.S., Arif, A.B., Zulkifli, H.S., Tong, C.C., Hassan, M.A., Karim, M.I.A. (1997). The treatment of oil palm empty fruit bunch for subsequent use as substrate for cellulase production by Chaetomium globosum kunze. Bioreour. Technol. 62: 1–9. [CSA], [CROSSREF]
- Alam, M.Z., Mahamat, M.E., Muhammad, N. (2005). Production of cellulase from oil palm biomass as substrate by solid state bioconversion. American Journal of Applied Sciences 2: 569–572. [CSA]
- Krishna, C. (1996). Microbial enzyme production utilizing banana wastes. Ph.D thesis, Cochin University of Science and Technology, India.
- Alam, M.Z., Fakhru'l-Razi, A., Abd-Aziz, S., Molla, A.H. (2003). Optimization of compatible mixed cultures for liquid state bioconversion of municipal wastewater sludge. Water, Air and Soil Pollution 149: 113–126. [CSA], [CROSSREF]
- Erikson, K.E., Grunewald, A., Vallander, L. (1980). Studies of growth condition in wood for three white-rot fungi and their cellulose less mutants. Biotechnol. Bioeng. XXII: 363–376. [CSA], [CROSSREF]
- Ghosh, V.K., Deb, J.K. (1988). Production and characterization of xylanase form Thielaviopsis basicola. Applied Microbiol. Biotechnol. 29: 44–47. [CSA], [CROSSREF]
- Tien, M., Kirk, T.K. (1984). Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H2O2 requiring oxygenase. Proc Nat Acad Sci USA 81: 2280–2284. [CSA]
- Zheng, Z., Shetty, K. (1998). Solid state production of beneficial fungi on apple processing wastes using glucosamine as the indicator of growth. Journal of Agricultural and Food Chemistry 46: 783–787. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Miller, G.L. (1959). Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31: 426–428. [CSA], [CROSSREF]
- Fujian, X., Hongzhang, C., Zuohu, L. (2001). Solid state production of lignin peroxidase (LiP) and manganese peroxidase (MnP) by Phanerochaete chrysosporium using steam exploded straw as substrate. Bioresour. Technol. 80: 141–151. [CSA], [CROSSREF]
- Del Pilar Castillo, M. (1997). Degradation of pesticides by Phanerochaete chrysosporium in solid substrate fermentation. Department of Microbiology, Swedish University of Agricultural Sciences.
- Toshiko, K., Mari, T., Ishii, T., Itoth, Y., Kirimura, K., Usami, S. (1989). Production of lipases by Rhizopus Oligosporous. A newly isolated fungus. J Hokoku Waseda 50: 61–65. [CSA]
- Lonsane, B.K., Ghidyal, N.P., Budiatman, S., Ramakrishna, S.V. (1985). Engineering aspects of solid state fermentation. Enzyme Microb. Technol. 7: 258–265. [CSA], [CROSSREF]
![Figure 3 Effect of inoculum size on ligninase production. 5% inoculum (▴), 10% inoculum (▪), 15% inoculum (♦) and 25% inoculum (•). [Co-substrate: 2% w/w; moisture content: 70%].](/cms/asset/7d26ddf2-92ab-4a88-8b73-974d5cbc8a6a/ianb19_a_129014_f0003_b.gif)
![Figure 4 Effect of co-substrate (wheat flour) on LiP activity. 1% wheat flour (▪), 2% wheat flour (♦), 3% wheat flour (▴) and 5% wheat flour (•). [Inoculum size: 5% v/w; moisture content: 70%].](/cms/asset/756661c9-27a1-4909-bc6f-930d14d51331/ianb19_a_129014_f0004_b.gif)
![Figure 5 Variation of moisture content on ligninase activity. 50% moisture (▪), 60% moisture (♦), 70% moisture (▴) and 80% moisture (•). [Inoculum size: 5% v/w; Co-substrate: 2% w/w].](/cms/asset/bc81fbdd-00cd-4d7a-880b-6b6735806c3e/ianb19_a_129014_f0005_b.gif)