Abstract
This study was designed to determine the ability of human umbilical vein endothelial cells (HUVEC) in dermal equivalent (DE) to form microvessel-like tubes after transplantation into normal rats. A mixture of rat fibroblasts and HUVEC was inosculated into collagen-chitosan sponges to prepare endothelialized dermal equivalents (EDE). After culture in vitro for 24 hours, inosculated cells dispersed throughout the sponges and the equivalents were transplanted subcutaneously into the back of normal Lewis rats. Anti-human specific CD31 antibody was used for immunohistochemical localization of human endothelial cells in sections of EDE excised from rats after grafting. HUVEC in EDE organized into microvessel-like tubes at the end of the first week after transplantation, which still persisted after two weeks. The host microvessels began to pervade both DE and EDE during the second week after transplantation.
These results demonstrated that HUVEC in EDE was able to persist and form microvessel-like tubes after transplantation into normal rats, and this is the first time to transplant DE containing HUVEC into normal rats.
INTRODUCTION
Skin is one of the first generation of tissue-engineered organs that has been successfully developed in the laboratory and clinically applied [Citation[1-5]]. Recent advances in tissue engineering have made cultured skin (CS) a viable option for the treatment of acute and chronic skin wounds due to burns and nagging ulcers [Citation[6-9]]. But clinical performance of currently available human skin equivalents lacking intrinsic vasculature is limited by slow vascularization in the initial phase after transplantation. Autologous split-thickness skin grafts containing intrinsic vasculature can become perfused in several days by inosculation of preexisting graft vessels with those of the recipient, but avascular CS must get perfused entirely by neovascularization from the wound bed, which requires 14 days or more under ideal circumstances [Citation[10], Citation[11]]. During this time the oxygen and nutrients provision of the graft is entirely dependent on diffusion, which is often not enough [Citation[10], Citation[12]]. CS may become ischemic and nutrient-deprived after grafting, thus increasing the rate of graft failure. For example, early clinical failures in the use of Integra were due to slower than expected vascularization of this material. Although this problem was solved later, two operations were required [Citation[13]]. In other clinical experiments, the problem of slow vascularization has been addressed by giving extensive nutrients and using antimicrobial dressing fluids after grafting [Citation[12], Citation[14]], which is labor-intensive and may add mechanical stresses to the grafts.
Various strategies have been explored to accelerate vascularization of CS. For example, scaffolds containing angiogenic factors including vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) could enhance angiogenesis of CS after transplantation [Citation[15-19]]. Genetic modification of cells in CS was also attempted to accelerate the rate of graft vascularization [Citation[20], Citation[21]]. For instance, seeding human acellular dermis grafts with human umbilical vein endothelial cells (HUVEC) transduced with the survival gene Bcl-2 accelerated the rate of graft vascularization [Citation[22]]. Another way to accelerate CS vascularization is constructing CS containing microvascular networks formed prior to implantation. In 1998, co-culture of HUVEC with dermal fibroblasts in a collagen biopolymer promoted the spontaneous formation of a capillary-like network by HUVEC in vitro [Citation[23]]. In another study CS containing human keratinocytes, fibroblasts, and human dermal microvascular endothelial cells (HDMEC) were grafted to athymic mice and formed vascular analogues in vivo [Citation[24]]. Although these various strategies for vascularization of CS have been proved effective in vitro or in immunodeficient animal models, there is no report whether HUVEC in endothelialized dermal equivalents (EDE) could form microvessel-like tubes after being transplanted into a normal host.
In this study, a mixture of rat fibroblasts and HUVEC were inosculated into collagen-chitosan sponges to prepare EDE. Then the EDE was transplanted subcutaneously into the back of immuncompetent Lewis rats. Immunosuppression was obtained with cyclosporin-A. Collagen-chitosan matrices were chosen for scaffold making, as their structure allowed fibroblasts and endothelial cells to migrate [Citation[25-27]]. Tissue-engineered organs have relied on natural and synthetic biodegradable matrices [Citation[28-30]] to provide a scaffolding effect and mechanical strength. Natural materials including collagen, Matrigel, and alginates have been used as scaffolds [Citation[31-33]]. Since these materials compose the naturally occurring extracellular matrix, it seems reasonable to use them as the basis for engineered tissues. These matrices function as temporary scaffolds that maintain transplanted cells in a defined space and therefore guide new tissue growth and organization. Endothelial cells were isolated from human umbilical veins, which is a discarded and therefore widely available tissue source. Grafts were excised from rats for histological and immunohistochemistrical examination. The results demonstrated that HUVEC in EDE formed microvessel-like tubes after transplantation.
MATERIALS AND METHODS
Cell Isolation and Culture
Animal studies were performed with the approval of the Institutional Animal Care and Use Committee. Dermal fibroblasts were isolated from neonatal Lewis rat skins according to the methods described by Auger et al. [Citation[34]]. Briefly, the skin was cut into 2 cm × 0.25 cm strips, and the dermis and epidermis were enzymatically separated by incubation with 0.025 g/ml dispase (Gibco) for 2.75 hours. The dermal tissue strips were minced and incubated for 1 hour with 0.025 g/ml collagenase I (Gibco) for fibroblast isolation. The digested dermal tissue was centrifuged at 1000 × g for 10 minutes and the fibroblasts were inoculated in flasks containing Dulbecco's modified Eagle's medium (DMEM, Sigma). HUVEC was obtained from healthy newborns by the method of Jaffe et al. [Citation[35]]. Briefly, human umbilical cords were collected in ice-cold culture media, veins were cannulated at both ends, washed with PBS and infected with a warm collagenase I solution (0.01 g/ml, Gibco). The cord was placed in HEPES with 5 mM CaCl2 at 37°C. After 15 minutes incubation, the veins were gently massaged and vigorously perfused with M199 medium (Sigma). The isolated endothelial cells were centrifuged and suspended in M199 medium supplemented with 20% fetal bovine serum (FBS, Sigma), 2 mM L-glutamine, 10 ng/ml VEGF (Sigma) and 100 U/ml of penicillin G, and then plated into flasks. The endothelial nature of these cells was confirmed by von Willebrand factor expression [Citation[36], Citation[37]]. The culture medium was changed three times a week and the cells were used at passages three or four.
Collagen/chitosan Sponge Preparation
Collagen (0.6%, w/v)/chitosan (0.06%, w/v) sponges were prepared according to published protocols [Citation[38], Citation[39]]. Briefly, 428 g of 1.4% bovine collagen was dispersed in 900 ml of 0.05 M acetic acid solution by blending at 2000 × g for 90 minutes at 4°C in a refrigerated homogenizer. The collagen/chitosan copolymer was formed by adding 100 ml of 0.6% (w/v) chitosan solution (95% deacetylated) to the collagen dispersion, and then blended for additional 90 minutes. The collagen/chitosan dispersion was degassed by centrifugation at 2000 × g for 20 minutes. The final solution was poured into 12-well plates, frozen overnight at − 70°C and lyophilized. Collagen/chitosan matrices of 50 mg dry weight were incubated in 20 ml 40% (v/v) ethanol containing 50 mM 2-morpholinoethane sulfonic acid (MES, pH 5.5) for 30 minutes at room temperature. Matrices were subsequently immersed in 20 ml 40% (v/v) ethanol containing 50 mM MES (pH 5.5), 24 mM 1-ethyl-3(3-dimethyl amino-propyl) carbodiimide, 5 mM N-hydroxysuccinimide and 0.2% (w/v) chondroitin sulfate. After reaction for 4 h, the matrices were washed twice in 0.1 M Na2HPO4 (pH 9.1) for 2 h, twice in 1 M NaCl for 2 h, in 2 M NaCl for 1 day (with 6 changes of washing solution), followed by washing with distilled water and lyophilization.
DE, EDE Preparation
For EDE preparing, collagen/chitosan sponges were inosculated with a suspension of 2 × 107 fibroblasts/cm2 mixed with 2 × 107 HUVEC/cm2 in a 1:1 mixture of fibroblast growth medium and endothelial cell growth medium supplemented with 10 ng/ml vascular endothelial cell growth factor. For dermal equivalents (DE) preparing, collagen/chitosan sponges were inosculated with a suspension of 2 × 107 fibroblasts/cm2 in fibroblast growth medium. All equivalents were cultured at 37°C, 5% CO2. A total of 80 equivalents were produced. After 24 hours in culture, EDE and DE were collected either for light and scanning electron microscopic observation or for transplantation into rats.
Light and Scanning Electron Microscopy of Cultured Equivalents
Histological characterization of equivalents was studied by H + E staining and neutral red staining. For H + E staining, harvested equivalents were snap-frozen in liquid nitrogen and then placed in OCT compound. Sections of 5 µmin thickness were cut and fixed in paraformaldehyde. Frozen sections were stained with H + E using standard procedures [Citation[25], Citation[40]]. For neutral red staining, harvested equivalents were directly stained with neutral red and observed with an XDS-1B light microscope.
Cellular morphology of equivalents was examined using the way of George [Citation[41]]. Briefly, harvested equivalents were fixed in a 3% glutaraldehyde/4% paraformaldehyde solution, postfixed with a 1% osmium tetroxide solution, dehydrated with increasing concentrations of ethanol, then dried with increasing concentrations of acetonitrile. Samples were sputter coated with a thin layer of gold-palladium and viewed with a JSM 6700 F NT scanning electron microscope.
Transplantation of Equivalents
Equivalents were transplanted into 6- to 8-week-old male normal Lewis rats. Animals were anaesthetized with ether. Transplantation sites on the back of rats were prepared by removing all visible fur with scissors. A 1.5 cm incision to the level of fascia was made on the lateral back. A subcutaneous pocket was created by blunt dissection, into which the equivalent was then placed. The rat skin was closed with sterilized surgical needles. These rats were kept in sterile conditions and fed antibiotics during the study period.
Immunosuppression was obtained with cyclosporin-A (60 mg/kg daily) [Citation[42]]. Rats were killed day 3, day 5, week 1 and 2 after surgery (n = 5 per group per time point) after transplantation. Graft biopsies were collected at death for histological and immunohistochemistrical analyses.
Histology and Immunohistochemistry of Transplanted Equivalents
Harvested equivalents were snap-frozen in liquid nitrogen and then placed in OCT compound. For histology, sections of 4 µm in thickness were cut, fixed in paraformaldehyde and then stained with H + E. For immunohistochemistry, the specimen was cut into 10 µm sections. Cryostat sections were fixed in cold acetone, rinsed twice with PBS, blocked using 3% hydrogen peroxide in PBS for 12 minutes, washed three times with PBS and incubated for 20 minutes at room temperature with 1% normal goat serum. After incubation overnight with polyclonal mouse anti-human CD31 antibody (Zymed) at 4°C, sections were incubated with biotinylated anti-mouse IgG (1/100 dilution) (Zymed) for 30 minutes, followed by incubation with streptavidin-HRP (Zymed). For antigen-antibody complexes detecting, sections were incubated with 3, 3-diaminobenzamine (Sigma) at room temperature for 10 minutes and counterstained with Gil's hematoxylin (Sigma).
RESULTS AND DISCUSSION
Seeded Cells Dispersed through Collagen/chitosan Matrices After 24 Hours Culture In Vitro
The result of H + E and neutral red staining showed that after 24 hours in culture seeded cells dispersed through collagen/chitosan matrices (A), but no microvessel-like tubes formed (B). Scanning electron microscopy of the EDE displayed that seeded sells formed aggregates both on the surface of the sponges (A) and inside the sponges (B). At the same time cell-cell and cell-matrices junctions were observed (B). The results of neutral red staining, H + E staining and scanning electron microscopy of DE revealed no significant histological differences from that of EDE (data not shown) at this time.
Figure 1 Seeded cells dispersed through collagen/chitosan matrices in EDE after 24 hours culture. A) Three-dimensional equivalents stained with neutral red (original magnification × 250). B) Cryostat section stained with H + E, note that no microvessel-like tubes were formed in equivalents at this time (original magnification × 100).
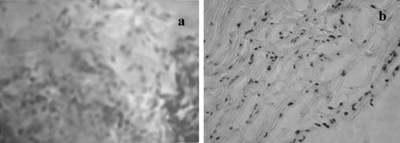
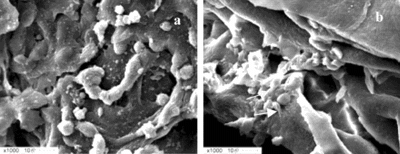
Figure 2 Scanning electron microscopy of equivalents after 24 hours culture. A) Cells formed aggregates on the surface of the sponges. B) Cells formed aggregates inside the sponges. Intercellular junctions (upper arrow) and cell-matrices junctions were seen (lower arrow).
Many in vitro models have been developed to study the formation of capillary tubes by endothelial cells. For example, chitosan/collagen biopolymers were seeded with a suspension of fibroblasts and HUVEC (1:1) to construct CS, and capillary-like tubes formed by HUVEC were clearly visible from days 15 to 31 in culture [Citation[23]]. In a similar study, endothelialized dermis was prepared by coculturing human dermal fibroblasts and HUVEC in collagen/glycosaminoglycan/chitosan biopolymer, and the capillary tube formation is apparent after 10 days of culture [Citation[17]]. In another study HMVEC monolayers were plated and overlayed with human type I collagen followed by a second overlay of collagen with embedded fibroblasts. The fibroblasts stimulated HMVEC to survive and form capillary networks after 4 to 5 days [Citation[43]]. All these studies demonstrated that cultured human endothelial cells could form stable human endothelium-lined, capillary-like structures in living skin equivalents in vitro. On the other hand these models also displayed that the capillary tube formation would appear in no less than 4 to 5 days in culture. In our experiments the equivalents were cultured for only 24 hours before transplantation, so capillary tubes should not be formed at this time, as was confirmed by the result of H + E and neutral red staining.
HUVEC in EDE Formed Microvessel-like Tubes after Transplantation into Normal Rats
For in vivo experiments, equivalents were transplanted into 6- to 8-week-old male normal Lewis rats after 24 hours in culture and retrieved at death. H + E staining and anti-human specific CD31 antibodies were used to detect and determine the identity of microvessel-like tubes in frozen sections of equivalents excised from rats. CD31 (also referred to as platelet endothelial adhesion molecule-l, PECAM-l), a member of the Iglike superfamily, is considered to be a standard marker for the presence of endothelial cells [Citation[44-46]].
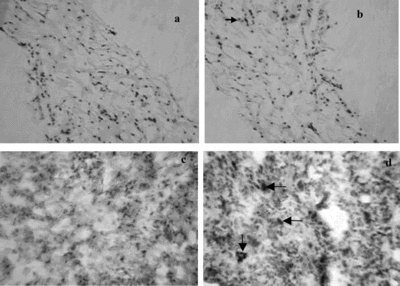
The results of histological and immunohistochemistrical detection showed that no tubes appeared in EDE or DE on day 3 or day 5 after transplantation. At the end of the first week after transplanting, microvessel-like tubes were seen in EDE (B), but not in DE (A). Immunohistochemistrical detection showed that the microvessel-like tubes in EDE were formed completely by HUVEC. Simultaneously, individual cells that had CD31 reactivity were also observed in EDE ( D). No cells in DE had CD31 reactivity (C).
Figure 3 Histology and immunohistochemistry of equivalents at 1 week after grafting into rats. A) H + E staining of DE, no microvessel-like tubes were seen (original magnification × 100). B) H + E staining of EDE, microvessel-like tubes were observed (arrow) (original magnification × 100). C) Immunohistochemistry of DE, no cells had CD31 reactivity (original magnification × 250). D) Immunohistochemistry of EDE, both CD31 positive microvessel-like tubes (vertical arrow) and CD31 positive individual cells (horizontal arrows) were seen (original magnification × 250).
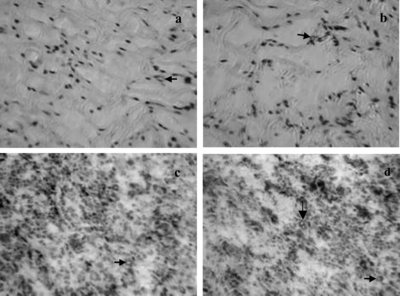
Microvessel-like tubes were seen in both DE (A) and EDE (B) two weeks after transplantation. But immunohistochemistry showed that no microvessel-like tubes in DE had CD31 reactivity (C). Microvessel-like tubes stained or not stained with antihuman CD31 antibody appeared in EDE. Fewer or no individual cells had CD31 reactivity (D), suggesting aggregation of the human endothelial cells into primitive vascular structures and/or loss of individual cells. These data revealed that 1 week after grafting HUVEC in EDE organized into microvessel-like tubes, and these tubes still persisted 2 weeks after grafting. During the second week, the rat microvessels began to pervade both the EDE and the DE.
Figure 4 Histology and immnohitochemistry of equivalents at the second week after grafting into rats. A) H + E staining of DE, microvessel-like tubes were observed (arrow). B) H + E staining of EDE, microvessel-like tubes were observed (arrow). C) CD31 staining of DE, microvessel-like tubes not stained with antihuman CD31 antibody were seen (arrow). D) CD31 staining of EDE, microvessel-like tubes formed by HUVEC in EDE still persisted, and rat microvessel, not stained with antihuman CD31 antibody (horizontal arrows), were seen (original magnification × 250).
A review of the literature shows that there are just a few published works reporting the angiogenesis of endothelialized CS in vivo. Yet the published results of these studies are encouraging, since the aggregates of endothelial cells in vitro could lead to vascular analogues in vivo. For example, CS containing human keratinocytes, fibroblasts, and dermal microvascular endothelial cells (HDMEC) were grafted to athymic mice after 16 days in culture. By the fourth week after grafting, HDMEC were found in linear and circular organizations resembling vascular analogs [Citation[24]]. In another study, human devitalized dermis seeded with Bcl-2-transduced HUVEC were implanted subcutaneously into mice, and become perfused prior to murine neovascularization [Citation[22]]. However, even if these in vivo models have been successful, most of these studies have been done in immunoincompetent animal models. So in our experiments dermal equivalents containing HUVEC were transplanted into normal rats after 24 hours culture in vitro. It's well known that endothelial cells are antigenic, so cyclosporin-A was used to limit immunoreaction. At the end of the first week after transplantation, HUVEC in EDE organized into microvessel-like tubes and these tubes still persisted at the end of the second week after transplantation. It was confirmed that before transplantation there were no microvessel-like tubes formed in EDE, so the HUVEC formed microvessel-like tubes detected in our experiments were formed completely in vivo.
Although HUVEC formed microvessel-like tubes and plenty of individual HUVEC were observed in EDE at 1 week, the density of microvessel-like tubes formed by HUVEC did not have significant improvement by the end of 2 weeks. These data suggested that though a proportion of the HUVEC in EDE formed microvessel-like tubes after transplantation, a great deal of the cells lost over time. Endothelial cell loss by apoptosis in tissue-engineered vascular constructs was also observed by other investigators [Citation[25]], and it is a major limitation in the construction of human synthetic microvessels [Citation[47]]. Some methods have been tried to address this question. For example endothelial cells were genetically modified with Bcl-2 to delay apoptosis and promote survival after grafting [Citation[33]]. Yet still more work needs to be done to address this question.
We used HUVEC to construct EDE. In the study of vascularized skin equivalents construction, the optimal source of endothelial cells remains unresolved. Most investigators used HDMEC and HUVEC [Citation[17], Citation[23], Citation[24], Citation[38]] for this purpose. As endothelial cells are highly antigenic, it is ideal to use autologous cells to limit potential immunoreactivity for clinical use. Although it might be desirable for some applications, the time needed to produce adequate number of EC for perfusing the reconstructed skin prior to grafting precludes the use of this technology for treatment of patients with acute injuries. For example, successful culture of HDMEC usually needs 6 weeks for sufficient expansion of cell numbers [Citation[48]]. Furthermore, in the case of autologous HDMEC, a painful and often scarring secondary procedure is required to harvest adequate numbers of cells from adults. In contrast, HUVEC are isolated from a dispensable and widely available tissue source-the human umbilical vein-thus facilitating the use of HUVEC in preclinical studies. Furthermore, studies related to organ transplantation are elucidating the signals involved in T-cell activation. Consequently, it may be possible to engineer immune acceptance by regulating factors in the costimulatory pathways, such as the binding of CD28 on T cells to B7 ligands on antigen-presenting cells, or creating immunochimerism [Citation[49]].
In this study, it was not determined whether the microvessel-like tubes formed by HUVEC get maturation. During new capillary blood vessels formation, newly formed vessel maturation is accomplished by reconstitution of the basement membrane and by investment with mesenchymal cells such as pericytes and smooth-muscle cells [Citation[50], Citation[51]]. In immunoincompetent animal models, as mentioned above, Bcl-2 transduced HUVEC in human skin equivalents formed vessels after implantation in immunodeficient mice. These vessels acquired several characteristics of mature vessels such as reactivity with antibodies directed against basement membrane components laminin and type IV collagen and investiture with smooth muscle-actin-expressing cells [Citation[22]]. In this primitive study, whether the microvessel-like tubes in EDE formed by HUVEC get maturation was not definitively determined. Additional study is needed to address this question.
To our knowledge, this is the first time to transplant dermal equivalents containing HUVEC into normal rats. Our short-term grafting experiment demonstrated that HUVEC in EDE could form microvessel-like tubes after transplantation. At the same time, the EDE allowed for the ingrowth of the existing rat vasculature. The microvessel-like tubes formed by HUVEC may enhance initial vascularization of EDE after transplantation and so reduce graft failure caused by deficiency of vascular structures in tissue engineered dermal equivalents.
This work was mainly supported by the National High Technology Research and Development Program of China (863 Program) 2002AA326040. The authors thank Mrs. Xiaoli Hu for technical support with the histochemistry work.
REFERENCES
- Rheinwald, J.G., Green, H. (1975). Serial cultivation of stains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell 6: 331–344.
- Bell, E., Sher, S., Hull, B., Merril, M.S., Neveux, Y. (1983). The reconstruction of living skin. J. Invest. Dermatol. 81: 2s–10s.
- Archambault, M., Yaar, M., Gilchrest, B.A. (1995). Keratinocytes and fibroblasts in a human skin equivalent model enhance melanocyte survival and melanin synthesis after ultraviolet irradiation. J. Invest. Dermatol. 104: 859–867.
- Shahabeddin, L., Berthod, F., Damour, O., Collombel, C. (1990). Characterization of skin reconstructed on a chitosan-cross-linked collagen-glycosaminoglycan matrix. Skin Pharmacol. 3: 107–114.
- Cooper, M.L., Hansbrough, J.F. (1991). Use of a composite skin graft composed of cultured human keratinocytes and fibroblasts and a collagen-GAG matrix to cover full-thickness wounds on athymic mice. Surgery 109: 198–207.
- Eaglstein, W.H., Iriondo, M., Laszlo, K. (1995). A composite skin substitute (graftskin) for surgical wounds. A clinical experience. Dermatol. Surg. 21: 839–843.
- Falabella, A.F., Valencia, I.C., Eaglstein, W.H., Schachner, L.A. (2000). Tissue-engineered skin (Apligraf) in the healing of patients with epidermolysis bullosa wounds. Arch. Dermatol. 136: 1225–1230.
- Sheridan, R.L., Morgan, J.R., Cusick, J.L., Petras, L.M., Lydon, M. M., Tompkins, R.G. (2001). Initial experience with a composite autologous skin substitute. Burns 27: 421–424.
- Balasubramani, M., Kumar, T.R., Babu, M. (2001). Skin substitutes a review. Burns 27: 534–544.
- Young, D.M., Greulich, K.M., Weier, H.G. (1996). Species-specific in situ hybridation with fluorochrome-labeled DNA probes to study vascularization of human skin grafts on athymic mice. J. Burn Care Rehabil. 17: 305–310.
- Supp, D.M., Supp, A.P., Bell, S.M., Boyce, S.T. (2000). Enhancd vascularization of cultured skin substitutes genetically modified to overexpress vascular endothelial growth factor. J. Invest. Dermatol. 114: 5–13.
- Boyce, S.T., Goretsky, M.J., Greenhalgh, D.G., Kagan, R.J., Rieman, M.T., Warden, G.D. (1995). Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns. Ann. Surg. 222: 743–752.
- Boyce, S.T., Kagan, R.J., Meyer, N.A., Yakuboff, K.P., Warden, G.D. (1999). The 1999 clinical research award: Cultured skin substitutes combined with Integra Artificial SkinTM to replace native skin autograft and allograft for the closure of excised full-thickness burns. J. Burn Care Rehabil. 20: 453–61.
- Boyce, S.T., Supp, A.P., Harriger, M.D., Greenhalgh, D.G., Warden, G.D. (1995). Topical nutrients promote engraftment and inhibit wound contraction of cultured skin substitutes in athymic mice. J. Invest. Dermatol. 104: 345–349.
- Montesano, R., Vassalli, J.D., Baird, A., Guillemin, R., Orci, L. (1986). Basic fibroblast growth factor induces angiogenesis in vitro. Proc. Natl. Acad. Sci. 83: 7297–7301.
- Atkins, B.Z., Hueman, M.T., Meuchel, J.M., Cottman, M.J., Hutcheson, K.A., Taylor, D.A. (1999). Myogenic cell transplantation improved in vivo regional performance in infarcted rabbit myocardium. J. Heart Lung Transplant. 18: 1173–1180.
- Hudon, V., Berthod, F., Black, A.F., Damour, O., Germain, L., Auger, F. A. (2003). A tissue-engineered endothelialized dermis to study the modulation of angiogenic and angiostatic molecules on capillary-like tube formation in vitro. Br. J. Dermatol. 148: 1094–104.
- Hutmacher, D.W., Garcia, A.J. (2005). Scaffold-based bone engineering by using genetically modified cells. Gene 347(1): 1–10.
- Peirce, S.M., Price, R.J., Skalak, T.C. (2004). Spatial and temporal control of angiogenesis and arterialization using focal application of VEGF164 and Ang-1. Am. J. Physiol Heart Circ. Physiol. 286: 918–925.
- Supp, D.M., Boyce, S.T. (2002). Overexpression of vascular endothelial growth factor accelerates early vascularization and improves healing of genetically modified cultured skin substitutes. J. Burn Care Rehabil. 23: 10–20.
- Schwarz, E.R., Speakman, M.T., Patterson, M., Hale, S.S., Isner, J.M., Kedes, L.H., Kloner, R.A. (2000). Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat: Angiogenesis and angioma formation. J. Am. Coll. Cardiol. 35: 1323–1330.
- Schechner, J.S., Crane, S.K., Wang, F., Szeglin, A.M., Tellides, G., Lorber, M.I., Bothwell, A.L., Pober, J.S. (2003). Engraftment of a vascularized human skin equivalent. FASEB J. 17: 2250–2256.
- Black, A.F., Berthod, F., L'Heureux, N., Germain, L., Auger, F.A. (1998). In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 12: 1331–1340.
- Supp, D.M., Wilson-Landy, K., Boyce, S.T. (2002). Human dermal microvascular endothelial cells form vascular analogs in cultured skin substitutes after grafting to athymic mice. FASEB J. 16: 797–804.
- Jacques, E.N., Martin, C.P., Joan, B.C., Michelle, M.S., Stephanie, L., Mohamed, K.K., Christina, L.A., David, J.M., Peter, J.P. (2001). Engineering and characterization of functional human microvessels in immunodeficient mice. Lab. Invest. 81: 453–463.
- Kim, B.S., Mooney, D.J. (1998). Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 16: 224–230.
- Freyman, T.M., Yannas, I.V., Yokoo, R., Gibson, L.J. (2001). Fibroblast contraction of a collagen-GAG matrix. Biomaterials 22: 2883–2891.
- Guest, J.D., Rao, A., Olson, L., Bunge, M.B., Bunge, R.P. (1997). The ability of human Schwann cell grafts to promote regeneration in the transected nuderat spinal cord. Exp. Neurol. 148: 502–522.
- Chen, G., Sato, T., Ohgushi, H., Ushida, T., Tateishi, T., Tanaka, J. (2005). Culturing of skin fibroblasts in a thin PLGA-collagen hybrid mesh. Biomaterials 26(15): 2559–66.
- Cassell, O.C., Morrison, W.A., Messina, A., Penington, A.J., Thompson, E.W., Stevens, G.W., Perera, J.M., Kleinman, H.K., Hurley, J.V., Romeo, R., Knight, K.R. (2001). The influence of extracellular matrix on the generation of vascularized, engineered, transplantable tissue. Ann. N. Y. Acad. Sc. 944: 429–442.
- Marijnissen, W.J., van Osch, G.J., Aigner, J., van der Veen, S.W., Hollander, A.P., Verwoerd-Verhoef, H.L., Verhaar, J.A. (2002). Alginate as achondrocyte-delivery substance in combination with a nonwoven scaffold for cartilage tissue engineering. Biomaterials 23: 1511–1517.
- Kubota, Y., Kleinman, H.K., Martin, G.R., Lawley, T. (1988). Role of laminin and basement membrane in the morphological differentiation of human endothelial cells in capillary-like structure. J. Cell Biol. 107: 1589–1598.
- Schechner, J.S., Nath A.K., Zheng L., Kluger, M.S., Hughes, C.C., Sierra-Honigmann, M.R., Lorber, M.I., Tellides, G., Kashgarian, M., Bothwell, A.L. (2000). In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc. Natl. Acad. Sci. 97: 9191–9196.
- Auger, F.A., Lopez Valle, C.A., Guignard, R., Tremblay, N., Noel, B., Goulet, F., Germain, L. (1995). Skin equivalents produced by tissue-engineering using human collagens. In Vitro Cell. Dev. Biol. 31: 432–439.
- Jaffe, E.A., Machman, R.L., Bekcer, C.G., Minick, C.R. (1973). Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 52: 2745–2758.
- L'Heureux, N., Germain, L., Labbé, R., Auger, F.A. (1993). In vitro construction of human blood vessel from cultured vascular cells: A morphologic study. J. Vasc. Surg. 17: 499–509.
- Voyta, J.C., Via, D.P., Butterfield, C.E., Zetter, B.R. (1984). Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J. Cell Biol. 99: 2034–2040.
- Pieper, J.S., Hafmans, T., Veerkamp, J.H., van Kuppevelt, T.H. (2000). Development of tailor-made collagen-glycosanminoglycan matrices: EDC/ NHS crosslinking, and ultrastructural aspects. Biomaterials 21: 581–593.
- Damour, O., Gueugniaud, P.Y., Bertin-Maghit, M., Rousselle, P., Berthod, F., Sahuc, F., Collombel, C. (1994). A dermal substrate made of collagen-GAGchitosan for deep burn coverage: First clinical uses. Clin. Mater. 15: 273–276.
- Basadonna, G.P., Auersvald, L., Khuong, C.Q., Zheng, X.X., Kashio, N., Zekzer, D., Minozzo M., Qian, H., Visser, L., Diepstra, A., Lazarovits, A.I., Poppema, S., Strom, T.B., Rothstein, D.M. (1998). Antibody-mediated targeting of CD45 isoforms: A novel immunotherapeutic strategy. Proc. Natl. Acad. Sci. 95(7): 3821–3826.
- Pins, G.D., Toner, M., Morgan, J.R. (2000). Microfabrication of an analog of the basal lamina: Biocompatible membranes with complex topographies. FASEB J. 14(3): 593–602.
- Scorsin, M., Hagege, A., Vilquin, J.T., Fiszman, M., Marotte, F., Samuel, J.L., Rappaport, L., Schwartz, K., Menasche, P. (2000). Comparison of the effects of fetal cardiomyocyte and skeletal myoblast transplantation on postinfarction left ventricular function. J. Thorac. Cardiovasc. Surg. 119: 1169–1175.
- Velazquez, O.C., Snyder, R., Liu, Z.J., Fairman, R.M., Herlyn, M. (2002). Fibroblast-dependent differentiation of human microvascular endothelial cells into capillary-like, three-dimensional networks. FASEB J. 16(10): 1316–8.
- Albelda, S.M., Muller, W.A., Buck, C.A., Newman, P.J. (1991). Molecular and cellular properties of PECAM-1 (endoCAM/CD31): A novel vascular cell-cell adhesion molecule. J. Cell Bio. 114(5): 1059–1068.
- Newman, P.J., Berndt, M.C., Gorski, J., White, G.C., 2nd., Lyman, S., Paddock, C., Muller, W.A. (1990). PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science 247: 1219–22.
- Barbera-Guillem, E., Nyhus, J.K., Wolford, C.C., Friece, C.R., Sampsel, J.W. (2002). Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Research 62: 7042–7049.
- Ilan, N., Mahooti, S., Madri, J.A. (1998). Distinct signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis. J. Cell Sci. 111: 3621–3631.
- Sahota, P.S., Burn, J.L., Heaton, M., Freedlander, E., Suvarna, S.K., Brown, N.J., Mac Neil, S. (2003). Development of a reconstructed human skin model for angiogenesis. Wound Repair Regen. 11: 275–84.
- Bour-Jordan, H., Blueston, J.A. (2002). CD28 function: A balance of costimulatory and regulatory signals. J. Clin. Immunol. 22: 1–7.
- Risau, W. (1997). Mechanisms of angiogenesis. Nature 17: 386 (6626), 671–4.
- Hanahan, D. (1997). Signaling vascular morphogenesis and maintenance. Science 277: 48–50.