Abstract
Neural stem cells (NSCs) were widely used for studying the cell's replacement after transplantation in nervous system because of its specific characteristics. However, Stracing the cells after transplantation was still a problem. In the present study, we isolated and cultured the neural stem cells from the C57BL/6J EGFP transgenic mouse (EGFP mice), and identified the capacity for self-renewal and differentiation into the three CNS lineages (neurons, astrocytes, and oligodendrocytes). Then we transplanted the single neural stem cell into the lesion spinal cord. Expression of GFP and differentiation was evaluated at two weeks post-transplantation. The data showed that these neural stem cells derived from the EGFP mice could maintain transgene expression and could differentiate into the MAP2 positive cells after transplantation into the injured spinal cord. The results suggested that NSC expressing EGFP was a useful marker for tracing the cells after transplantation in vivo and functional in the treatment to spinal cord injury.
1. INTRODUCTION
Neural stem cells (NSCs) were immature, multipotent cells characterized by their capacity for self-renewal and capacity to differentiate into the three CNS lineages (neurons, astrocytes, and oligodendrocytes). Numerous recent reports had demonstrated the high degree of plasticity these cells possess, specifically in the setting of neuronal transplantation [Citation[1], Citation[2]].
The common marker for the NSCs in the early embryonic stages was nestin [Citation[3]]. Nestin-positive NSCs could be isolated from the embryonic brain and multiplied in vitro by supplementing serum-free medium with epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF). The response of NSCs to these growth factors seemed dependent on the age and on the region of their origin [Citation[4]]. In EGF/bFGF supplement medium the proliferating NSCs from floating aggregates, namely neurospheres. By transferring the NSCs to an EGF/bFGF-free medium on a laminin substrate they adhered and spontaneously differentiated into neurons, astrocytes, and oligodendrocytes. In many studies, serum had been used to enhance this differentiated process [Citation[5]].
Transplantation of neural precursor cells was a promising therapeutic strategy for the treatment of CNS injuries and neurodegenerative disorders [Citation[6]]. Transplanted cells offered a number of possible therapeutic uses, including delivery of therapeutic factors to provide trophic support or missing gene products, mobilization of endogenous NSCs and replacement of lost or dysfunctional cells [Citation[7]]. Traumatic spinal cord injury could specifically benefit from the engraftment of NSCs. Transplanted cells may remyelinate denuded axons, decrease glia scar formation, prevent secondary cell loss, promote regeneration, and replace neural cells. Nevertheless, previous reports suggested that NSCs existing in the adult rat spinal cord proliferate and differentiate exclusively into astrocytes, but not into neurons [Citation[8-12]].
The EGFP transgenic mouse was a kind of transgenic mouse line with an “enhanced green fluorescence protein” (EGFP) cDNA under the control of a chicken beta-chain promoter and cytomegalovirus enhancer. All of the tissue from this transgenic mouse, with the exception of erythrocytes and hair, was green under excitation light [Citation[13]].
In this study we had isolated and cultured NSCs from embryonic 57BL/6J EGFP transgenic mouse embryos (E14), and investigated differentiation of mouse embryonic NSCs progeny in vitro. After transplantation into the leisured spinal cord, we could estimate whether NSCs could differentiate into neuron in vivo.
2. EXPERIMENTAL PROCEDURE
2.1. Isolation and Culture of NSCs
Hippocampus was removed from C57BL/6 E-GFP transgenic mouse embryos (E14) and digested with pre-warm 0.025% trypsin-EDTA. Cells were plated at density of 2 × 105 cells/ml into 10 cm culture dishes with no substrate pretreatment. The culture medium was composed of DMEM-12 (InvitrogenTM, USA), including 1% N2 supplement, 20 ng/mlEGF (InvitrogenTM, USA) and 20 ng/ml bFGF (InvitrogenTM, USA). From the 2nd day of plating, the cultures started to form neurospheres. Half of the medium was changed every 3 days and cells were passaged every 7 days by mechanical dissociation.
2.2. Differentiation of Neurospheres
Neurospheres of passage 4 ∼ 6 were dissociated mechanically into single cell suspension, and then were plated at 5 × 105 cells/ml onto poly-L-lysine (0.25 mg/ml) coated coverslips in a 6-well plate to differentiate. The cells were differentiated in DMEM/F12 medium containing 1% fetal bovine serum (FBS) without EGF and bFGF. The cells were fixed for immunofluorescence staining after 7 days culture. The phenotype of differentiated NSCs was determined by staining with monoclonal antibodies to glial fibrillary acidic protein (GFAP) for astroglia lineage, 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) for oligodendrocyte lineage, and microtubule associated protein-2 (MAP2) for neuronal lineage.
2.3. Immunofluorescence
The cells were processed for immunofluorescence staining after fixing. Coverslips were fixed in 4% paraformaldehyde (in phosphate-buffered saline, PBS, PH 7.2) for 30 minutes followed by 3 (10 minutes each) washes in PBS (PH 7.2). Cells were incubated in 10% nomal goat serum/PBS 1 hour for blocking non-specific reaction, and then incubated with primary antibody solutions overnight at 4°C. The following primary antibodies were used: mouse monoclonal Nestin (1:200 dilution, Chemicon), mouse polyclonal GFAP (1:200 dilution, Chemicon), mouse monoclonal CNPase (1:200 dilution, Chemicon), and mouse monoclonal MAP2 (1:200 dilution, Chemicon). Coverslips were washed in PBS 3 times (10 minutes each). Appropriate secondary antibodies (Rabbit ati-mouse Cy3, Chemicon) were added and incubated for 1 hour at RT. Cells were rinsed with PBS (3 times, 10 minutes each), placed on glass slides, and covered with mounting medium. Fluorescence was detected and photographed on a Leica DMRXA2 photomicroscope. The software for this system was Leica DC500 and IM500.
2.4. Cell Counting and Analysis
Five coverslips labeled of every antibody were used for statistical analysis. Five photos of each coverslip were taken using a 40 × Plan-Neofluor objective with a 1.0 numerical aperture. The number of immunoreactive cells in each photo was counted and compared with the total number of green cells found in the same photos. Data were expressed as mean (%)±S.E.
2.5. Transplantation of NSCs into the Injured Spinal Cord
Adult wild type C57BL/6J male mice approximately 2 months old and weighing 25–30 g (n = 12) were used in this study. Prior to surgery, mice were anaesthetized with 2% pentobarbital sodium intraperitoneally and fixed on a surgical table. Under aseptic conditions, a midline skin incision was made on the dorsal side and the spinal columm was exposed from the T8 to T12 levels. The lamina of T9 and T11 were carefully removed using microroneurs. Microscissor cuts were created at the lateral half spinal cord, and then the NSCs derived from the hipocampus of GFP transgenic mice were transplanted into the injured site using 10 µl microinjector. One-2 µl injections of DMEM/F12 media containing 1 × 105 single cell were delivered to the injured site of normal wild type mice. As a control, PBS was also injected. After two weeks, spinal cords were sectioned at 5 µm on a cryostat. Sections were processed with primary antibodies mouse monoclonal MAP2 overnight at room temperature, followed by reaction with species-specific IgG conjugated to Cy3 (1:150) for 1 h. Micrographs of sections were taken by Leica DC500 and IM500.
3. RESULTS
3.1. Identification of EGFP-NSCs In Vitro
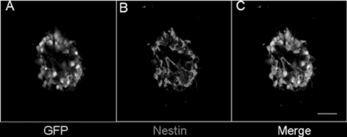
The cells were isolated from the hippocampus and cultured in the media supplemented with EGF; these neurospheres expressed EGFP, exhibiting green color under excitation light (A) and maintained undifferentiated state characterized by nestin immunoreactivity (Nestin, a marker of NSCs, B). Cells could be maintained in this state for up to one year in culture, generating a large number of cells, without losing their proliferate and multilineage potential. In culture of EGFP embryonic hippocampus, we found that EGF includes the proliferation of a single precursor cell, which gave rise to form neurospheres of undifferentiated cells that could generate neurons and glia.
3.2. Differentiation and Characterization of NSCs In Vitro
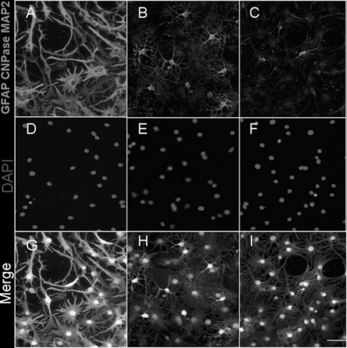
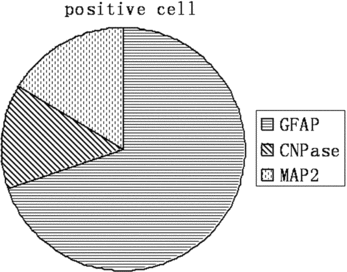
To examine the differentiation ability of EGFP NSCs in vitro, the NSCs were cultured in the medium containing 1% FBS without EGF and bFGF for 7 days. These cells after differentiation were stained with the astrocyte marker GFAP (A), the oligodendrocyte marker CNPase (B), and the neuron marker MAP2 (C). Quantification of these 3 cellular phenotypes showed 71.6% GFAP positive cells, 13.4% CNPase positive cells, and 16.5% MAP2 positive cells (; ). This data was consistent with the previous reports, and suggested that these EGFP expressing NSCs could be used as a marker for tracing the cell's fate after transplantation.
Table 1. Percentage of the glia and neuron cells after 7-day differentiation of EGFP NSCs in vitro
3.3. Transplantation of NSCs into the Spinal Cord In Vivo
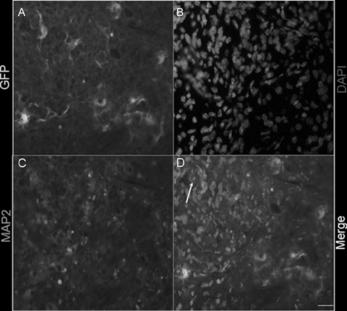
In order to test the characteristics of EGFP-NSCs in vivo, we further transplanted them into the injured spinal cord. Two weeks after grafting, EGFP positive cells were found in the spinal cord. These EGFP NSCs (A) could differentiate into MAP-2 positive cells (C). This demonstrated that EGFP positive cells could be easily identified by differentiation to neuron and become a useful marker for the grafting cells.
4. DISCUSSION
In the present study, we isolated and cultured the neural stem cells from the C57BL/6J EGFP transgenic mouse (EGFP mice), and identified the capacity for self-renewal and differentiation into the three CNS lineages (neurons, astrocytes, and oligodendrocytes). The data showed that these neural stem cells derived from the EGFP mice could differentiate into the MAP2 positive cells after transplantation into the leisured spinal cord. The results suggested that NSC expressing EGFP was functional after transplantation into the injured spinal cord in vivo and could become a useful marker for tracing the cells after transplantation.
In recent years, the neural stem cells could be obtained from the different parts of the brain both in the embryos and adults. These cells could be expanded in vitro with EGF and bFGF, and had the capacity to differentiate into neurons, astrocytes, and oligodendrocytes, which provided a great hope for treatment of the CNS degenerative diseases. Lots of studies had shown that these neural stem cells could be isolated from normal mice brain and passaged for more than 10 times. In our study, we isolated the NSCs from the C57BL/6J EGFP transgenic mouse. We demonstrated that the NSCs derived from the EGFP transgenic mouse were green under the 488 nm irritation wave. In addition, the EGFP expressing NSCs had the capacity for self-renewal and differentiation into the three CNS lineages. This suggested that the EGFP expressing NSCs could be used as a marker for tracing the cell's fate after transplantation in vivo.
The C57BL/6J EGFP transgenic mouse was generated by Takada in 1997 [Citation[14]]. Because all of the tissue from this transgenic mouse was green under excitation light, many kinds of cells isolated from this mouse were commonly used for cell tracing in vitro and in vivo. For example, the single bone marrow cells (BMCs) and hematopoietic stem cells or marrow stromal cells were injected into the ischemic brain and lesioned site, and used to promote functional recovery [Citation[15], Citation[16]]. Someone had transplanted the neural spheres derived from the EGFP mice brain into the eyes of B6 mice, and found that these cells could differentiate into the neuron and repair the injured eye.
Developing effective protocols for the transplantation of NSCs was a promising therapeutic strategy for repairing the CNS not only in the traditional sense of cell replacement but also by providing trophic support and protection. Ideally, differentiation neurons could contribute to plasticity and formation of new circuits, oligodendrocytes could participate in remyelination, and new astrocytes could reduce the extent of cell death and injury. Thus, effective transplantation of NSCs had a potential for treating CNS injury and neurondegenerative disorders. In fact, previous studies have reported that neural progenitors or NSCs could not differentiate into neurons when transplanted back into the spinal cord. EGFP was a useful marker for tracing the cell after transplantation. In this study, we transplanted the single neural stem cells' suspension from EGFP transgenic mice into the injured spinal cord. Then we estimated the survival and differentiation of NSCs in vivo. We found that the NSCs could survive and differentiate into MAP2 positive cells. Taken together, these studies indicated that EGFP expressing NSCs were a useful marker for cell tracing in the transplantation and could differentiate into neurons.
Funded by the Chinese National 973 Project (No. 2003 CBS 15306) and the Chinese National Natural Science Fund for Outstanding Youth (No. 30625036).
REFERENCES
- Brustle, O., Choudhary, K., Karram, K., Huttner, A., Murray, K., Dubois-Dalcq, M., McKay, R.D. (1998). Chimeric brains generated by intraventricular transplantation of fetal human brain cells into embryonic rats. Nature Biotechnology 16(11): 1040–1044.
- Gage, F.H., Coates, P.W., Palmer, T.D., Kuhn, H.G., Fisher, L.J., Suhonen, J.O., Peterson, D.A., Suhr, S.T., Ray, J. (1995). Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A 92(25): 11879–83.
- Lendahl, U., Zimmerman, L.B., McKay, R.D. (1990). CNS stem cells express a new class of intermediate filament protein. J. Cell 60(4): 585–595.
- Tropepe, V., Sibilia, M., Ciruna, B.G., , et al. (1999). Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telecephalon. J. Dev Biol. 208(1): 166–188.
- Kilpatrick, T.J., Bartlat, P.F. (1993). Cloning and growth of multipotential neural precursors: Requirements for proliferation and differentiation. J Neuron. 10(2): 255–265.
- Fischer, I. (2000). Candidate cells for transplantation into the injured CNS. Prog. Brain Res. 128: 253–257.
- Rao, M.S., Mayer-Proschel, M. (2000). Precursor cells for transplantation. Prog. Brain Res. 128: 273–292.
- Namiki, J., Tator, C.H. (1999). Cell proliferation and nestin expression in the ependyma of the adult rat spinal cord after injury. J Neuropathol Exp Neurol. 58: 489–98.
- Horner, P.J., Power, A.E., Kempermann, G., Kuhn, H.G., Palmer, T.D., Winkler, J., , et al. (2000). Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 20: 2218–28.
- Shihabuddin, L.S., Horner, P.J., Ray, J., Gage, F.H. (2000). Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 20: 8727–35.
- Yamamoto, S., Nagao, M., Sugimori, M., Kosako, H., Nakatomi, H., Yamamoto, N. , et al. (2001). Transcription factor expression and notch-dependent regulation of neural progenitors in the adult rat spinal cord. J Neurosci. 21: 9814–23.
- Chow, S.Y., Moul, J., Tobias, C.A., Himes, B.T., Liu, Y., Obrocka, M., , et al. (2000). Characterization and intraspinal grafting of EGF/bFGF-dependent neurospheres derived from embryonic rat spinal cord. Brain Res. 874: 87–106.
- Masaru, O., Masahito, I., Katsuya, K. , et al. (1997). “Green mice” as a source of ubiquitous green cells. J FEBS Letters. 407: 313–319.
- Takada, T., Iida, K., Awaji, T., Itoh, K., Takahashi, R., Shibui, A., Yoshida, K., Sugano, S., Tsujimoto, G. (1997). Selective production of transgenic mice using green fluorescent protein as a marker. Nat Biotechnol. 15(5): 458–61.
- Dinh, A.T., Kubis, N., Tomita, Y., Karaszewski, B., Calando, Y., Oudina, K., Petite, H., Seylaz, J., Pinard, E. (2006). In vivo imaging with cellular resolution of bone marrow cells transplanted into the ischemic brain of a mouse. Neuroimage 33(3): 1028.
- Koda, M., Okada, S., Nakayama, T., Koshizuka, S., Kamada, T., Nishio, Y., Someya, Y., Yoshinaga, K., Okawa, A., Moriya, H., Yamazaki, M. (2005). Hematopoietic stem cell and marrow stromal cell for spinal cord injury in mice. Neuroreport 16(16): 1763–7.