Abstract
A lactose biosensor was developed by immobilizing lactase and galactose oxidase in a polyvinyl formal membrane and was attached to the oxygen electrode of a dissolved oxygen analyzer for estimation of lactose in milk and food products. The enzyme immobilized polyvinyl formal membrane was characterized by atomic force microscopy. The biosensor showed the linearity for 1–7 g dl−1 of lactose and can be reused for up to 20 measurements. The effects of pH, temperature and the stability of the immobilized lactase and galactose oxidase in PVF membrane were also studied. The enzyme membrane was found stable up to 35°C and had a shelf-life of more than three months at 4°C.
INTRODUCTION
Lactose is hydrolyzed by lactase to glucose and galactose, which are further metabolized. Lactase (β-Galactosidase) is produced in the cells of the epithelium of the small intestine. It is most concentrated in the mucosal cells of the brush border of the jejunum. Production of lactase begins to decline in most children between the ages of 2 to 5 years. Most adults retain only 10 percent of infant-level lactase activity. Lactose intolerance occurs in children after weaning, when the cell line in the small intestine decreases the production of lactase [Citation[1], Citation[2]]. Symptoms which begin about 30 min to 2 h after consuming foods containing lactose result as the unmetabolized lactose passes through the small intestine to the large intestine. Facultative bacteria present in intestine splits lactose into acetic, butyric, propionic and other short-chain fatty acids, which can be absorbed by intestinal cells and used as metabolites. The by-products such as carbon dioxide, hydrogen, and methane are produced, and can cause a gassy, bloated or nauseous feeling [Citation[3], Citation[4]]. The lactose-intolerant people are discouraged from consuming lactose-containing milk. An excessive amount of lactose in blood indicates gastrointestinal malignancy. Effective control of the lactose in food stuffs is also important to those who are unable to digest lactose. The inability of humans to digest lactose has enormous health consequences, particularly among the poor population of the world where milk is the only economical and fairly available source of nutrition [Citation[5]]. Therefore, it becomes essential to detect lactose in milk, food products and biological fluids.
There are various methods for the determination of lactose, including spectrophotometry, polarometry, infrared spectroscopy, titrimetry and chromatography, etc. These methods are tedious and time-consuming due to long sample preparation including lactose extraction and filtration. Many techniques have been used for the immobilization of enzymes in different matrices for the fabrication of biosensors [Citation[6-14]]. Several types of biosensors involving different enzymes are reported in the literature [Citation[15-24]]. Lactose biosensors can also be used for the detection of galactose in milk. Mostly, non-hydrolyzed native milk contains a very small amount of galactose (<0.004 g dl−1), which does not interfere for estimation of lactose, whereas hydrolyzed milk may contain >2.0–3.5 g dl−1 galactose, which may give false results for lactose estimation. Therefore it is not recommended for lactose estimation in hydrolyzed milk sample. The biosensor may be used for estimation of galactose where lactose is not present.
Here, we describe a lactose sensor based on immobilized lactase and galactose oxidase in polyvinyl formal membrane. Lactose biosensor can also be developed with immobilized glucose oxidase instead of galactose oxidase but it interferes with free glucose, which is present together with lactose. Therefore, the use of galactose oxidase with lactase in polyvinyl formal membrane is an improvement over the previously reported lactose biosensors [Citation[25]]. A biostrip and LB film based biosensor for the detection of lactose and galactose in milk was also developed in our laboratory [Citation[26-28]].
MATERIALS AND METHODS
Chemicals and Instruments
Polyvinyl formal (PVF), lactase (β-galactosidase) and galactose oxidase were purchased from Sigma-Aldrich, USA. O-Nitrophenyl β-D-galactopyranoside (ONPG) and o-tolidine were obtained from Sisco Research Lab, India. All other chemicals were of analytical reagent grade.
Amperometric measurements were conducted by using Aqualytic dissolved oxygen meter, Model OX-24, Germany. Atomic force microscopy was carried out using Nanoscope workstation II Digital, USA.
Immobilization of Lactase and Galactose Oxidase
Lactase (80 units, lyophilized powder) and galactose oxidase (170 units, lyophilized powder) were dissolved in 200 µl of 100 mM sodium phosphate buffer, pH 7.0. Polyvinyl formal (40 mg) was dissolved in 1 ml of chloroform/ethylene dichloride mixture (1:1 v/v) in a tube. The enzyme solution and polyvinyl formal solution were mixed quickly and spread evenly onto a clean glass plate at room temperature (25°C) for 2 h. A thin membrane was formed on glass surface. The lactase and galactose oxidase immobilized polyvinyl formal membrane (11 × 5 cm2) was then carefully scraped from the glass plate. The membrane was washed several times with distilled water to remove unbound enzymes. The immobilized enzyme membrane was stored at 4°C till further use. The aqualytic dissolved oxygen meter contains a single electrode to which the immobilized enzyme PVF membrane is attached at the sensing tip of the electrode by o-ring. The PVF membrane with and without immobilized enzymes was studied by atomic force microscopy.
Enzyme Activity Assay
The presence of lactase and galactose oxidase in the immobilized PVF membrane was carried out spectrophotometrically by keeping the film (I cm2) in 1 ml reaction mixture using ONPG and o-tolidine, respectively, as substrates. ONPG gives a yellow colored product, which absorbs at 410 nm [Citation[29]] and o-tolidine, with galactose oxidase also gives a colored product that absorbs at 425 nm [Citation[30]]. The response (dissolved oxygen mg l−1 depletion) of immobilized enzyme membrane was carried out at different temperatures, pH, storage conditions and in the presence of interferents.
RESULTS AND DISCUSSION
Activity of Lactase and Galactose Oxidase in PVF Membrane
The enzymes membrane was washed several times to observe leaching out lactase and galactose oxidase. After several washings it was found that membrane retained 90–95% activity of both enzymes.
Atomic Force Microscopy
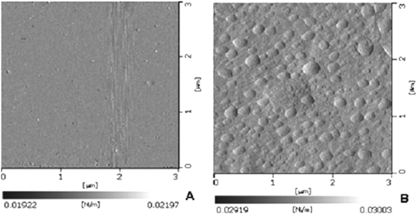
Atomic force micrographs (AFM) of polyvinyl formal and enzymes immobilized in polyvinyl formal membranes are shown in (A) and (B), respectively. The AFM of polyvinyl formal membrane without entrapped enzymes (A) indicates uniform polymeric layer, whereas globular structures due to entrapment of lactase and galactose oxidase are seen in B. This suggests that the enzyme molecules are uniformally distributed and packed in the PVF membrane.
Response Measurements
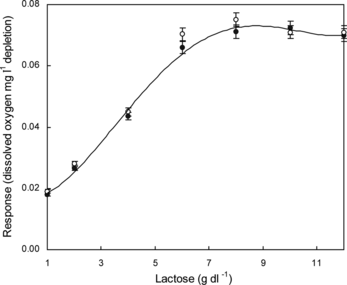
The response (dissolved oxygen mg l−1 depletion) of the immobilized enzymes in a PVF membrane attached to an oxygen electrode (lactose sensor) at different concentrations of lactose in a 100 mM phosphate buffer of pH 6.5 as well as in lactose/ galactose free milk (10 g solid powder dl−1 water) was determined at 25°C (). It showed linearity from 1 to 7 g dl−1 after which a limiting value of response was obtained. After measuring the response, the membrane was washed thoroughly with water and subsequently dried. The same electrode was used for testing the working stability (reusability) and the response was compared with a fresh membrane. It was observed that the membrane may be used 20 times, with 5–10% loss in response (data not shown). Beyond this, there was severe loss (40–50%).
Effect of Temperature and pH
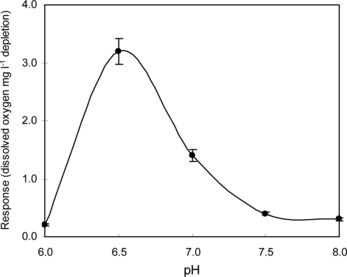
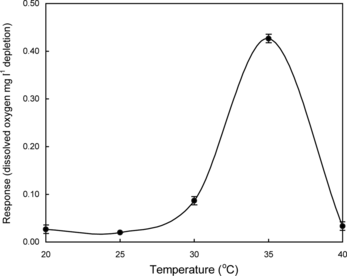
Thermal stability and effect of pH on immobilized enzymes in PVF membrane were investigated at 3 g dl−1 lactose in a 100 mM phosphate buffer of pH 6.5 by response (dissolved oxygen mg l−1 depletion) measurements. The enzyme membrane was kept at the desired temperature for 2 min. shows the result of the immobilized enzymes (lactase and galactose oxidase) response measurements as a function of temperature at a 3 g dl−1 lactose solution in a 100 mM phosphate buffer of pH 6.5. It was observed that response increased up to 35°C and then it decreases drastically. Therefore, the immobilized enzyme membrane is used below 35°C to prevent inactivation of the immobilized lactase and galactose oxidase enzymes.
Figure 3 Response of the immobilized enzymes in PVF membrane at different temperatures in the presence of 3 g dl−1 lactose in a 100 mM phosphate buffer of pH 6.5. The enzyme membrane was kept at the desired temperature for 2 min. Five readings were taken at each measurement.
The activity of immobilized enzymes is highly influenced by the change in pH. Lactose solutions (3 g dl−1) were made in 100 mM phosphate buffers of different pH and response measurements were taken at room temperature (25°C) using immobilized enzymes in PVF membrane (). The response increased gradually from pH 6.0 to pH 6.5 and after that decreased sharply, indicating that the optimum pH 6.5 can be used for lactose estimation.
Shelf Life
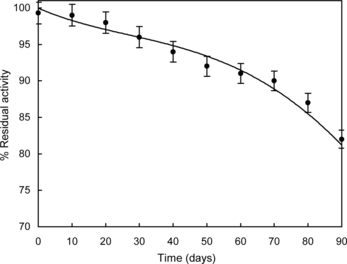
Enzymes immobilized in PVF membrane were tested for stability under the same operating conditions as for response measurements (dissolved oxygen mg l−1 depletion). The response of the enzyme membrane attached with an oxygen electrode was measured once in 10 days. The enzyme membrane was stored at 4°C when not in use. shows the response of the enzyme membrane on different days of storage. It showed that the response of the membrane was almost the same for 20 days and after that decreases gradually. This may be due to partial decay in the activity of the immobilized enzymes lactase and galactose oxidase. The shelf life of the immobilized enzymes in PVF was found to be more than three months.
Role of Interferents on Response
Different interferents, which are mostly present in milk such as ascorbic acid, calcium and uric acid, were tested with immobilized enzymes in PVF membrane fabricated lactose biosensor. These substances were added at their physiological normal level in the presence of 3 g dl−1 lactose solution. There was no significant effect of these interferents on the performance of the enzymes PVF membrane based lactose biosensor.
CONCLUSIONS
Lactase and galactose oxidase were immobilized in polyvinyl formal membrane. The PVF membrane was attached to the electrode of a dissolved oxygen analyzer for the fabrication of a lactose biosensor. The biosensor can be used repeatedly, 20 times for the estimation of lactose and shows linearity for 1–7 g dl−1 lactose. The enzymes immobilized in polyvinyl formal membrane were stable up to 35°C and had a shelf life of more than three months at 4°C. No significant effect of interferents such as ascorbic acid, calcium chloride and uric acid was observed. Lactose biosensor works only in the presence of lactose containing samples. If a sample contains galactose contamination it gives a false result. Therefore, lactose biosensor is not recommended for use in galactose containing samples, especially in hydrolyzed milk. For the detection of galactose in sample, galactose biosensor (without immobilized lactase) was developed in our laboratory.
The authors are thankful to Prof. S. K. Brahmachari, Director IGIB, Delhi, for providing necessary facilities and Prof. T. Srikhirin and Dr. Sukanya, Physics Department, Mahidol University, Bangkok, Thailand, for AFM studies. This work was supported by a fellowship to SKS from CSIR, India.
REFERENCES
- Paige, D.M., Bayless, T.M., Huang, S., Wexler, R. (1975). Lactose hydrolysed milk. Am. J. Clin. Nutr. 28: 818–822.
- Unger, N.S., Scrimshaw, N.S. (1981). Comparative tolerance of adults of differing ethnic backgrounds to a lactose free and lactose containing dairy drink. Nut. Res. 1: 1227–1233.
- Friedl, J. (1981). Lactose difficiency: Distribution associated problems and implications for nutritional policy. Eco. Food Nutr. 11: 37–48.
- Suarez, F.L., Savaiano, D.A., Levitt, M.D. (1995). A comparison of symptoms after the consumption of milk or lactose hydrolysed milk by people with self reported severe lactose intolerance. The New Eng. J. Med. 333: 1–4.
- Scrimshaw, N.S., Murray, E.B. (1988). The acceptability of milk and milk products in populations with a high prevalence of lactose intolerance. Am. J. Clin. Nutr. 48: 1079–1159.
- Trinder, P. (1969). Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 6: 24–27.
- Umana, M., Waller, J. (1986). Protein modified electrodes: The glucose oxidase/polypyrrole system. Anal. Chem. 58: 2979–2983.
- Pickup, J.C. (1989). In Applied Biosensors, L.W. Donald, Ed., Butterwort Publishers: MA, Stometham, pp. 227–237.
- Macholan, L. (1991). In Bioinstrumentation and Biosensor, L.W. Donald, Ed., Marcel Dekker: New York, pp. 349–379.
- Griffiths, D., Hall, G. (1993). Biosensors: What real progress is being made. Trends in Biotech. 1: 122–125.
- Carrara, C.R., Rubiolo, A.C. (1996). Determination of kinetic parameters for free and immobilized β-galactosidase. Process Biochem. 31: 243–248.
- Ates, S., Mehmetoglu, G. (1997). A new method for immobilization of β-galactosidase and its utilization in a plug flow reactor. Process Biochem. 32: 433–436.
- Allison, M.J., Bering, C.L. (1998). Immobilised lactase in the biochemistry laboratory. J. Chem. Edu. 75: 1278–1280.
- Mishima, Y., Motonaka, J., Maruyama, I., Nakabayashi, I., Ikeda, S. (2000). Glucose sensors based on titanium dioxide electrode modified with potassium hexacyanoferrate (III). Sensors Actuators B 65: 343–345.
- Guilbault, G.G., Neto, G.O. (1985). In Immobilized Cells and Enzymes: A Practical Approach, J. Woodward, Ed., IRL Press, Oxford: Oxford, pp. 55–74.
- Barker, S.A. (1987). In Biosensor-Fundamental and Application, A.P.F. Turner, I. Karube, G.S. Wilson, Eds., Oxford University Press: New York, pp. 85–99.
- Pandey, P.C., Misra, A.P. (1988). Conducting polymer coated enzymes. Analyst 113: 329–332.
- Pfeiffer, D., Ralis, E.V., Makower, A., Scheller, F.W. (1990). Amperometric bi-enzyme based biosensor for the detection of lactose-characterization and application. J. Chem. Technol. Biotechnol. 49: 255–265.
- Svorc, J., Miertus, S., Barlikova, S.A. (1990). Hybrid biosensor for the determination of lactose. Anal. Chem. 62: 1628–1631.
- Carlsson, H., Ljungcrantz, P., Lindbladh, C., Persson, M., Bulow, L. (1994). Use of genetically prepared enzyme conjugates in lactose and galactose analysis, Anal. Biochem. 218: 278–283.
- Narang, U., Prasad, P.N., Bright, F.V., Ramanathan, K., Kumar, N.D., Malhotra, B.D., Kamalasanan, M.N., Chandra, S. (1994). Anal. Chem. 66: 3139–3142.
- Svitel, J., Curilla, O., Tkac, J. (1998). Microbial cell based biosensor for sensing glucose, sucrose or lactose. Biotech. Appl. Biochem. 27: 153–158.
- Eshkenazi, I., Maltz, E., Zion, B., Rishpon, J. (2000). A three cascaded enzymes biosensor to determine lactose concentration in raw milk. J. Dairy Sci. 83: 1939–1945.
- Gould, B.J., Rocks, B.F. (2000). In Hand Book of Enzyme Biotechnology, A. Wiseman, Ed., Haisted Press: New York, pp. 424–425.
- Sharma, S.K., Singhal, R., Malhotra, B.D., Sehgal, N., Kumar, A. (2004). Lactose biosensor based on Langmuir-Blodgett films of poly(3-hexylthiophene). Biosens. Bioelectron. 20: 651–657.
- Sharma, S.K., Sehgal, N., Kumar, A. (2002). A quick and simple biostrip technique for detection of lactose. Biotech. Lett. 24: 1737–1739.
- Sharma, S.K., Singh, S.K., Sehgal, N., Kumar, A. (2004). Biostrip technique for detection of galactose in dairy foods. Food Chem. 88: 299–303.
- Sharma, S.K., Singhal, R., Malhotra, B.D., Sehgal, N., Kumar, A. (2004). Langmuir-Blodgett film based biosensor for estimation of galactose in milk. Electrochim. Acta. 49: 2579–2485.
- Craven, G., Steers, E., Anfinsen, C. (1965). Purification, composition and molecular weight of the β-galactosidase of E.coli K12. J. Biol. Chem. 240: 2468–2472.
- Worthington, V. (1972). Worthington Enzyme Manual, Worthington Biochemical Corporation: NJ, Freehold, p. 21.