Abstract
Urease was immobilized activated PEG-5000 and encapsulated urease and PEG modified urease within alginate beads. Encapsulated urease and PEG-urease were thoroughly characterized for pH, temperature and stabilities and these properties were compared with free and PEG modified enzyme. A 34 and 57% mass and 19.3 and 43% activity yield of urease and PEG-urease resulted following encapsulation. The average diameter for the beads was found to be 2.5 mm for urease bead and 1.8 mm for PEG-urease bead. The stabilities of PEG-urease beads at pH 6.0 were higher than those of urease beads. PEG-urease beads retained 70% of initial activity after reusing seven times at pH 6.0. Also time dependency of substrate conversion was determined for enzyme beads.
INTRODUCTION
Urease enzyme catalyzes the hydrolysis of urea to ammonia and carbondioxide. Determination of urea in physiological fluids is of great interest in medicine. Urea is a major metabolic end product and the removal of its excess has been a major problem for the patients suffering from renal failure. One of the major applications of immobilized urease is the direct removal of urea from blood for detoxification or in the dialysate regeneration systems of the artificial kidney machines [Citation[1]].
Many immobilization techniques for urease have been reported and reviewed recently but covalent bonding and microcapsulation techniques have been usually prefered [Citation[2], Citation[3]]. Encapsulation technology is designed to entrap materials within a semi-permeable polymeric membrane and/or a gel matrix. Capsules are generally spherically with diameters ranging from 1 µm to several millimeters and are manufactured from a variety of polymeric materials. Microcapsules, consisting of a membrane surrounding a liquid core, have been synthesized with nylon [Citation[4]], polyethyleneimine [Citation[5]] and other synthetic and natural polymeric membranes [Citation[6]].
Alginate is used extensively for the encapsulation of biologically active materials since it is biochemically inert and gels under mild conditions. As a naturally occurring polysaccharide produced by Brown algae, it is a linear polyanion of 1–4 linked β-D-mannuronic and α-L-guluronic acid residues. Alginate exists as a soluble salt in the presence of monovalent cations such as Na+, and forms an ionotropic gel with divalent cations such as Ca+2, Ba+2 or Al+3. beads are commonly prepared by extruding sodium alginate dropwise into a Ca+2 solution [Citation[7]].
The use of poly(ethylene glycol) (PEG), which is dihydroxy terminated, its sone-end-capped derivative monomethoxy poly(ethylene glycol) (MPEG) to reduce protein immobilization is well documented [Citation[8]]. PEG was reported to be non-toxic, amphipathic and non immunogenic and is used frequently as a polymeric matrix for urease immobilization [Citation[2], Citation[9]].
2. MATERIALS AND METHODS
Urease (EC 3.5.1.5) from jack beans(Type III), bovine serum albumine (BSA), MPEG-5000, sodium alginate and calcium chloride were supplied by Sigma (St. Louis, MO, USA). All other chemicals were of analytical grade and were purchased from Merc (Germany).
2.1. Preparative of PEG-Urease
MPEG-5000 was activated with cyanuric chloride and coupled to urease (1:1 molar ratio Urease-Lys/MPEG) by our previously study [Citation[2]].
2.2. Preparation of Alginate Beads Containing Urease and PEG-urease
Solutions were refrigerated to + 4°C prior to use and the temperature of the calcium chloride solution was maintained at 8–10°C throughout bead formation. 2 ml (0.03 mg/ml) alginate was mixed 0.5 ml urease and PEG-urease(10 mg/ml). The mixture was vortexed and it was extruded dropwise through a syringe needle. The drops were collected in 0.05 M calcium chloride. After stirring for 4 h at 4°C, beads of calcium alginate with entrapped enzyme were collected. Beads were washed with bidestiled water to remove any calcium chloride solution and were stored suspended in bidestiled water at 4°C. The amount of encapsulated urease and PEG-urease was determined by measuring the initial and final concentrations of protein within encapsulation medium using Biure methods [Citation[10]] and determination of absorbance amount at 280 nm. A calibration curve constructed with BSA (bovine serum albumine) solution of known concentration 1–10 mg/ml for biure methods and 0, 1–2.5 mg/ml for 280 nm was used in the calculation of protein in the enzyme and wash solutions.
2.3. Urease Activity Assay
The concentration of ammonia liberated was determined by the Berthelot assay method [Citation[11]] using a Pharmacia spectrophotometer at 630 nm wavelength. A standard curve with ammonia in the range of 0–12.0 µmol/ml should be drawn under similar conditions to estimate the urea concentration of unknown samples.
Enzyme and PEG-enzyme beads (0.051 U/mg-protein/beads) were pre-incubated in activity assay solution at 37°C 10 min intermittent shaking. After the desired time interval, the enzyme beads were separated from the reaction mixture. An aliquot of the reaction mixture was used to develop color with Berthelot assay method as described above for the soluble enzyme.
Each experiment was repeated twice under the same conditions.
2.4. Determination of Particle Size
The particle size of the urease and PEG-urease beads was determined with the help of a magnifying glass. This was roughly checked by suspending the beads in water contained in a 10 ml of graduated cylinder and measuring the increase in volume.
2.5. Determination of Optimum pH and Temperature
Effect of pH on the activity of uerase beads and PEG-urease beads preparations was investigated in the different buffer solution of pH ranging from 4 to 9(pH 4–5, 50 mM citrate buffer, pH 6–8, 50 mM phosphate buffer, pH 9, 50 mM borate buffer).
The effect of temperature on the activity of both encapsulated enzymes was also studied between 8–55°C at pH 6.0, 50 mM phosphate buffer.
2.6. Stability Tests
The activities of urease, PEG-urease and enzyme beads preparations after storage in bidestiled water at 4°C were measured with the experimental conditions given above.
To determine operational stabilities of the urease and PEG-urease beads, the activity of ten beads were assayed at standard conditions repeatedly.
Urease and PEG-urease beads were incubated with 50 mM pH 6.0 phosphate buffer at 0–400 min. After incubation time, enzyme beads activity was investigated under the standard activity assay conditions.
2.7. Time Dependent of Substrate Conversion
Urease and PEG-urease beads (2.55 U/mg-protein) were incubated 100 ml of assay solution including 0.15 mg/ml urea in pH 6.0, 50 mM phosphate buffer at 37 C° 0–200 min 150 rpm. After the desired time interval, the 500 µl sample was separated from the batch system. An aliquat of the reaction mixture was used to develop color with phenol-hypocloride reagent.
3. RESULTS AND DISCUSSION
3.1. Urease Immobilization on Activated PEG-5000
In the our previous study, urease was modified to varying degrees with PEG-5000 [Citation[2]]. The covalent attachment of PEG-5000 to urease increases the size of the molecule substantially so urease was immobilized on activated PEG-5000 using molar ratio 1:1 of urease containing lysine and activated PEG-5000. The activity of free urease was 20.6 U/mg-protein at pH 7.0 and 37°C. Approximately 12.4% of the activity was lost when urease was immobilized on activated PEG.
3.2. Preparation of Alginate Beads Containing Urease and PEG-urease
The property of calcium alginate gel that restricts its applicability to non-covalent protein immobilization is its open lattice structure that leads to protein leakage. Dashevsky reported that entrapment of the enzyme lactase into the alginate beads resulted in 36.0% loss of the protein [Citation[12]]. Martinsen found that minimum 50.0% of the entrapped proteins are lost from the alginate beads during the 1st h [Citation[13]]. However, this rate of diffusion decreased with increasing size of the protein. For larger solutes such as PEG-urease entrapped enzymes are not lost from the alginate beads during 400 min at pH 6.0.
Urease and PEG-urease were encapsulated within alginate beads. summarizes the mass (protein) and activity yields of encapsulated urease and PEG-urease. The yield on a mass basis is 34.0% for urease beads and 57% for PEG-urease beads but recovery of activity is approximately 19.3% for urease beads and 43.0% PEG-urease beads.
Table 1. Efficiency of Urease and PEG-Urease within Alginate beads
The average diameter for the beads was found to be 2.5 mm for urease beads and 1.8 mm for PEG-urease beads. They were relatively uniformly spherical in shape.
3.3. Effect of pH and Temperature on Catalytic Activity
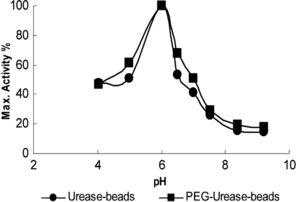
The effect of pH on the activity of the enzyme beads was examined in the pH range 4–9 at 37°C and the results are presented in . The optimal pH for free and PEG modified urease was found pH 7.0 in our previous study [Citation[2]]. On the other hand, the optimal pH for the urease beads was found to have shifted to pH 6.0. This observed displacement toward the acidic region for the immobilized urease preparation is because pH conditions in the pore space of the polymeric matrix are different from those in the rest of the solution. The enzymatic product (ammonium ions) is a charged molecule that will effect the local pH.
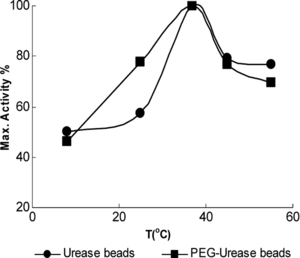
The activities obtained in a temperature range of 8–55°C were expressed as a percentage of the maximum activity. As is shown in , the optimum temperature for all urease preparations was 37°C. This indicates that immobilization and encapsulation procedure has no effect on the optimum temperature of the urease.
3.4. Stability Tests
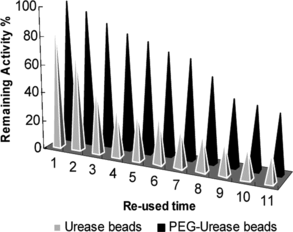
The operational stability test for urease and PEG-urease beads is shown in . The PEG-urease beads are stable in operational conditions with considerable urea degradation. In addition, a high operational stability obtained with PEG-urease bead indicates that the PEG-urease bead could successfully be used for the degradation of urea from biological fluids or estimation of blood urea concentration.
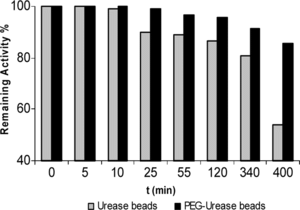
pH 6.0 stability experiments were carried out with the urease beads and PEG-urease beads, which were incubated in the absence of substrate at pH 6.0. The urease beads and PEG-urease beads retained their activity to a level of 53.9% and 85.6% during a 400 min incubation period. The PEG-urease bead was inactivated at a much slower rate than that of urease bead form ().
The free urease, PEG-urease and encapsulated urease preparations were stored in bidestiled water at 4°C for a predetermined period. Under the same storage conditions, the activity of PEG modified urease decreased slower than that of the free urease; the free urease lost approximately 75% activity within 6 days. PEG modified urease lost 68% activity within 8 days. On the other hand, urease and PEG-urease bead preserved about 12% and 13% of its initial activity during a 1-month storage period.
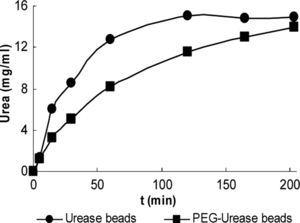
3.5. The Dependent of Substrate Conversion
50 urease and the same activity amount of PEG-urease beads were incubated in 100 ml of assay solution at 37°C, 150 rpm. Samples were taken from the outlet and analyzed for ammonium ion concentration. As is shown in the batch system result () about 15 and 10 mg urea was degradated during a 100 min period for urease and PEG-urease beads, respectively. The degration of urea using urease bead is slower than using PEG-urease bead.
CONCLUSION
Urease was covalently immobilized on activated PEG and it lost about 12.4% of the activity during the immobilization procedure. Alginate was used as an encapsulation material for urease and PEG-urease. Mass and activity yields were 34 and 30% for urease beads, 57 and 43% for PEG-urease beads, respectively. The PEG-urease and urease beads were more stable than free and immobilized urease at 4°C. After washing with bidestiled water, the PEG-urease beads can be re-used several times (after seven uses there was only 30% loss of activity) for urea estimation, thus making it a very economical procedure compared to conventional clinical methods using diacetylmonoxime, which is still widely used [Citation[14]]. Also, PEG-urease beads could successfully be used in a continuous system for the degradation of urea from biological fluids.
REFERENCES
- Elçin, M., Sungur, S., Akbulut, U. (1992). Bioorganic and Medicinal Chemistry Letters 5: 31–40.
- Hamarat, Ş., Uslan, A.H. (1996). Artif. Cells Blood Subst. Imm. Biotechnol. 24(3): 273–276.
- Bayramoğlu, G., Altmok, H., Bulut, A., Denizli, A., Arica, M.Y. (2003). Reactive and Functional Polymers 56: 111–121.
- Monshipouri, M., Neufeld, R. (1991). Enzyme Microb. Technol. 13: 309–313.
- Poncelet, D., Alexakis, T., Poncelet de Smet, B., Neufeld, R.J. (1994). Methods in Enzymology 11: 31–40.
- Das, N., Kayastha, A.M., Malhotra, O.P. (1998). Biotechnol. Appl. Biochem. 27: 26–29.
- Hearn, E., Neufeld, R.J. (2000). Process Biochemistry 35: 1253–1260.
- Gombotz, W.R., Guanghui, W., Hoffman, A.S. (1989). J. Appl. Polym. Sci. 37: 91–107.
- Hamarat Baysal, Ş., Uslan, A.H. (2002). Artif. Cells Blood Subst. Imm. Biotechnol. 30(1): 71–79.
- Uslan, A.H., Telefoncu, A. (1984). Izmir: Laboratory of Biochemistry, Ege Üniversitesi Bornova.
- İmren, H. (1985). Klinik Tanida Laboratuvar 637: 716–723.
- Dashevsky, A. (1998). Int. J. Pharm. 161: 1–5.
- Martinsen, A., Skjak-Braek, G., Smidsrod, O. (1989). Biotechnol. Bioeng. 33: 79–89.
- Evans, R.P. (1968). J. Clinical Pathology 21: 527–529.