Abstract
The objective of this study is to investigate the effect of bioabsorbable Calcium alginate film in guided bone regeneration by the study of Haversian remodeling. Circular bone defects of 5 mm diameter were created in the corners of mandibles in 35 rabbits. The defects were covered with calcium alginate film (CAF) served as the experimental group, or collagen membrane (CM) as the control group, respectively. Healing condition was analyzed with gross, histological and immunohistochemical studies after 1, 2, 4, 6 and 8 weeks. The experimental group appeared more and earlier Haversian remodeling and osteoinductive factors leading to better bone regeneration. The control group showed more macrophages, less and later Haversian remodeling, absorbled slowly, while collected fewer osteoinductive factors in the early stage. Calcium alginate film, which is a relatively cheaper material, provides better effect than the collagen membrane in bone regeneration, Haversian remodeling and quantity of osteoinductive factors.
The Haversian canal is not only the important component of the tensile cortical bone but also the basic unit of its structure and function [Citation[1]], which contains blood vessels, nerves and some connective tissue. The remodeling of the Haversian canal of tensile cortex in guided bone regeneration (GBR) [Citation[2-5]] model is intimately relevant to the bone repair and healing. By observation of the newly regenerated Haversian canal and assessment of osteoinductive factors in the healing cause of adult rabbit's mandible defect, the objectives of this investigation were to study the mechanism and the effectiveness of our newly introduced CAF-calcium alginate film [Citation[6-10]], a material that does not require a second-stage surgery for its removal and has an enhanced benefit in guided bone regeneration, and to compare the findings with those obtained from the collagen membrane (CM) [Citation[11-13]].
METHODS
All animal protocols were in accordance with Institutional Animal Care and Use Committee (IACUC) approval following NIH guidelines for the use of animals in research. Permission was also obtained from the Zhejiang University Conjoint Ethics Committee.
Thirty-five adult male Japanese white rabbits weighing 2.2 to 3.0 kg were used in this study. CAF were self-manufactured [Citation[10]]. CM was bought from Tian Jin Institute of Biomedical Engineering, the Chinese Academy of Medical Sciences and Peking Union Medical College. Medical glue 508 was a product of Bai Yun Medical Glue Head Company, Guang Zhou, China. The monoclonal antibodies Transformation Growth Factor beta (TGF-β, 1: 300) and Vascular Endothelial Growth Factor (VEGF, 1: 200) were bought from Maixin Biotech, Inc., Fu Zhou City, China.
Surgical Procedure
The rabbits were anesthetized with an intraperitoneal injection of 4 ml/kg of body weight of urethane. After shaving the skin and disinfecting the surgical group in each animal, a skin incision was made along the inferior border of the mandible in both sides. After exposing the masseter muscle, a deep mesio-distal incision (approximately 4 mm coronal to the border of the mandibular ramous) was made through the muscle. The bone surface was exposed after separating the tissues and the periosteum both buccally and lingually. Under constant, copious irrigation with saline, a slowly rotating trephine bur was used to create standardized [Citation[10]], circular, 5-mm-diameter defects in the incisor region anterior to the jaw angles. The defect on one side of the jaw (experimental group) was covered both buccally and lingually by CAF, which was cut to extend 2 to 3 mm beyond the defect margins under the periosteum. On the contra lateral side (control group), the same procedure was performed with the placement of CM. The membranes were tightly adapted to the bone surface and stabilized with medical glue 508. Sutured in layers, the animals were given an intramuscular injection of penicillin every day in the first 3 days. The 35 animals were sacrificed euthanasia in groups after 1, 2, 4, 6, and 8 weeks of healing with 7 in each group. The 2-week time point refers to 18 days operately.
Preparation of Specimen
The mandible specimens, along with the surrounding soft tissues, were harvested and immediately immersed in a 10% formalin fixative solution for histological preparation. The specimens were then decalcified in 5.5% EDTA (PH = 7.2). After dehydration, the specimens were embedded in paraffin wax, and sections of 5 µm in thickness were made in a buccolingual direction, perpendicular to the external surface of the mandible. The sections were stained with routine hematoxylin and eosin (HE) or with demonstration solution A and B (DAB) after immunohistochemical antibody reaction by S-P, showing brown as positive, keeping a uniform terminal point consistently. A group of multiple independent examiners who have been calibrated made the histological analysis. Two sections of each side and a total 140 sections for each antibody to VEGF or TGF-β were randomly taken under 40 × 40 observation field with ten fields randomly selected for each section. Mean grey value of each antibody in defects was measured to indicate the quantity of the corresponding antibody through the MIAS-300 type image pattern analysis technique. Results were expressed as means±standard deviations (SD) and were considered as significant at p = 0.05 (critical level). Statistical calculations were carried out using the SPSS software package.
RESULTS
Gross Examination
All rabbits recovered well and remained healthy, gaining body weight during the procedures.
CAF Group
One week after surgery in the CAF group, the defects were covered by the intact CAF, with none of them displaced. Congestion was not obvious in the defects. After 18 days of healing, the CAF shape was desalinated, starting to be degraded, and the defects were fairly hard. Four weeks after surgery, the membrane had been absorbed with a smooth defect surface. No residual defect was found. Six weeks postoperatively, the reconstructed bone appeared to be the same as a normal bone in quality and color.
CM Group
One week after surgery, CM was also intact and not displaced, but hyperemia was obvious. Eighteen days post surgery, CM shape was still intact and the defects diameter had not shrunk. Four weeks after surgery, CM shape was incomplete and the membrane began to be degraded; the soft residual defects were visible. After six weeks of healing, there remained CM residuals. The regenerated area was not smooth on its edges. After eight weeks of healing, the defects were darkly red, and membranes were just resorbed.
Histological Observation
CAF Group
Seven days after surgery, we observed a blood coagulum, sparse primitive blood vessels, and a few membrane crumbs (which were dyed as homogeneous) in the secluded space, with several inflammatory cells and osteoblasts at the rims. After 18 days of healing, the quantity of osteoblasts increased, and bone trabeculae appeared. Four weeks postoperatively, the defect was filled with reticular trabeculae, and Haversian system remodeling tended to be stable. After six to eight weeks, lamellar new bone was mature and membrane bits disappeared ().
CM Group
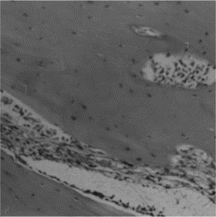
After 7 days of healing, coagulum was clear, along with a few stripped reticular membrane bits and phagocytes. Osteoblasts and bone trabeculae were few in number at 18 days post surgically. Four weeks after surgery, phagocytes, inflammatory cells and bone trabeculae were obvious. Six weeks postoperatively, newly regenerated bone was still immature, and remnants of the membrane were still visible. And Haversian system remodeling was still unstable. After 8 weeks of healing, many proliferated collagen fibers were visualized as uncalcified above the surface of the defect. Cartilage and bone trabeculae were still immature ().
Figure 2 Six weeks postoperatively, newly regenerated bone was still immature, and the Haversian system remodeling was still unstable in CM group (× 200).
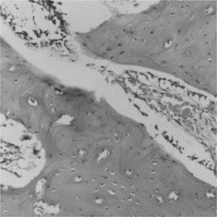
There were two CAF group defects completely closed with regenerated new bone at 4 weeks post healing, while there was only one CM group defect completely closed 6 weeks after operation. Another three CM defects had no advance in bone healing from 4 to 6 weeks postoperatively, with slow proliferating and calcifying of collagen.
Immunohistochemical Staining and Assessments
VEGF was positively stained as early as 1 week post operation in CAF group, in the nuclear of osteoblasts of intramembranous ossification, indicating that the regeneration capability was activated (). It was strongly stained in Haversian canals in the early 4 weeks of healing (), and then decreased. However, the expression sequence of VEGF in CM group was almost contrary. TGF-β was mainly observed as positively stained in the endotheliocyte of Haversian canal and in the plasm of cells inside canal in the new bone of 4-week CAF group defect. It was also observed in the matrix among the new trabeculae near the normal bone around the CM group defect. TGF-β staining intensity was low in the fibroblasts and osteoblasts in the CM defect. Image assessment results showed that both the two respective osteoinductive factors were significantly higher in CAF group than in CM group statistically (p < 0.05) ().
Figure 3 VEGF was positively stained as early as a week post operation in CAF group, in the nuclear of fibroblasts of intramembranous ossification (× 400).
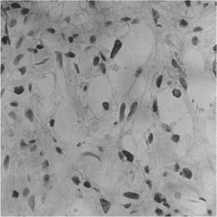
Figure 4 TGF-β was observed as positively stained in the 4-week group, in the endotheliocyte of Haversian canal and in the plasm of cells inside canal in the new bone of CAF group defect (× 200).
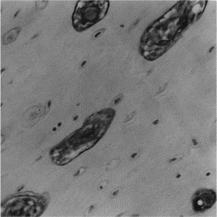
Table 1. Total quantities of antibodies TGF-β and VEGF summed from each time point in CAF group and CM group (*P < 0.05)
DISCUSSION
When a fracture or bone defect happens, TGF-β is released from its storage in bone matrix and periost, stimulating the osteogenitor cells, osteoblast and other cells in the bioactive layer of periost to multiplicate and to synthesize the bone matrix by the function of diffusion, and so to promote the new bone generation and maturity [Citation[14]]. Therefore, TGF-β is expressed in the new or old bone matrix instead of in fibro granule tissue.
The mature type of TGF-β is a homology diploid with a molecular weight of 25 k, which has a relatively high content in blood platelets and bone tissues. It can accelerate the synthesis of cell DNA and type I collagen, and inspire the formation of cartilage and endochondrol ossification, and regulate the moving or dissociation and accumulation of osteoblasts. Its main super family members BMP2 and BMP7 can induce endochondrol ossification and intramembranous ossification [Citation[15-16]]. The probable functions of TGF-β in bone regeneration are mainly to mediate the effect of systemic factors in a whole body on osteocyte, and to regulate the effects among the osteogrowth factors in local sites. TGF-β may stimulate the proliferation of mesenchymal cell, cartilage cells and osteoblasts, as well as inspire the synthesis of excellular matrix, while inhibiting the formation of osteoclasts.
The key factor that affects bone rehabilitation lies in the local blood supply. On the one hand, blood vessels furnish the tissues with nutrition, oxygen, other growth factors and productions of inflammation reactive; on the other hand, the endotheliocytes develop into osteoblasts and osteoclast, while the leaf mesectoderm cells in the blood vessel wall also develop into fibroblast, osteoblast and collagen. VEGF is a strongly effective and peculiar mitogen of endotheliocyte, which inspires the endotheliocyte proliferation and development while the blood plasma proteins exosmose out to become the temporary matrix for blood vesselgenisis [Citation[17-19]]. These are the prerequisite requirements for blood vesselgenisis. Haversian canal remodeling is closely correlated with bone healing and regeneration. The essence of bone healing is the reconstruction of Haversian system, during which osteoclasts are absorbed in bone and osteoblasts turn into osteogenisis directly [Citation[1]].
It can be inferred that the molecular biological events [Citation[20]] and reconstruction velocities of bone defect healing courses in different groups are intimately relevant to local blood supply characteristics. The VEGF expressions were relatively late in the control group of our current experiment. Moreover, the expressions of osteoinductive factors were variable with time and among groups, instead of being a single linear relationship, which was the appearance of the quantity and density of new bone in regeneration course. This is coincident with Tastuyama's report [Citation[21]]. It was the CAF group, which started the osteogenisis earlier at the molecular standard. This offers a theoretic basis of gene therapy for bone regeneration, and confirms the simplicity in gene therapy of bone, which only requires a short-term and early-stage gene expression to induce the osteogenesis [Citation[22-24]].
The stained positions of osteoinductive factors were different between the CAF group and CM group even at the same time point post surgically, just as the bone regeneration activations are different in regard to a time between the two groups. VEGF and TGF-β were observed in immunohistochemical sections under a microscope to be stained in matrix cells of bone marrow and blood vessel wall in Haversian canal in the experiment. The osteogenisis activities were observed in regular histological sections under a microscope as marching from the defect rim to the defect center, and developing from fibroblast cells to osteoblast and then to bone trabeculae progressively. It can also be inferred that the directions of Haversian system reconstruction and bone regeneration is coincident with that of the first stage of fracture healing and the segmental defect regeneration of a pipe-like bone.
Furthermore, the remodeling of the Haversian canal in CAF group occurred in a larger quantity. It happened earlier and in a better order than in the CM group. The collagen fibroses and bone trabeculae in CAF group were also more in quantity, earlier and better organized than in the CM group under a scanning electromicroscopic observation as reported in another article [Citation[10]]. In fact, the orderly developing of collagen fibroses Haversian canal is significantly important to bone healing, which confirms the neat arrangement of later lamellar bone and the quicker regeneration of bone defect. In conclusion, the remodeling of the Haversian canal and the bone regeneration of a bone defect healing were coincidental [Citation[25]] in the study, which showed the advantages of CAF; the key factor of quick repair for better guided bone regeneration lies in the sufficient inductive factor products of gene expression.
The authors would like to express their gratitude to Yongping Zhang, Lijie Fan, Songying Li and Professor Ji-an Hu, Stomatology Hospital, College of Medicine, Zhe Jiang University, for their assistance and advice during the animal preparations and the interpretation of histologic specimens. We also thank Professor Gongxiang Chen, Department of Clinical Laboratory, Second Affiliated Hospital, College of Medicine, Zhe Jiang University, for his help in article preparation. This study was supported by a fund of the Public Health Office, Zhe Jiang Province (2002B026), China.
REFERENCES
- Mohsin, S., O'Brien, F.J., Lee, T.C. (2006). Three dimensional reconstruction of Haversian canals using x-ray micro-computed tomography. Bone 38, Supplement 1: 16.
- Lioubavina-Hack, Natalia, Thorkild Karring, Samuel E Lynch, Jan Lindhe. (2005). Methyl cellulose gel obstructed bone formation by GBR: An experimental study in rats. Journal of Clinical Periodontol 32: 1247–1253.
- Ryuji, H., Kenji, K., Tomohide, K., Takashi, M., Daisuke, C., Masayoshi, W. , et al. (2000). Controlled local application of basic fibroblast growth factor (FGF-2) accelerates the healing of GBR, an experimental study in beagle dogs. Clin. Oral Implants Res. 11: 345–353.
- Donos, Nikolaos, Lambros Kostopoulos, Maurizio Tonetti, Thorkid Karring. (2005). Long-term stability of autogenous bone grafts following combined application with guided bone regeneration. Clin. Oral Implants Res. 16: 133–139.
- Hammerle, Christoph, H.F., Jung, Ronald E. (2003). Bone augmentation by means of barrier membranes. Periodontol 33: 36–53.
- Ishikawa, K., Ueyama, Y., Mano, T., Koyama, T., Suzuki, K., Matsumura, T. (1999). Self-setting barrier membrane for guided tissue regeneration method: initial evaluation of alginate membrane made with sodium alginate and calcium chloride aqueous solutions. J. Biomed. Mater. Res. 47: 111–115.
- Calafiore, Riccardo, Giovanni Luca, Mario Calvitti. (2001). Cellular support systems for alginate microcapsules containing islets as composite biocartificial pancreas. Ann. N. Y. Acad. of Sci. 11,944: 240–252.
- Rokstad, Anne, Bard Kulseng, Berit Strand. (2001). Transplantation of alginate microcapsules with proliferating cells in mice. Capsular overgrowth and survival of encapsulated cells of mice and human origin. Ann. N. Y. Acad. of Sci. 11,944: 216–225.
- Kenley, R., Marden, L., Turek, T. (1994). Osseous regeneration in the rat calvarium using novel delivery systems for recombinant human bone morphogenetic Protein-2 (rh-BMP-2). J. Biomed. Res. 28: 1139–1147.
- Huang, Jianqi, Hong He, Lieping Sheng, Genghua Gu. (2002). Comparison of calcium alginate film with collagen membrane for guided bone regeneration in mandibular defects in rabbits. J. Oral. Maxillofac. Surg. 60: 1449–1454.
- von Arx, Thomas, Robert K Schenk, Daniel Buser, David L Cochran, Joachim S Hermann. (2001). Lateral ridge augmentation using different bone fillers and barrier membrane application. A histologic and histomorphometric pilot study in the canine mandible. Clin. Oral Implants Res. 12: 260–269.
- Taguchi, Yuya, Norio Amizuka, Masayoshi Nakadate, Hideo Ohnishi, Noritaka Fujii, Kimimitsu Oda, , et al. (2005). A histological evaluation for guided bone regeneration induced by a collagenous membrane. Biomed. 26: 6158–6166.
- Fujihara, K., Kotaki, M., Ramakrishna, S. (2005). Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomed. 26: 4139–4147.
- Bonewald, L.F., Mundy, G.R. (1990). Role of transfomation growth factor beta in bone remodeling. Clin. Orthop. Res. 250: 261.
- Lee, J.Y., Peng, H., Usas, A. (2002). Enhancement of bone healing based on ex vivo gene therapy using human muscle-derived cells expressing bone morphogenetic protein 2. Human Gen. Therapy 13: 1201–1211.
- Reddi, A.H. (1998). Initiation of fracture repair by bone morphogenetic proteins. Clin. Orthop. Relat. Res. 355 Suppl: S66–S72.
- Mayr-Wohlfart, U., Waltenberger, J., Hausser, H., Kassler, S., Gunther, K.P., Dehio, C., , et al. (2002). Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone 30: 472–477.
- Pufe, Thomas, Katharina E Scholz-Ahrens. (2003). The role of vascular endothelial growth factor in glucocorticoid-induced bone loss: evaluation in a minipig model. Bone 33: 869–876.
- Uchida, Soshi, Akinori Sakai, Hideaki Kudo, Hajime Otomo, Makato Watanuki, Masahiro Tanaka, , et al. (2003). Vascular endothelial growth factor is expressed along with its receptors during the healing process of bone and bone marrow after drill-hole injury in rats. Bone 32: 491–501.
- Dimitriou, Rozalia, Eleftherios Tsiridis, Giannoudis, Peter V. (2005). Current concepts of molecular aspects of bone healing. Injury 36: 1392–1404.
- Tatsuyama, K., Maezawa, Y., Baba, H. (2000). Expression of various growth factors for cell proliferation and cytodifferentiation during fracture repair of bone. Eur. J. Histochem. 44: 269–278.
- Boden, S.D., Hair, G.A., Viggeswarapu, M. (2000). Gene therapy for spine fusion. Clin. Orthop. 379 Suppl: S225–S233.
- Evans, C.H., Ghivizzani, S.C., Robbins, P.D. (2004). Orthopaedic gene therapy. Clin. Orthop. 429: 316–329.
- Fransceschi, R.T. (2005). Biological approaches to bone regeneration by gene therapy. J. Den. Res. 84: 1093–1103.
- Jardini, Maria Aparecida Neves, Andrea Carvalho De Marco, Luiz Antonio Lima. (2005). Early healing pattern of autogenous bone grafts with and without e-PTFE membranes: A histomorphometric study in rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 100: 666–673.