Abstract
“Atomic Force Microscope” (AFM) tips (cantilevers) carrying a pseudo-specific ligand, i.e., histidine were prepared and investigated for detection of Human Immunoglobulin-G (HIgG) in aqueous media. The AFM tips (cantilevers) were first treated with HNO3 and silanized to create amino groups; then glutaraldehyde (GA) was bonded via these surface amino groups; and finally, histidine molecules were immobilized by reaction of the amino groups of histidine with the free aldehyde groups of GA. Optimal immobilization conditions were described. Immobilizations were observed both by optical and confocal laser scanning microscopy. Interactions between the histidine carrying AFM tips (cantilevers) and the aqueous medium containing HIgG with different concentrations were quantified by “the separation distance” measured with the AFM system as the main variable. A quite nice linear correlation between the HIgG concentration and the separation distance was measured with AFM system. Interactions were also followed by an alternative “Modified Lowry” method, in which similar behavior was observed. We were able to measure HIgG concentration in aqueous media down to 0.055 pmol/μ l (8mg/dl) concentration with this AFM based novel immunosensor.
1. INTRODUCTION
“Atomic Force Microscopy” (AFM) has made a great impact on the concept of microscopy, especially in life sciences Citation[1]. AFM is a widespread technique for high-resolution imaging in the molecular/submolecular range and can be operated in aqueous environments, allowing the observation of dynamic molecular events in real-time and under somewhat physiological conditions Citation[2], Citation[3], Citation[4], Citation[5], Citation[6], Citation[7]. AFM generates an image of a molecule by probing its surface with a sharp tip integrated to the end of a flexible cantilever. The interaction signal, between the tip and the surface, is acquired and digitized to provide a three-dimensional image of the surface.
AFM has the ability to measure forces of 10 pN or less and this ability makes it an essential tool for measuring biological interaction. A number of studies have been performed to investigate interactions of antigens-antibodies Citation[8], Citation[9], Citation[10], Citation[11], Citation[12], Citation[13], Citation[14], Citation[15], Citation[16], Citation[17], Citation[18], Citation[19], Citation[20], Citation[21], streptavidin–biotin interactions have been studied with AFM Citation[22], Citation[23], Citation[24], Citation[25], Citation[26], Citation[27], Citation[28], Citation[29], Citation[30], and the strands of DNA Citation[31]. In this approach one of the complementary molecules (the ligand) is immobilized onto the cantilever (or tip) of the AFM, and the other (the target) is covalently attached to a substrate surface, and then interaction forces are measured with AFM.
Recently, the concept of cantilever-based biosensor techniques driven from AFM was released Citation[32], Citation[33], Citation[34], Citation[35]. Here, the ligand molecules are immobilized onto the AFM cantilevers, and then are interacted with the target molecules in the medium, which causes bending of the cantilever (due to interaction forces), which in turn can be used for identification of the target molecule within the medium.
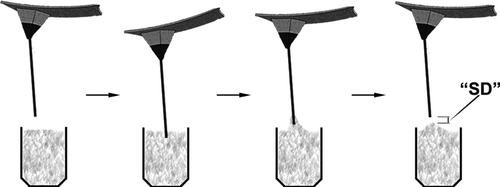
We have also attempted to develop novel molecular biosensors based on AFM, in which we have a much simpler and very economical approach to do the same measurements with the previous approach. Here, we measured the “separation distance” (SD) as the main parameter to follow the interaction between the ligand molecules immobilized on the cantilever (the tip) and the target molecules within the medium. In order to do this one only needs an AFM head with a cantilever, a sensitive approching unit, and a CCD camera. With this simple system, designed and constructed in our lab, we were able to develop a novel nucleic acid sensor Citation[36]. Following this preliminary study, we applied a similar strategy and used the same system for the detection of HIgG in aqueous media, and reported the results in this article. Note that we selected histidine as a pseudospecific bio-ligand, which has a specific affinity especially to HIgG, and has been used for affinity separation and identification by us and also others Citation[37], Citation[38], Citation[39].
2. MATERIALS AND METHODS
2.1. Materials
L-Histidine HCl·H2O (Biological Industries, Israel) was used as the pseudo-specific bio-ligand for detection of HIgG. All other chemicals, including the target molecule “Human Immunoglobulin-G” (HIgG), were purchased from Sigma (USA) and used without any pretreatment. Standard silicon nitride tips (cantilevers) were purchased from Digital Instruments (USA).
2.2. Immobilization of Histidine onto AFM Tips
The pseudo-specific ligand, i.e. histidine, was immobilized by covalent bonding via its primary amine groups onto the AFM cantilevers with a four-step procedure, which is a slight modification of the protocol applied by Chowdhury and Lunckham (for antibody immobilization) Citation[16], and almost the same that we have used for ssDNA immbolization Citation[36] onto AFM tips/cantilevers. These four steps are briefly as follows: (i) the silicon nitride tips were placed into 10% nitric acid solution, which was left in a silicon oil bath at 80°C for 20 min. This results in the formation of surface hydroxyl groups on the silicone nitride cantilevers; (ii) the cantilevers were placed into 10% 3-aminopropyltrimethoxysilane (APTS) solution with a pH of 7 adjusted with acetic acid at 80°C for 240 min, thus the reactive primary amine groups are formed on the nitride surface; (iii) gluteraldehyde (GA), as a bridging agent (and also a spacer arm) between the solid support and the histidine-ligand, was attached via surface primary amine groups (coming from APTS). Here, the cantilevers were reacted in 10% GA solution for 1 h. Note that in this third step, it is assumed that most of the GA molecules were covalently bonded to amine groups via one end, leaving the aldehyde group at the other end free for further reactions; and (iv) at the last step, histidine was covalently bonded (“immobilized”) to the cantilivers by the reaction of the free aldehyde groups on the cantilevers (coming from the GA molecules) and the primary amino groups of histidine. After preliminary studies Citation[40], histidine immobilization was achieved in aqueous medium buffered with sodium tetra borate (pH: 9) containing 2.5 nmol/μ L histidine, at room temperature for 1 h. Note that the cantilevers were thoroughly rinsed with distilled water after each step described above.
In order to follow changes on the AFM cantilever surfaces after the first three treatment steps, images of the surfaces were taken with an optical microscope, with 200× magnification (with 20× objective and 10× photoocular) (Axiosckop 20, Zeiss, Germany). The brightness, contrast was adjusted and some software filters (explained below each figure) were applied using PhotoShop v3.0 (Adobe Systems, CA, USA) to enhance details of images.
Histidine immobilizations on the AFM cantilevers were also observed on the images taken with a Confocal Laser Scanning Microscope (CLSM, Leica SP2, Heidelberg-Germany with the excitation wave-length of 488 nm). The cantilevers were incubated in a phosphate buffer solution (pH: 7) containing 1-ethyl-3-carbodiimide (EDAC, 10 mg/ml) for about 60 min at room temperature. 2 mg of pyrenmethylamine hydrochloride dissolved in 1 ml acetone + 0.1 ml of trimethylamine (TME) + 5 ml distilled water was added to this solution, and incubated (magnetically agitated) for 2 h at room temperature, in a dark room. The cantilevers were then thoroughly rinsed with distilled water, and CLSM images were obtained.
2.3. HIgG Detection
The solutions (50 μ L) containing different amounts of the target molecule, HIgG (0.39, 0.78, 1.56, 3.11, 6.25, 12.45, 24.90 and 49.80 μ g/ml) in phosphate buffer (pH: 7.4) were used. In order to follow the extent of interaction of the histidine carrying AFM tips with the target solution containing HIgG, a simple and novel approach was applied, which was decribed in detail in our previous paper Citation[36]. The apparatus used was a homemade atomic force microscope Citation[41]. The main part that allowed us to perform these tests is a Burleigh Aris 11 Approach Module (USA), which is simply an inchworm motor that provides precise approach positioning in Z-direction with high stiffness and holds position even with the power turned off. This type of motor allowed us to control the approach of the target solution (placed on the stage) to the AFM cantilevers to with a resolution of 1 nm and a speed range of 1 nm/s to 0.5 mm/s, which was achieved/controlled with the software that we have developed. We were also able to observe the position of the cantilever with a camera attached to the fiber optic unit of the AFM system, and to measure its distance from the surface of the target solution.
In a typical test, the sample containing the target solution (pure buffer solution or the buffer solution containing the target molecules) was driven at +z-direction to the AFM cantilevers with a controlled/selected speed until the cantilever touched the solution. Just at this position we waited about 10 min until it reached equilibrium (in the case of original measurements for HIgG determination to complete coupling reaction of histidine on the surface with the HIgG molecules in the solution), and then the sample was driven at the opposite direction (−z-direction) with a predetermined rate, about 900 nm/s Citation[41]. The “separation point” in which the AFM tip gets rid of the solution that sticks on its surface, was observed with the camera (with a 20X ocular) of the AFM system. The “separation distance” (in nm), was measured by counting the steps via computer (). Note that each step corresponds one nm distance.
Alternatively, HIgG adsorption (coupling) onto the AFM cantilevers carrying histidine was also measured by a modified form of the “Lowry” protein determination method Citation[40], Citation[42]. Typically, after the AFM cantilever was interacted with the HIgG solution at the conditions described above, the supernatant was removed and diluted with distilled water to 1 ml. 0.1 ml deoxycholate (DOC, 15%) was added to this solution and stirred for 10 min. 0.1 ml copper tartrate carbonate (72%) was added, and stirred for another 10 min. All these were done at room temperature. Then this solution was centrifuged at 1400 rpm for 30 min, the supernatant was discarded and the precipitate was dissolved in 0.2 ml distilled water and 0.2 ml Reagent A (a mixture of copper tartarate carbonate, NaOH, sodium deodecyl sulfate and water). Reagent B (a mixture of folin ciocalteu and water) was added to this solution and stirred magnetically for 30 min at room temperature in a dark room. Then, absorbance was measured at 750 nm with a UV-spectrophotometer (Shimadzu, UV-1601, Japan).
3. RESULTS AND DISCUSSION
3.1. Functionalization of AFM Cantilevers
The AFM cantilevers were modified by using an amino-containing silane compound (APTS), which was used for silylation of the tips/cantilevers by us and others in similar studies for modification of AFM tips and silica surfaces in general Citation[32], Citation[36], Citation[43], Citation[44]. There are some other possible reactions in silylation of surfaces with APTS, which may interfere with successful modifications, such as hydrolysis of APTS to form silanols and siloxane bonds Citation[36]. The reaction of APTS with the silanol surface may produce the desired amino functional groups on the surface, but the self-condensation reaction of APTS with itself will lead to cross-linking and “uncontrollable polymerization.” However, the latter, cross-linked polymer will remain suspended in the water phase and is usually removed (washed away) while the aminosiloxane that was grafted to the surface is covalently bonded and is permanently attached. The other possible reaction is a combination of self-condensation and grafting with the surface. However, the product in this case is essentially the same as the grafting reaction.
Trimethoxy functional silanes have been successfully grafted to silica surfaces from aqueous solutions over a wide pH range, including the pH values that we have used here Citation[45]. Water is needed in this procedure in order to hydrolize the methoxy groups of the silane. Usually, a catalyst is added to the water in order to facilitate the condensation and grafting reaction. Amines are examples of such catalysts. However, in our case, there was no need for additional catalyst since the amine on the APTS molecules was sufficient.
After the silanization reaction, we have further modified AFM cantilevers by glutaraldehyde (GA) attachment. Note that GA has six carbon atoms (a linear chain) and two aldehyde groups at the two ends; therefore it has been very widely used as a bridging agent and also a spacer arm for immobilization of several bio-ligands onto solid support in which usually the primary amine groups on both bio-ligand and surface are bridged. In this study we have selected the same strategy for immobilization of histidine (carries primay amine groups) onto the AFM cantilevers via surface primary amine groups (coming from APTS).
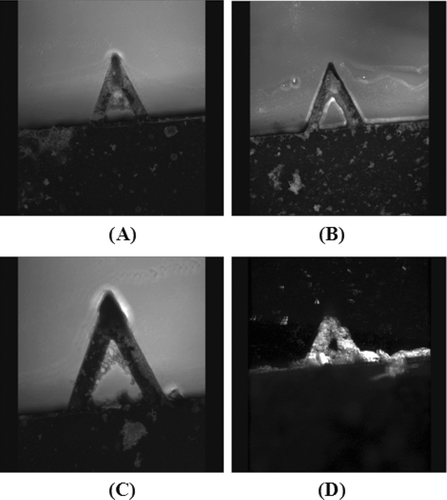
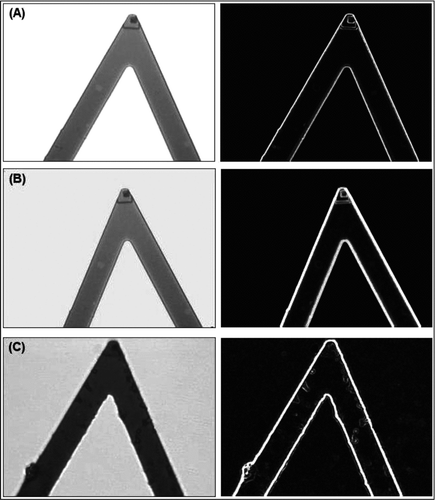
Representative micrographs of the AFM cantilevers taken with an optical microscope before and after chemical modifications are given in . There were no changes after the silanization step, while structures with different sizes on different parts of the cantilever surface, including at the top corner where the tip is located, were very much observable. It is not possible to see the single glutaraldehyde (GA) molecules by optical microscopy; therefore, most probably these structures are some GA polymeric (rather oligomeric) chains or aggregates that may occur during the treatment the surfaces with GA. From these images we assumed that the other parts of the surface of both the cantilever and tip were covered with GA surface groups that are available for further modifications.
Figure 1 Representative optical micrographs of the AFM cantilever with 200X magnification (with 20X objective and 10X photoocular): (A) untreated; (B) after silanization step; and (C) after glutaraldehyde attachment. Pictures at the left column are the images without using filters, while the ones at the right column were after filtering by using Photoshop, glowing edges.
Histidine is an amino acid, and a well known pseudo-specific bio-ligand that was used in affinity separation and biospecific recognition of immunoglubulins, especially HIgG Citation[37], Citation[38], Citation[39]. In this study we have also attempted to develop an AFM-based affinity sensor for HIgG detection in aqueous media by using histidine as the bio-ligand. It has a primary amine group; therefore we were able to covalently immobilize onto the GA attached AFM cantilevers, described in the previous section via the free aldehyde groups (coming from GA) on the surfaces. Histidine immobilization was successfully achieved from buffer solution (sodium tetraborate, pH: 9) containing 2.5 nmol/μ L at room temperature in 1 h. . First reference to shows representative confocal microscopy images of the histidine immobilized AFM cantilevers. It seems that almost all of the tip and cantilever are covered with histidine molecules. These histidine-carrying AFM cantilevers were taken to the HIgG detection experiments discussed below.
3.2. Detection of HigG
Recently, we were able to develop a very simple and novel detection system (based on AFM) for following hybridization of target single strand oligonucleotides with their complementary strains immobilized onto AFM tip (cantilever) surfaces, as a molecular nucleic acid sensor Citation[36]. Here, in this study, by following the same approach we have attempted to design a biosensor to detect HIgG in aqueous media, in which AFM cantilevers carrying histidine as the pseudospecific bio-ligand were used.
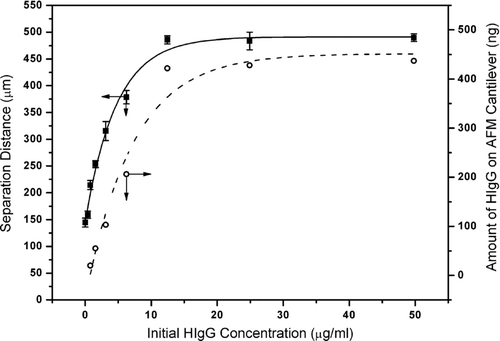
It is well known from the surface tension (contact angle) measuring systems that when a surface is dipped in (“descent”) and out (“ascent”) of a liquid, forces representing the interaction between the surface and liquid are measured, which are related to the surface tension of the liquid and depend on not only the liquid phase properties but also on the surface properties (hydrophobicity, ionic charges, etc.) Citation[46]. Our approach is based on this well-known basic information. As described in the experimental part, 50 μ L solutions containing different amounts of HIgG were interacted with AFM cantilevers carrying histidine, and the “separation distance” values were measured (see also Scheme 1). The values measured for the solutions containing different amounts of HIgG are given in . As seen here there are two parts of the curve. The first part is in the HIgG concentration range of 0.39–12.45 μ g/ml, in which there is a quite nice linear correlation between the HIgG concentration and the separation distance measured with the AFM system. It means that with this novel system one could not only qualitatively (existing or not) but also quantatively (the amount of HIgG) detect/measure HIgG in the aqueous media quite sensitively in the concentration range described above. As expected, when the concentration was high enough (more than about 12–15 μ g/ml) the separation distance reached a plateau and did not change with further increase in the HIgG concentration, which represents the whole coverage of the AFM cantilever surface.
In parallel to the AFM measurements we have also looked at the changes of the amount of HIgG adsorbed onto the histidine carrying AFM cantilevers after interaction with HIgG solutions with different concentrations. The dotted-line curve in gives this data. The shape of the whole curve is very nicely fit (in parallel) with the previous curve, which proves that the simple AFM sensor developed in this study measures the HIgG concentration quite effectively/correctly.
Figure 3 Change of separation distance (measured by the AFM system; average of ten repeated measurements with each five cantilevers and the standard deviation) and amount of HIgG adsorbed onto histidine carrying AFM cantilevers (obtained by the modified Lowry method).
We have investigated repeated use of the AFM sensors in repeated experiments. Unfortunately, we were not able to measure close values even after the first desorption of HIgG from the cantilever surfaces and adsorption, even if we have used the aqueous solution with the same HIgG concentration. The AFM cantilevers/tips that we have used were not mechanically strong enough to use more than a few times. We are still working to find a better approach for the desorption/adsorption cycle, which would be more effective and allow repeated use. However, in the meantime we concluded that either cheaper AFM tips for single use should be preferred, or the tips resisting to high mechanical forces should be designed. Our efforts on using these novel sensors for measuring HIgG in biological fluids (e.g., blood) are under investigation.
4. CONCLUSIONS
Today, AFM is considered an important device of modern nanotechnology for imaging of surfaces at the submicron level, even down to a few nanometers Citation[1], Citation[2], Citation[3], Citation[4], Citation[5], Citation[6], Citation[7]. Recent studies have exhibited that AFM could be used as a tool to measure the interaction forces between biological molecules including antibody-antigen, streptavidin-biotin, oligonucleotides with their complementaries Citation[8], Citation[9], Citation[10], Citation[11], Citation[12], Citation[13], Citation[14], Citation[15], Citation[16], Citation[17], Citation[18], Citation[19], Citation[20], Citation[21], Citation[22], Citation[23], Citation[24], Citation[25], Citation[26], Citation[27], Citation[28], Citation[29], Citation[30], Citation[31]. In this approach one of these complementary molecules is covalently immobilized onto the cantilever (or tip) of the AFM while the other onto a substrate surface, and then interaction forces between these two are measured with AFM. Alternative studies have also demonstrated that the ligand molecules are immobilized onto the AFM cantilevers, and then are interacted with the target molecules in the medium, which causes bending of the cantilever (due to interaction forces), which in turn can be used for identification of the target molecule within the medium Citation[32], Citation[33], Citation[34], Citation[35]. Here, the forces should be quantified, which requires all the complex hardware (optical and mechanical features) and software of the AFM system. Very recently, we have also attempted to develop molecular biosensors based on AFM Citation[36]. In our approach, we apply a very simple strategy as depicted in Scheme 1, and measure the “separation distance” (SD) as the main parameter to follow the interaction between the ligand molecules immobilized on the cantilever (the tip) and the target molecules within the medium. Note that for this type of measurement a quite simple/economic hardware consisting of an AFM head with a cantilever, a sensitive approching unit, and a CCD camera would be enough to obtain the SD values. We were able to design and construct this simple system in our labs and applied it first for detection of single strand oligonucleotides using their complementary strands immobilized onto AFM tips (cantilevers) as biorecognition element (ligand) Citation[36]. Here, in this study we made one further step, and used a pseudo-specific ligand, histidine, as bio-ligand, which was immobilized onto the AFM tips (cantilevers) for detection of HIgG within aqueous media. This paper demonstrates that histidine can be immobilized onto the AFM tips (cantilevers) effectively, and then can be used even for quantitative determination of HIgG concentration. We have also observed some limitations, which were mainly due to mechanical strength of the AFM tips, which did not allow us to use the same tips for repeated application (desorption-adsorption).
The authors would like to thank S. Ali Tuncel and M. Tuncel for confocal imaging. Prof. Erhan Pişkin was supported by the Turkish Academy of Sciences as a full member.
REFERENCES
- Binnig G., Quate C. F., Gerber C. Phys Rev Lett 1986; 56: 930
- Rugar D., Hansma P. K. Phys Today 1990; 43: 23
- Radmacher M., Tillmann R. W., Fritz M., Gaub H. E. Science 1992; 157: 1900
- Henderson E., Haydon P. G., Sakaguchi D. S. Science 1992; 257: 1944
- Rief M., Gautel M., Qesterheit F., Fernandez J. M., Gaub H. E. Science 1997; 276: 1109
- Oesterhelt F., Oesterhelt D., Pfeiffer M., Engel A., Gaub H. E., Muller D. Science 2000; 288: 143
- Charras C., Lehenkari P., Horton M. Methods Cell Biol. 2002; 68: 171
- Liu H. Y., Fan F.-R., Lin C. W., Bard A. J. J Am Chem Soc. 1986; 108: 3838
- Ducker W. A., Senden T. J., Pashley R. M. Nature 1991; 353: 239
- Williams R. A., Blanch H. W. Biosens. Bioelectron. 1994; 9: 159
- Stuart J. K., Hlady V. Langmuir 1995; 11: 1368
- Wu G., Datar R. H., Hansen K. M., Thundat T., Cote R. J., Majumdar A. Nat. Biotechnol. 2001; 19: 856
- Stuart J. K., Hlady V. Langmuir 1995; 11: 1368
- Dammer U., Hegner M., Anselmetti D., Wagner P., Dreier M., Huber W., Guentherholdt H.-J. Biophys. J. 1996; 70: 2437
- Allen S., Chen X., Davies J., Davies M. C., Dawkes A. C., Edwards J. C., Roberts C. J., Sefton J., Tendler S. J., Williams P. M. Biochemistry 1997; 36: 7457
- Chowdhury P. B., Luckham P. F. Colloid Surface A 1998; 143: 53
- Hinterdorfer P., Schilcher K., Baumgartner W., Gruber H. J., Schindler H. Nanobiology 1998; 4: 39
- Ros R., Schwesinger F., Anselmetti D., Kubon M., Schaefer R., Pluckthun A., Tiefenauer L. Proc. Natl. Acad. Sci. 1998; 95: 7402
- Willemse O. H., Snel M. M. E., van der Werf K. O., De Grooth B. G., Greve J., Hinterdortfer P., Gruber H. J., Schindler H., van Kooyk Y., Figdor J. G. Biophys. J. 1998; 75: 2220
- Stuart J. K., Hlady V. Biophys. J. 1999; 76: 500
- Harada Y., Kuroda M., Ishida A. Langmuir 2000; 16: 708
- Lee G. U., Kiddwell D. A., Colton R. J. Langmuir 1994; 10: 354
- Florin E.-L., Moy V. T., Gaub H. E. Science 1994; 264: 415
- Chilkoti A., Boland T., Ratner B., Stayton B. S. Biophys. J. 1995; 69: 2125
- Wong S., Joselevich E., Woolley A. T. Nature 1998; 394: 52
- Chunbo Y., Chen A., Kolb P., Moy V. T. Biochemistry 2000; 39: 10219
- De Paris R., Strunz T., Oroszlan K., Guntherodt H.-J., Hegner M. Single Mol. 2000; 1: 285
- Lo Y.-S., Zhu Y.-J., Beebe T. B. Langmuir 2001; 17: 3741
- Busse S., Scheumann V., Menges B., Mittler S. Biosens. Bioelectron 2002; 17: 704
- Zhang X., Moy V. T. Biophys. Chem. 2003; 104: 271
- Lee G. U., Chrisey L. A., Colton R. J. Science 1994; 266: 771
- Moulin A. M., O'Shea S. J., Welland M. E. Ultramicroscopy 2000; 82: 23
- Fritz J., Baller M. K., Lang H. P., Rothuizen H., Vettiger P., Meyer E., Güntherodt H.-J., Gerber C., Gimzewski J. K. Science 2000; 288: 326
- Battiston F. M., Ramseyer J.-P., Lang H. P., Baller M. K., Gerber C., Gimzewski J. K., Meyer E., Güntherodt H. J. Sensor. Actuat. B-Chem 2001; 77: 122
- McKendry R., Zhang J., Arntz Y., Strunz T., Hegner M., Lang H. P., Baller M. K., Certa U., Meyer E., Güntheodt H. J., Gerber C. Proc. Natl. Acad Sci. 2002; 99: 9783
- Koçum C., Ülgen S. D., Çubukçu E., Pişkin E. Ultramicroscopy 2006; 106: 326
- El-Kak A., Manjini S., Vijayalakshmi M. A. J. Chromatography A 1992; 604: 29
- Kalaycıoğlu E., Patır S., Pişkin E. Langmuir 2003; 19: 9538
- Denizli A., Alkan M., Garipcan B., Özkara S., Pişkin E. J. Chromatography B 2003; 795: 93
- Çınar O. M.Sc. Thesis, Hacettepe University, Bioengineering Division, Ankara, Turkey 2006
- Ülgen S. D. M.Sc. Thesis, Hacettepe University, Bioengineering Division, Ankara, Turkey 2003
- Lowry O. H. J. Biol. Chem. 1951; 193: 265
- Leyden D. E. Silanes, Surfaces and Interfaces, First ed. Gordon and Breach, New York 1986
- Plueddemann A. P. Silicone Compounds, Silylating Agents, Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed. Wiley, New York 1982; vol. 20: 962
- Pludedmann E. P., Stark G. L. Mod. Plast. 1974; 92: 74
- Principles of Colloid and Surface Chemistry, Second ed., P. C. Hiemenz. Marcel Dekker, Inc., New York 1986