Abstract
To investigate the possibility of constructing artificial peripheral nerves using de-acetyl chitin conduit, the sciatic nerves defect model was built at left legs in SD rats. They were divided into 3 groups randomly: group A: nerve graft in situ (n = 12, gap distance 10 mm); group B: biological chitin conduit bridging the peripheral nerve defect (n = 12, gap distance 10 mm); group C: biological chitin conduit bridging the peripheral nerve defect with nerve fibers in conduits (n = 12, gap distance 10 mm). Electrophysiological examination, histological examination and re-myelinated axons counting were applied after 6th and 12th week after operation, respectively. Regenerated nerve fibers were seen in the distal nerve segments of all three groups. The nerve conduction velocity and the re-myelinated axons counting of group A were better than that of group C at both 6th and 12th week time points (p < 0.05). The nerve conduction velocity and the re-myelinated axons counting of group C were better than that of group B at both 6th and 12th week time points (p < 0.05). The repair effects of chitin conduit with nerve fibers in conduit bridging peripheral nerve defect (10 mm) were better than that of simple conduit bridging group, and that of group A (nerve graft group) was better than that of group C.
Keywords:
The bridging of a long segment peripheral nerve defect was always a big problem in clinics. The autologous nerve graft is almost the only and best way to repair the long segment defect of peripheral nerves. But the new injury and functional defection will appear when we apply the autologous nerve graft to repair the peripheral nerve defect. The preparation of artificial nerves to substitute for the self nerve is always the main project of medical scientists all over the world Citation[8]. Our research used de-acetyl chitin conduit (State Intellectual Property Office of P.R.C, Patent Number: 01136314.2) on the basis of selective regeneration theory in the peripheral nerve regeneration process. In the experiment, we applied the single nerve fasciculus with de-acetyl chitosan conduit to repair the defect of peripheral nerve in order to explore the feasibility of applying the chitin conduit in clinical cases.
MATERIALS AND METHODS
Material and Grouping
Material: midheaven chitin conduit (de-acetyl chitosan conduit invented by People's Hospital of Peking University and Chinese Textile Academy, Patent Number: 01136314.2). The length was 12 mm, the inner diameter was 1.5 mm, and the wall thickness was 0.1 mm.
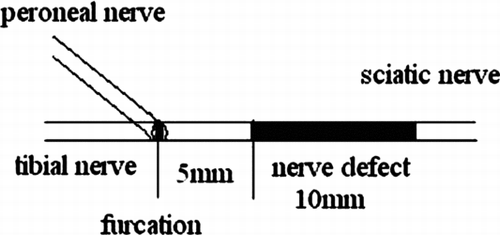
Grouping: 36 healthy, mature, male SD rats weighing 200∼ 250 g were divided into 3 groups randomly. We established the defect model in the right sciatic nerve; the length of defect was 10 mm, the distance from distal end of defect to furcation of sciatic nerve was 5mm. Group A: nerve graft in situ (n = 12,10 mm); group B: biological conduit bridging the peripheral nerve defect (n = 12, with 10 mm); group C: biological conduit bridging the peripheral nerve defect with nerve fibers in conduits (n = 12, with 10 mm).
Experiment Methods
The rats were anesthetized by 5% Ketamine (0.2 ml/100 g) injected into the abdominal cavity. The right sciatic nerve was exposed, establishing the defect model with 10 mm defect; the distance from distal end of defect to furcation of sciatic nerve was 5 mm ().
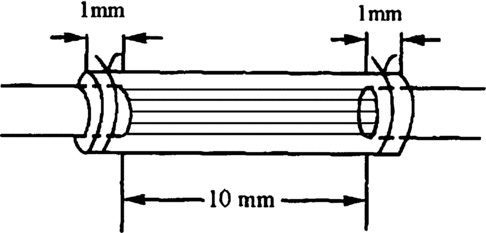
The suture method of nerve graft in situ (group A): cut off 10 mm nerve in 4× operating microscope, the distance from distal end of defect to furcation of sciatic nerve was 5 mm, suture it in situ by 9-0 nylon suture. The suture method of conduit (group B and group C): made the same defect in rats; selected the suitable chitin conduit to bridge the defect. A sewing needle threaded the conduit from outer to inner, 1 mm distance to conduit, then threaded the perilemma and conduit from inner to outer, 1 mm distance to proximum broken ends of nerve, tensing two suture line, ligaturing, the broken ends of nerve could insert to conduit about 1mm. Both the distal end and proximate were all inserted to the conduit about 1mm, 10 mm distance between the broken ends of the nerve.
The suture method of nerve fibers in conduits (group C): dissect the nerve (cut down in establishing nerve defect) carefully; choose 5 single nerve fasciculus in 4 multiple operating microscopes, first suture the 5 nerve fasciculus to the broken ends of nerve, every fasciculu suture one needle. Then we bridged the nerve defect with conduits; the suture method was the same as group B. The block diagram is described in .
Detection Items
Observations in general: 6 weeks and 12 weeks after operation, every group selected 6 rats, revealed the sciatic nerve of the right leg, and observed the appearance in general at suture and conduit.
Electrophysiologic study (EPS): 6 weeks and 12 weeks after operation, selected 6 rats were taking EPS. We put the electrode in group A in two sides of the nerve stoma, and in group B and group C in two sides of the conduit about 1∼ 2 cm. The center side was stimulating electrode; the periphery side was leading electrode and measured the distance between electrodes. We determined the nerve conduction velocity (NCV) of sciatic nerves by electromyogram instrument (Oxford).
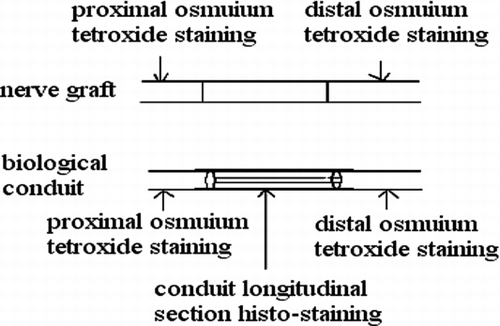
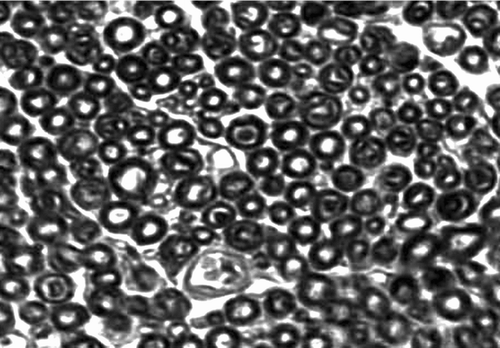
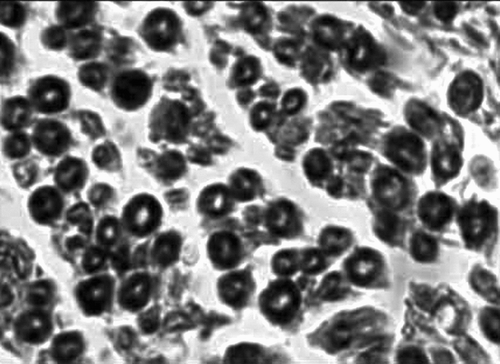
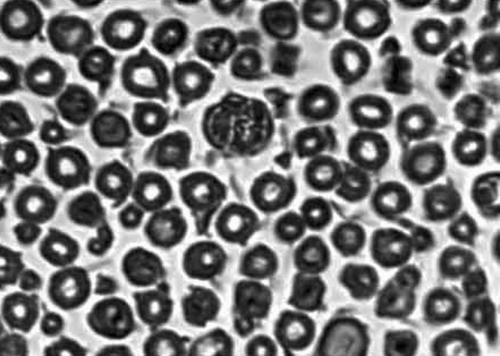
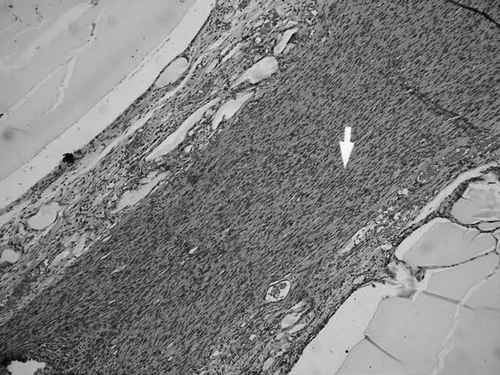
Observed in light microscope: 6 weeks and 12 weeks after operation, we executed the rats and finished the electromyogram determination. Group of conduit: we observed the regeneration of nerve fasciculus in longitudinal section of conduit; 5 mm nerve segment of distal conduit stained by osmic acid to count the number of re-myelinated nerve axons; 5 mm nerve segment of proximal conduit as comparison. Group of nerve graft: we chose the distal end of distal anastomosis's 5 mm nerve segment stained by osmic acid to count the number of re-myelinated nerve axons; the proximal end of proximate anastomosis's 5 mm nerve segment was used as comparison. shows how to deal with the nerve tissue.
The specimen was fixed by 10% formalin, osmic acid stained, paraffin imbedding, and then sliced into 3μ m sections. We observed under a light microscope (amplify 10× 40), and counted the number of myelinated nerve fibers in the unit visual field. Three groups drew the materials in different times (6 weeks, 12 weeks), and 6 nerve sections were counted and analyzed. We took the left upper, right upper, right lower, left lower, and meso five places as the visual field units to count. We used the mean in 5 unit visual fields as the number of myelinated nerve fibers. The area of distal nerve section was calculated by pathological image manipulation software designed by BeiHang University, calculating the mean of the single fiber neurite area after counting 100 neurite areas.
Statistical analysis: statistical data was expressed by x ± s, SPSS11.0 Statistical software was used to apply statistical analysis. Comparison in multiple mean applied the “q” test; comparison in two groups applied the “t” test; p < 0.05 was regarded to be of statistical significance. We compared the difference of the mean value of NCV, and re-myelinated nerve axons in the unit visual field and area section.
RESULTS
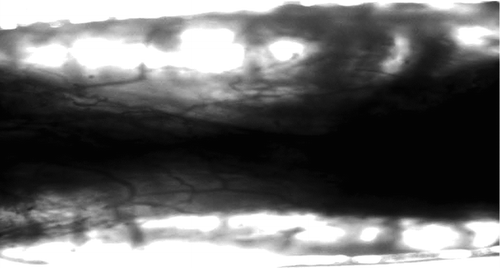
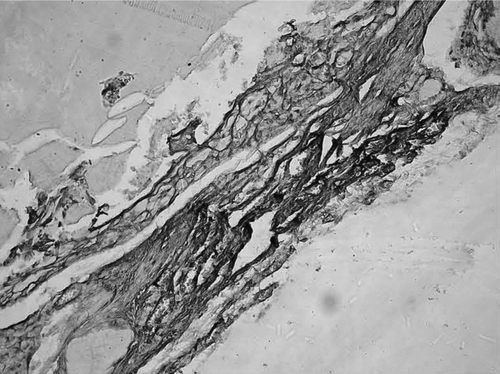
Observations in general: at 6 weeks there was no apparent conglutination around the nerve conduit and acroteric tissue after the operation. At 12 weeks: the surface of chitin conduit was a little crude, part of the conduit had been absorbed, but still could sustain the inherent lumens shape. The vascular net on the nerve conduit was quite clear ( and ).
Motor nerve conduction velocity (MNCV): shows the results of NCV about 3 groups in time point of 6 weeks and 12 weeks. Seen from the MNCV recovery trend, MNCV of three groups at 12th week were better than that at 6th week, but MNCV of nerve graft group was obviously better than that of the fasciculu + conduit group (p < 0.05); MNCV of fasciculu + conduit group was better than that of the simple conduit group (p < 0.05). After 12 weeks, MNCV of three groups at the 12th week were better than that at the 6th week, but MNCV of the nerve graft group was obviously better than that of the fasciculu + conduit group (p < 0.05); MNCV of the fasciculu + conduit group was better than that of the simple conduit group (p < 0.05).
Re-myelinated nerve axons counting in the visual field unit: showed every group's myelinated nerve fiber counting in the 6th and 12th weeks. From the tendency of recovery of re-myelinated nerve axons counting in every group, the number of re-myelinated nerve axons in 12 weeks is less than that in 6 weeks in the nerve graft group; the number of re-myelinated nerve axons in the 12th week was more than that in 6 weeks in the conduits group and in the fasciculu + conduit group. At the time point of the 6th week, the difference between the nerve graft group and the fasciculu + conduit group was of significance (p < 0.05); the difference between the fasciculu + conduit group and the simple conduit group was of significance (p < 0.05). At the time point of the 12th week, the difference between the nerve graft group and the fasciculu + conduit group was of significance (p < 0.05); the difference between the fasciculu + conduit group and the simple conduit group was of significance (p < 0.05). So at the time point of the 6th week and 12th week, the re-myelinated nerve axons number of the nerve graft group was better than that of the fasciculu + conduit group; the re-myelinated nerve axons number of the fasciculu + conduit group was better than that of the single conduits group.
Cross section area of distal re-myelinated nerve axons: every group calculated cross section area of distal re-myelinated nerve axons at the 6th week and 12th week time point. From the tendency of recovery of the cross section area of the distal myelinated nerve fiber axon, the 12th week's average cross section area was bigger than that in the 6th week in 3 groups. At the time point of the 6th week, the nerve graft group's area was bigger than that in the fasciculu + conduit group, and the difference was of significance (p < 0.05); the fasciculu + conduit group's area was bigger than that in the simple conduit group's. At the time point of the12th week, the nerve graft group's area was bigger than that in the fasciculu + conduit group, and the difference was of significance (p > 0.05); the fasciculu + conduit group's area was bigger than that of the simple conduit group, and the difference was of significance (p < 0.05). shows all groups' data; , , show every group's quality of regeneration in distal nerve fiber axon (number and area).
Histological observationss: simple conduit group and fasciculu + conduit group could be found with complete regeneration nerve fibers at the 6th week. shows the HE staining. shows S100 staining to display the regenerated nerve fiber in conduit.
Table 1 The motor nerve conduction velocity of three groups
Table 2 Re-myelinated nerve axons counting in three groups
Table 3 Cross section area of distal re-myelinated nerve axons
DISCUSSION
The bridging of long segment nerve defects of the peripheral nerve is always a tough problem in clinics. The autologous nerve graft is almost the only and best way to repair the long segment peripheral nerve defect. But the new injury and functional defection will appear when we apply the autologous nerve graft to repair the peripheral nerve. The preparation of artificial nerves to substitute for the self nerve is always the main project of medical scientists all over the world Citation[5]. After using de-calcifying bone manufacture as the osseous conduit to bridge the defect of peripheral nerves, which Gluck and Vanlain first reported in the 1880s, there were several scholars using artery, anadesma vessel, vein and freeze drying artery to repair the peripheral nerve defect Citation[3], Citation[4], Citation[6]. With the wide use of artificial materials in biomedicine fields, people try to use artificial materials to make nerve conduit continously. According to the nerve regeneration biological features, the artificial conduit should have the below conditions: (1) specific three dimensional structure frame—nerve conduit; it can allow the growth of axons and has the aiming mechanical effect; (2) functional Schwann cells, biologic absorbable materials, and extra-cellular matrix can form a beneficial micro-environment and can facilitate the nerve fiber regeneration. Schwann cells play an important role during nerve regeneration process. If Schwann cells can move to broken nerve ends quickly, form a Bungner band by division growth and secret multiple neurotrophic factors, which can provide beneficial a micro-environment for nerve regeneration. In our experiment, the reserved fasciculu included Schwann cells, and Schwann cells can sustain secret neurotrophic factors and accelerate the nerve regeneration.
We use an absorbable de-acetyl chitin conduit compound with nerve fasciculus to construct artificial peripheral nerve bridging, basing it on the small gap artery sleeve bridging in peripheral nerve repair in our previous research Citation[1], Citation[2], Citation[7]. This artificial peripheral nerve bridging can maintain mechanical stabilization to some degree, avoiding scar tissue growing into the broken ends of nerve. The preserved cellular structure provided various nutrient substance exchanging and blood vessel growth, which can support the cell's survival. These materials were gifted with well biocompatibility and can fully degrade into water and carbon dioxide, which can avoid the second operation and compression to nerve from unabsorbable conduit. When we used the artificial peripheral nerve to bridge SD rats sciatic nerve10 mm defect, we found that the number of regeneration nerve fasciculus was less than that in 5 mm defect. The reason may be lack of supporting of Schwann cells. So we used the nerve fasciculus in conduit, aiming to preserve some functional Schwann cells in conduit for axon regeneration.
Our research found that the observation items of the three groups at 12th week (number of distal re-myelinated nerve axons, area of distal re-myelinated nerve axon, and MNCV) were all higher than that of the 6th week. We did not obtain the atrophy and decrease phenomenon of the number of regeneration axons during the peripheral nerve regeneration process. According to the classical peripheral nerve regeneration theory, there were plenty of nerve axons collateral erupted in proximate nerve fasciculus, and they grow into a distal newly formed myelin sheath by Schwann cells. If one axon grows into the myelin sheath successfully, others will atrophy and degenerate. Seen from this experiment and the classical regeneration theory, the only explanation was that the number change finished in 6 weeks. We laid particular emphasis on the comparison on the long-term effect of artificial peripheral nerve bridging, thus we select a later time point. It can be inferred from our experiment that the repairing nerve defect effect (10 mm in SD rats) of the artificial peripheral nerve with nerve fasciculus was better than that of artificial nerve bridging without nerve fasciculus, but the final results were not better than the autologous nerve graft in situ. It showed there was still a long way to go in artificial nerve bridging substitute nerve grafts to repair peripheral nerve defect.
We constructed this animal experiment model in order to find a way to bridge nerve defects. When the defect is a little longer (10 mm), we inserted the self nerve fasciculus to provide frame and axons growth targeting effectively. It's a cue that the space disposition and configuration in construction of artificial peripheral nerve should be more reasonable. In this experiment, the nerve fasciculus dissection was done under an operating microscope, which included dissecting nerve fasciculus' fascia and bridging nerve fasciculus into nerve conduit. The accuracy microsurgery operation can provide a reliable frame and targeting for regeneration nerve axons.
This paper was funded by Chinese National 973 Project (No: 2003CB515306) and Chinese National 973 Project (No: 2001CCA01300), and Chinese National Outstanding Youth Project (No. 30625036).
REFERENCES
- Baoguo Jiang, Jitian Taier. The histological observation after small gap artery bridging in peripheral nerve. Chinese Hand Surgery Journal 1998; 14: 50–52
- Baoguo Jiang, Shuhuan Wang, Chuanhan Feng. The comparison of small gap artery bridging and epineurial neurorrhaphy in peripheral nerve. Beijing Medical University Journal 1994; 26: 249–250
- Belkas J. S., Shoichet M. S., Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004; 26(2)151–160
- Gamez E., Goto Y., Nagata K., Iwaki T., Sasaki T., Matsuda T. Photofabricated gelatin-based nerve conduits: nerve tissue regeneration potentials. Cell Transplant 2004; 13(5)549–564
- Hisasue S., Kato R., Sato Y., Suetomi T., Tabata Y., Tsukamoto T. Cavernous nerve reconstruction with a biodegradable conduit graft and collagen sponge in the rat. J Urol 2005; 173(1)286–291
- Lundborg G. A 25-year perspective of peripheral nerve surgery: Evolving neuroscientific concepts and clinical significance. J Hand Surg [Am] 2000; 25: 391–414
- Jian Li, Baoguo Jiang, Dianying Zhan, et al. The experimental research of small gap bridging peripheral nerve injury with biological chitin conduit. Chinese Hang Surgery Journal 2003; 19(2)118–120
- Yang Y., Gu X., Tan R., Hu W., Wang X., Zhang P., Zhang T. Fabrication and properties of a porous chitin/chitosan conduit for nerve regeneration. Biotechnol Lett 2004; 26(23)1793–1797