Abstract
Microencapsulation may allow for immunosuppression-free islet transplantation. Herein we investigated whether human islets can be shipped safely to a remote encapsulation core facility and maintain in vitro and in vivo functionality. In non-encapsulated islets before and encapsulated islets after shipment, viability was 88.3±2.5 and 87.5±2.7% (n=6, p=0.30). Stimulation index after static glucose incubation was 5.4±0.5 and 6.3±0.4 (n=6, p=0.18), respectively. After intraperitoneal transplantation, long-term normoglycemia was consistently achieved with 3,000, 5,000, and 10,000 IEQ encapsulated human islets. When transplanting 1,000 IEQ, mice returned to hyperglycemia after 30-55 (n=4/7) and 160 days (n=3/7). Transplanted mice showed human oral glucose tolerance with lower glucose levels than non-diabetic control mice. Capsules retrieved after transplantation were intact, with only minimal overgrowth. This study shows that human islets maintained the viability and in vitro function after encapsulation and the inhomogeneous alginate-Ca2+/Ba2+ microbeads allow for long-term in vivo human islet graft function, despite long-distance shipment.
INTRODUCTION
Obstacles to the widespread use of islet transplantation for the treatment of juvenile diabetes include an insufficient number of appropriate donors, toxic immunosuppression protocols, recurrence of the underlying autoimmune disease, and sensitization of the recipient Citation[1], Citation[16], Citation[25], Citation[35], Citation[39]. Although new sources of cells and tissue may be developed in the future Citation[28], Citation[31], Citation[33], the destruction of the graft by the immune system may remain difficult to prevent without systemic immunosuppression. The side effects associated with long-term immunosuppression have been well documented. Enveloping the islets in semi-permeable capsules may enable transplantation of islets without immunosuppression. Lim and Sun first described this approach of embedding the pancreatic islets in alginate-PLL-alginate microcapsules and provided evidence that encapsulated islets could regulate blood glucose in rats Citation[13]. After this groundbreaking study, many investigations into encapsulated islet transplantation have been performed with documented immunosuppression-free graft survival in mice Citation[8], Citation[41], Citation[42], rats Citation[4], Citation[15], Citation[37], dogs Citation[12], Citation[27], monkeys Citation[6], Citation[32], and humans Citation[3], Citation[23].
A variety of semi-permeable membranes have been evaluated as immunoprotective barriers. Alginate has been the most frequently used material, as it is non-toxic and the encapsulation can be carried out under physiological conditions, conserving islet viability and function Citation[40]. Alginate composition, molecular weight, and concentration determine the microcapsule structure and properties, together with the composition of gelling and anti-gelling ions.
Stability, permeability, size, and biocompatibility are all considered important capsule properties. Stability and permeability can be further improved by adding a polycation layer to the surface of the alginate gel core, as coating was used in the original procedure of Lim and Sun. However, the addition of a polycation has been shown to provoke inflammation Citation[27], Citation[30] and is, quite likely, the major cause of capsule toxicity. Recently, Calafiore et al. initiated a phase I trial with proof of concept of preserving islet function without immunosuppression by using an alginate-PLO microcapsule Citation[3]. However, the low number of islets transplanted per patient has limited the success of this pilot trial and insulin independence has not been achieved.
In this study, the current knowledge of alginate structure – function relationship was optimized in a novel alginate capsule design. An alginate with a high molecular weight and a high content of G was used in combination with Ca2 + and a minimal concentration of Ba2 + as this allows for very strong and stable microcapsules Citation[14]. In addition, sodium was exchanged with mannitol as osmolyte in both the alginate and in the gelling solution, resulting in a higher concentratioin of alginate at the capsule surface than in the center Citation[36] that further improves the capsule stability. Hence, stable microcapsules were obtained without a proinnflammatory polycation Citation[30]. The microcapsules were permeable to IgG Citation[14], but microcapsules of similar permeability have previously been shown to protect both allo- and xenografts in mice Citation[5], Citation[17].
Since the field of immunoisolation involves expertise from different fields such as surgery, chemistry, engineering, and cell biology, an international approach was taken in the current study. The goal was to combine experience on islet isolation, microcapsule design, chemistry, engineering, and transplantation to improve the outcome of encapsulated islet transplantation. Human islets were isolated at the University of Illinois at Chicago (UIC), shipped to the Norwegian University of Science and Technology (NTNU) for microencapsulation, and then returned to Chicago. In this study, we assessed the viability and function of human islets in vitro and in vivo after being encapsulated in this novel inhomogeneous alginate-Ca2 + /Ba2 + beads and shipped trans-Atlantically in order to investigate the feasibility of a large international collaboration to advance encapsulated islet transplantation more rapidly into clinical trials.
RESEARCH DESIGN AND METHODS
Human Islet Isolation
Human pancreata were procured from heart-beating deceased multi-organ donors. The pancreata were sent in cold preservation solutions (UW, University of Wisconsin, or HTK, Histidine-Tryptophan Ketoglutarate) Citation[7], Citation[11], Citation[18] to the Cell Isolation Laboratory at UIC for islet isolation. Human pancreata from 6 donors were used in this experiment. Pancreatic islets were isolated using the method described by Ricordi et al. Citation[20]. Briefly, after cleaning the pancreas from the surrounding tissue, it was perfused with Liberase HI (Roche, Indianapolis, IN) with a concentration of 1.7 mg/ml in cold perfusion solution. The distended pancreas was then transferred to the Ricordi digestion chamber, connected to a modified, closed circulation tubing system, and warmed up to 37°C. During the digestion, the chamber was shaken gently. Samples were continuously taken to determine digestion progress. Once free islets were detected under the microscope, the digestion was stopped by flushing cold (4°C) Hank's Buffer Salt Solution (HBSS, Mediatech, Herndon, VA) into the circulation system to dilute the enzyme. After being collected and washed in M199, the tissue was incubated with UW solution before purification on a cell separator (COBE 2991, Cobe, Lakewood, CO). Following purification, islet yield (expressed as islet equivalents, IEQ), tissue volume, and purity were determined according to standard methods Citation[19]. Isolated islets were cultured in CMRL-1066 media (Mediatech, Herndon, VA), supplemented with 1.5% human albumin, 0.1% insulin-transferrin-selenium (ITS), and Pen/Strep in T175 flasks at 37°C overnight culture before islets were shipped in the same media at a maximal density of 1,000 IEQ per ml at room temperature.
Islet Shipment and Microencapsulation
After culturing overnight, the isolated islets were shipped by World Courier in supplemented CMRL-1066 medium at room temperature in a Styrofoam box to the NTNU in Trondheim, where they were microencapsulated in alginate, prepared from stipes of Laminaria hyperborea (Pronova UP-LVG from Nova Matrix, Oslo, Norway). The alginate had a content of 63% guluronic acid (G) units and the average length of G-blocks consisting of two or more continuous G units NG > 1=12.6 was measured by 1H-NMR. The weight average molecular mass of 243 kDa and the polydispersity (Mw/Mn) of 1.80 were measured by SEC-MALLS. The encapsulation method was previously described Citation[29]. Briefly, the islet suspension was centrifuged (1,000 rpm, 2 min, RT), and the islets were resuspended in a small volume of culture media (CMRL-1066 supplemented with 300 mg/L glutamine, 16.7 mM HEPES, Pen/Strep, and 15% heat inactivated A+ human serum). The islet suspension was mixed with a 2.0% sterile filtered alginate solution (in 0.3M mannitol, pH 7.2-7.4) to a final suspension of 10,000 IEQ per ml alginate in a 2.0% alginate solution. Gel beads with an alginate gradient were formed by dripping the alginate/cell suspension in a gelling solution of 50 mM CaCl2 and 1 mM BaCl2 (in 10 mM MOPS, 0.14 M mannitol and 0.05% Tween20, pH 7.2-7.4). Small droplet production was obtained using an electrostatic bead generator (7 kV, flow: 10 ml/hr per 0.4 mm needle used) Citation[29], resulting in alginate capsules of 509±30 µm in diameter (mean±SEM, n = 3 sets of experiments of 10 beads each). The alginate microcapsules were collected on a filter and washed three times with approximately 10 ml culture media before being transferred to Petri dishes for culturing at 37°C and 5% CO2. All encapsulation and culturing procedures were performed under sterile conditions. After being encapsulated, islets were shipped back to UIC for assessment. The encapsulated islets were shipped in culture medium CMRL-1066 supplemented with 300 mg/L glutamine, 16.7 mM HEPES, Pen/Strep, and 15% heat inactivated A+ human serum.
In Vitro Assessment
The encapsulated islets were assessed in vitro for viability and functionality after arrival in Chicago (after they were shipped from Chicago to Trondheim, encapsulated in Trondheim, and shipped back from Trondheim to Chicago). This corresponded to a period of 5-7 days after islet isolation. Non-encapsulated islets were assessed 1-2 days after islet isolation without any shipment.
Dithizone (DTZ) staining and viability: Encapsulated and non-encapsulated islets were assessed using DTZ for insulin granule zinc staining. Islet viability was assessed by fluorescent staining with Syto-Green/Ethidium Bromide Citation[2], Citation[43]. For the Syto-Green/Ethidium Bromide staining, double fluorescence was performed to assess the amount of live (green) versus dead (red) islet cells in a representative sample, where a minimum of 100 islets were counted per sample.
Static glucose incubation: A stimulation index (SI) was calculated by dividing insulin secretion during high glucose exposure by the insulin secreted during low glucose exposure Citation[38]. Non-encapsulated and encapsulated islets were challenged with Krebs-Ringer bicarbonate buffer (KRBB, pH 7.35) containing 0.5% BSA (Sigma) under low glucose concentration conditions (1.6 mM) and with high glucose concentration (16.7 mM). Groups of five handpicked, non-encapsulated or encapsulated islets were placed in five different wells of a 12-well plate and were then incubated with 1 ml of KRBB low glucose concentration (1.6 mM glucose final concentration) for 30 min, allowing them to stabilize insulin secretion before the supernatant was collected and discarded. Islets were then incubated for 60 min in KRBB low glucose at 37°C and 5% CO2, and supernatant was collected under a microscope, taking care not to remove any islets from the well. The same step was repeated by addition of KRBB high glucose solution (16.7 mM glucose final concentration) and incubation for 90 min. Supernatant was collected and frozen at -20°C for later measurement using an ELISA kit for human insulin (Mercodia, Uppsala, Sweden).
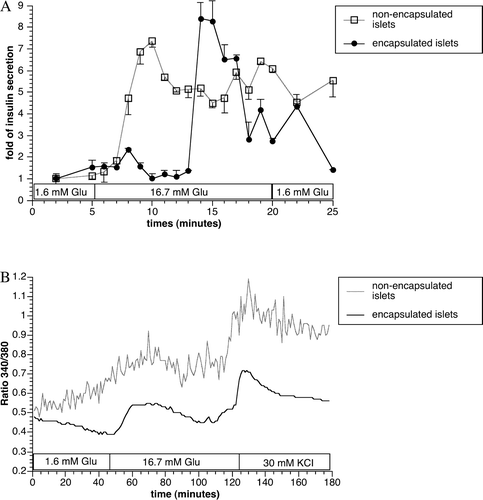
Dynamic insulin secretion: This was performed with non-encapsulated and encapsulated islets based on the method described by Roe et al. Citation[21], Citation[22]. Islets were transferred to a perifusion chamber and equilibrated by perifusing with KRBB containing 1.6 mM glucose for 15 min before glucose stimulation. After stimulation with high glucose solution (16.7 mM) for 15 min, 1.6 mM low glucose solution was infused in the chamber for 5 min. Aliquots from each perifusion chamber were collected at 1 min intervals throughout the experiment and frozen at –20°C for later insulin measurement.
Intracellular Ca2 + measurement: Intracellular Ca2 + was measured during glucose stimulation for functional evaluation of non-encapsulated and encapsulated islets using wide-field fluorescence imaging with dual-wavelength excitation fluorescent microscopy Citation[34],]. The islets were loaded with Fura-2/AM, a ratio-metric Ca2 + indicator (5 µM; Molecular Probes Inc., Eugene, OR), and incubated for 30 min at 37°C in KRBB (1.6 mM glucose). The coverslip was mounted in a microperifusion chamber (Medical System Inc., Paola, KA) on the apecium stage of an inverted microscope equipped for epifluorescence (TE-2000 U, Nikon Inc., Melville, NY) and perifused by a continuous flow (rate 2.5 ml/min of 5% CO2 -bubbled KRBB at 37°C (pH 7.4). KRBB containing high glucose and KCl concentration (16.7 mM and 30 mM) were administered to the islets and followed for 15 min. Single encapsulated and free islets were visualized with 10x objectives. Intracellular-free Ca2 + concentration after glucose stimulation was measured using a fluorescent video charge-coupled device camera (Hamamatsu, Hamamatsu City, Japan) and expressed as a ratio of fluorescence intensity of Fura-2 dual-wavelength excitation at 340 and 380 nm. Fluorescence emission at 519 nm was detected using Metafluor software (Universal Imaging, Downingtown, PA). The basal level of Ca2 + was estimated by measuring pre-stimulation levels.
In Vivo Assessment
Diabetic nude mice: Male nude mice (Harlan Industries, Indianapolis, IN) weighing 22-25 g were used as recipients for encapsulated human islets transplantation. Animals were housed and surgery was performed under a laminar flow hood located in “barrier” rooms at the Biologic Research Laboratory at UIC. Diabetes was induced by a single intraperitoneal (IP) injection of Streptozotocin (STZ, 220 mg/kg body weight; Sigma, St. Louis, MO) freshly dissolved in citrate buffer (pH, 4.5) at the day of human islet isolation. Animals were considered diabetic after two or more non-fasting blood glucose levels from the tail vein exceeded 350 mg/dl, which generally occurred after a maximum of 72 hours post injection. All procedures were performed under the guidelines of the National Institutes of Health and approval by the Animal Care Committee (ACC) at UIC.
Encapsulated human islet transplantation: In order to evaluate the in vivo function, we transplanted various amounts of encapsulated human islets intraperitoneally into diabetic nude mice, 10,000 (n = 3), 5,000 (n = 10), 3,000 (n = 9), and 1,000 IEQ (n = 7). Diabetic mice were placed under gas anesthesia (isoflurane) using a mini-mask (Viking Medical). A short midline abdominal incision (approximately 0.5 cm) was made and the encapsulated islets were slowly infused into the peritoneal cavity. Blood glucose and body weight were monitored every other day in the first week and twice a week afterwards.
Oral Glucose Tolerance Test (OGTT): The OGTT was performed at week 30 after transplantation in the mice transplanted with 3,000 IEQ encapsulated islets, non-transplanted diabetic, and normal controls. The OGTT was performed by the method described by Korbutt et al. Citation[10]. Briefly, after 4-6 hours fast, D-glucose (3 g/kg body weight) was administered as a 20% solution by gastric gavage into anaesthetized mice (isoflurane). Blood glucose levels were determined at 0, 15, 30, 60, 90, and 120 min by tail puncture.
Recovery of the capsules: For testing of the post-recovery microcapsule properties and histological analysis, encapsulated islets were removed at day 102 (n = 1), day 130 (n = 1), and day 200 (n = 2) post-transplantation. At the end of the study (at 260 and 329 days of post transplant), capsules were again removed from 13 mice transplanted with 1,000 to 10,000 IEQ of encapsulated human islets. Briefly, under gas anesthesia (isoflurane), a midline abdominal incision (approximately 0.5 cm) was made and 3 ml of warmed sterile HBSS was infused into peritoneal cavity to wash out the capsules. This procedure was repeated five times. Following the removal of the capsules, blood glucose was continuously monitored to confirm the achieved normoglycemia was due to the capsule transplant and not spontaneous recovery of the native islets. Islet function after explantation was tested by dynamic insulin secretion study using the encapsulated islets removed after 130 days, following the method described above.
Immunohistochemical study of the recovered capsules: Following the removal, the encapsulated islets were prepared for histological study using the method described by Kobayashi et al. Citation[9]. Briefly, the capsules were fixed in Bouin's solution for 2 hrs, dehydrated in graduated alcohol (70-100%), and embedded in paraffin. 5 µm sections were stained with H&E. In order to confirm the function of encapsulated islets, expression of insulin was assessed by immunohistochemical staining using polyclonal Guinea Pig anti-insulin antibody (1:100, Zymed Labs, San Francisco, CA) as the primary antibody. For detection of the primary antibody, FITC-conjugated Goat anti-mouse IgG (1:100, Sigma, St. Louis, MO, USA) secondary antibody was used according to the methods recommended by manufacturers. A Carl Zeiss LSM 510 confocal microscopy was used to visualize the antibody.
Statistical Methods
Statistical analysis was performed by Student's t-test. p values <0.05 were considered statistically significant. Results are expressed as mean±SEM when not indicated differently.
RESULTS
Viability and In Vitro Static Glucose Incubation Test
The encapsulation and shipment did not seem to have a measurable deleterious effect on the viability and insulin secretion in the static glucose stimulation assay of isolated human islets. These tests did not show any significant difference ( and ). Transport time of free islets before encapsulation was 32.7±4.8 hours and re-shipment duration after encapsulation was 38.4±7.7 hours.
Figure 1. DTZ-staining non-encapsulated (A) and encapsulated human islets (B) (light-microscope, 40x). Viability staining (Sytogreen, green for alive cells; Ethidium bromide, red for dead cells) non-encapsulated (C) and encapsulated islets (D).
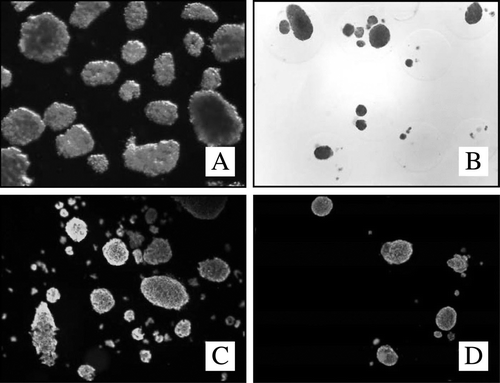
Table 1. Viability and stimulation index of the non-encapsulated control and encapsulated islets after shipment, encapsulation, and reshipment.
Dynamic Insulin Secretion and Intracellular Ca2 + Measurement
Following incubation with low and high glucose KRBB solution, we observed a discrete time lag of the acute release of insulin in encapsulated islets. However, apart from a lag-time of approximately five minutes, the results showed that the encapsulated islets retained a normal insulin secretion pattern and magnitude in response to glucose stimulation (A). The preserved function of the encapsulated islets was supported by similar intracellular Ca2 + level dynamics in response to glucose as compared to non-encapsulated islets (B).
In Vivo Assessment of the Encapsulated Human Islet
After transplantation, the blood glucose of diabetic nude mice reversed to normal values in all animals (). In three groups of mice transplanted with 3,000, 5,000, and 10,000 IEQ encapsulated human islets, 11 mice maintained normoglycemia until the end point of the study, which was from 260 to 329 days post transplantation. Seven mice died without hyperglycemia, presumably the end of their natural life span observed in athymic nude mice, and 4 mice were sacrificed after confirming hyperglycemia due to the removal of capsules. For animals transplanted with 1,000 IEQ, 4 mice returned to hyperglycemia 30-55 days after transplantation, and the remaining 3 mice also returned to hyperglycemia after 160 days of transplantation. OGTT showed that the encapsulated human islets regulated glycemia similar to human levels, and non-treated and non-diabetic mice had higher fasting and post-challenge glucose levels (A).
Figure 3. Non-fasting blood glucose levels and body weights change in the STZ-induced diabetic nude mice transplanted with different amounts of encapsulated human islets (10,000, 5,000, 3,000, and 1,000 IEQ).
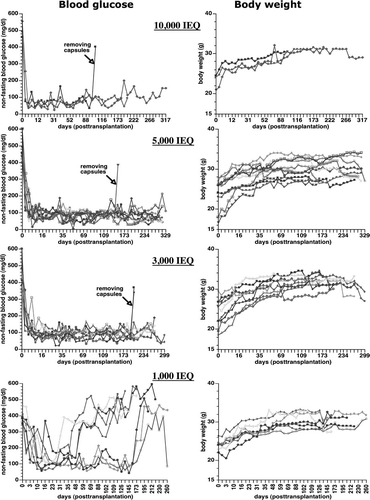
Figure 4. Oral glucose tolerance tests performed at 30 weeks after transplantation in the 3,000 IEQ encapsulated islets transplanted, diabetic non-transplanted, and normal control mice. Values were presented as mean±SEM (A). In vitro dynamic insulin secretion test of the retrieved capsules at 130 days after transplantation. Values were presented as mean±SEM from three different experiments (B).
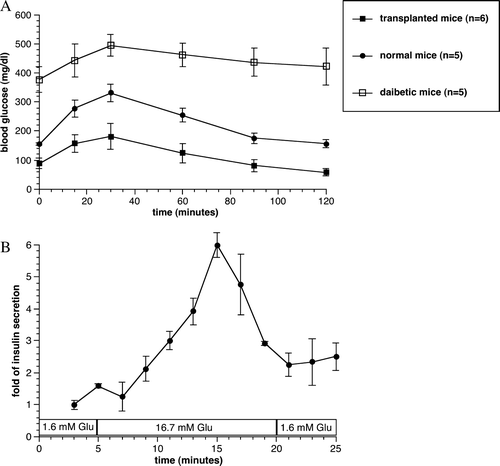
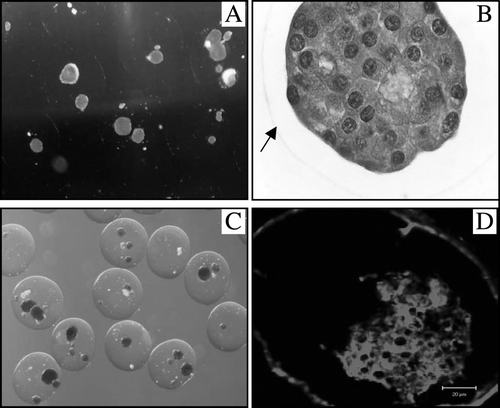
At the end of the study, capsules were retrieved from 13 mice transplanted with 1,000 to 10,000 IEQ encapsulated human islets by peritoneal lavage. In 12 out of 13 mice, the encapsulated islets were found to be free floating. The viability of retrieved encapsulated islets was 77±7% (n = 7). The retrieved capsules remained intact with a continuous smooth surface, and H&E staining showed the viable islet in the capsule. Moreover, fluorescent insulin staining for the retrieved capsules confirmed healthy insulin containing islets (). By testing the retrieved islets’ response to glucose in a static incubation assay, the encapsulated islets from all groups of mice were highly responsive, with a mean stimulation index of 9.5±3.7. From the perifusion study, we observed that the retrieved encapsulated human islets responded remarkably well with 5-fold increase in insulin secretion (B).
Figure 5. Direct light microscopy photos with DTZ (A, 40×) and H&E (B, 400×) staining of the retrieved capsules at 130 days after transplantation. The arrow indicates the border of the capsule. DTZ (C, 40×) and insulin fluorescent stained (D, Confocal Microscopy) pictures of the retrieved capsules at day 329 after transplantation.
In general, between the 4 groups differing by the number of encapsulated and transplanted IEQ, no differences were seen on the retrieved capsules in regard to overgrowth, viability, and the function of islets in a static incubation assay.
DISCUSSION
Our study demonstrated that human islets encapsulated in inhomogeneous alginate-Ca2 + /Ba2 + microcapsules were fully functional in vitro and in vivo, even after exposure to long shipments and an encapsulation process. The viability and excellent insulin secretion function after encapsulation and trans-Atlantic shipments have been demonstrated in vitro. Encapsulated human islets were then transplanted to diabetic nude mice to determine metabolic function and minimal mass required to achieve normoglycemia. For accurately evaluating the metabolic function, an immuno-incompetent nude mouse model was chosen to exclude the effects of rejection.
Human islets retained good viability (85%) after encapsulation. Good viability and function was expected, as omitting the polycation layer in the capsule simplifies the encapsulation process and is believed to cause minimal stress on the encapsulated islets. The maintenance of islet quality was also confirmed by both static and dynamic insulin secretion tests, showing similar insulin response for islets that underwent the two trans-Atlantic shipments and encapsulation as those of control islets. Good human islet function in vitro after several shorter shipment times and encapsulation has previously been described by Sandler et al. Citation[23]. Hence, our study confirms these findings also for longer shipment times.
In our in vivo study, diabetic nude mice were transplanted with different quantities of encapsulated islets. When using healthy islets, lower numbers are believed to be needed for achieving insulin independence than for non-healthy islets. There was no difference within the 3 groups (3,000, 5,000, and 10,000 IEQ) in terms of obtaining normoglycemia. Nevertheless, in the group of mice transplanted with 1,000 IEQ encapsulated human islets, 57% and 43% of the recipients returned to hyperglycemia after 55 and 160 days of transplantation, respectively. This indicates that 1,000 IEQ encapsulated human islets (=50,000 IEQ/kg) represent a minimal islet mass model when transplanting encapsulated human islets to STZ induced diabetic mice. The transplanted mice were able to control their blood glucose after a glucose challenge. However, the human islets responded as in humans and thus with lower glucose concentrations than for the control mice. No differences were seen between the groups on recovered islets or capsules from sacrificed mice. All capsules were found free of overgrowth with viable islets that responded in a static incubation assay.
It is important to note that in our previous study, transplantation of 1,000 IEQ of non-encapsulated human islets under the kidney capsule reverse diabetes in 50% of diabetic nude mice, and 2,000 IEQ usually renders all the transplanted mice normoglycemic. This indicates that despite the intraperitoneal location and the presence of the microcapsule, the encapsulated islets perform similarly to free islets. Other studies on encapsulated islets in mice have shown that a varying number of islets may be needed to normalize the blood glucose, depending on the source of the islets and whether the islets are syn-, allo-, or xenografts Citation[5], Citation[17]. For human islets, 1,800 IEQ encapsulated islets have been shown to restore normoglycemia in one out of three Balb/c mice for 40 weeks in a study performed by Schneider et al. Citation[24].
In conclusion, this study shows that human pancreatic islets preserve the viability and function through encapsulation in inhomogeneous alginate-Ca2 + /Ba2 + beads and distant transportation. Furthermore, the shipment of human islets is not a limitation for overseas collaboration and combined efforts to provide a functional cure for diabetes through encapsulated islet transplantation.
Acknowledgements
We are grateful to Dr. Louis H. Philipson (Department of Medicine, University of Chicago) for supporting the intracellular Ca2 + measurement and perifusion study.This study was conducted as part of the Chicago Project, an international effort for a functional cure for diabetes. The study was sponsored by the Christopher Foundation, the Efroymson Fund, the Wirtz Family, and the College of Medicine at UIC. JO is supported by NIH (ICR Grant 1 U42 RR023245-01) and JDRF (#5-2006-398 and #5-2006-424 and #5-2007-773), as well as by the Washington Square Health Foundation and the Grant Health Care Foundation. BLS is supported by the Norwegian Diabetes Association/Norwegian Foundation for Health and Rehabilitation. IL was supported by the Slovak Research and Development Agency under contract No. APVV-51-033205.
References
- Alejandro R., Lehmann R., Ricordi C., Kenyon N. S., Angelico M. C., Burke G., Esquenazi V., Nery J., Betancourt A. E., Kong S. S., Miller J., Mintz D. H. Long-term function (6 years) of islet allografts in type 1 diabetes. Diabetes 1997; 46: 1983–1989
- Avila J. G., Tsujimura T., Oberholzer J., Churchill T., Salehi P., Shapiro A. M., Lakey J. R. Improvement of pancreatic islet isolation outcomes using glutamine perfusion during isolation procedure. Cell Transplant 2003; 12: 877–881
- Calafiore R., Basta G., Luca G., Lemmi A., Montanucci M. P., Calabrese G., Racanicchi L., Mancuso F., Brunetti P. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care 2006; 29: 137–138
- De Vos P., Van Straaten J. F., Nieuwenhuizen A. G., de Groot M., Ploeg R. J., De Haan B. J., Van Schilfgaarde R. Why do microencapsulated islet grafts fail in the absence of fibrotic overgrowth?. Diabetes 1999; 48: 1381–1388
- Duvivier-Kali V. F., Omer A., Parent R. J., O'Neil J. J., Weir G. C. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes 2001; 50: 1698–1705
- Elliott R. B., Escobar L., Calafiore R., Basta G., Garkavenko O., Vasconcellos A., Bambra C. Transplantation of micro- and macroencapsulated piglet islets into mice and monkeys. Transplant Proc 2005; 37: 466–469
- Fridell J. A., Agarwal A., Milgrom M. L., Goggins W. C., Murdock P., Pescovitz M. D. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution for organ preservation in clinical pancreas transplantation. Transplantation 2004; 77: 1304–1306
- Kobayashi T., Aomatsu Y., Iwata H., Kin T., Kanehiro H., Hisanga M., Ko S., Nagao M., Harb G., Nakajima Y. Survival of microencapsulated islets at 400 days posttransplantation in the omental pouch of NOD mice. Cell Transplant 2006; 15: 359–365
- Kobayashi T., Harb G., Rayat G. R. Prolonged survival of microencapsulated neonatal porcine islets in mice treated with a combination of anti-CD154 and anti-LFA-1 monoclonal antibodies. Transplantation 2005; 80: 821–827
- Korbutt G. S., Mallett A. G., Ao Z., Flashner M., Rajotte R. V. Improved survival of microencapsulated islets during in vitro culture and enhanced metabolic function following transplantation. Diabetologia 2004; 47: 1810–1818
- Lakey J. R., Rajotte R. V., Warnock G. L., Kneteman N. M. Human pancreas preservation prior to islet isolation. Cold ischemic tolerance. Transplantation 1995; 59: 689–694
- Leu F. J., Chen C. F., Chiang W. E., Chern H. T., Shian L. R., Chung T. M., Wang J., Sun A. M. Microencapsulated pancreatic islets: a pathologic study. J Formos Med Assoc 1992; 91: 849–858
- Lim F., Sun A. M. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980; 210: 908–910
- Morch Y. A., Donati I., Strand B. L., Skjak-Braek G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006; 7: 1471–1480
- O'Shea G. M., Goosen M. F., Sun A. M. Prolonged survival of transplanted islets of Langerhans encapsulated in a biocompatible membrane. Biochim Biophys Acta 1984; 804: 133–136
- Oberholzer J., Triponez F., Mage R., Andereggen E., Buhler L., Cretin N., Fournier B., Goumaz C., Lou J., Philippe J., Morel P. Human islet transplantation: lessons from 13 autologous and 13 allogeneic transplantations. Transplantation 2000; 69: 1115–1123
- Omer A., Duvivier-Kali V., Fernandes J., Tchipashvili V., Colton C. K., Weir G. C. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation 2005; 79: 52–58
- Potdar S., Malek S., Eghtesad B., Shapiro R., Basu A., Patel K., Broznick B., Fung J. Initial experience using histidine-tryptophan-ketoglutarate solution in clinical pancreas transplantation. Clin Transplant 2004; 18: 661–665
- Ricordi C., Gray D. W., Hering B. J., Kaufman D. B., Warnock G. L., Kneteman N. M., Lake S. P., London N. J., Socci C., Alejandro R., et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat 1990; 27: 185–195
- Ricordi C., Lacy P. E., Scharp D. W. Automated islet isolation from human pancreas. Diabetes 1989; 38 Suppl 1: 140–142
- Roe, M. W., Worley, J. F., 3rd, Mittal, A. A., \Kuznetsov, A., DasGupta, S., Mertz, R. J., Witherspoon, S. M., 3rd, Blair, N., Lancaster, M. E., McIntyre, M. S., Shehee, W. R., Dukes, I. D. and Philipson, L. H. 1996. Expression and function of pancreatic beta-cell delayed rectifier K+ channels. Role in stimulus-secretion coupling. J Biol Chem 271: 32241–32246.
- Roe, M. W., Worley, J. F., 3rd, Tokuyama, Y., Philipson, L. H., Sturis, J., Tang, J., Dukes, I. D., Bell, G. I. and Polonsky, K. S. 1996. NIDDM is associated with loss of pancreatic beta-cell L-type Ca2+ channel activity. Am J Physiol 270: EE133–140.
- Sandler S., Andersson A., Eizirik D. L., Hellerstrom C., Espevik T., Kulseng B., Thu B., Pipeleers D. G., Skjak-Braek G. Assessment of insulin secretion in vitro from microencapsulated fetal porcine islet-like cell clusters and rat, mouse, and human pancreatic islets. Transplantation 1997; 63: 1712–1718
- Schneider S., Feilen P. J., Brunnenmeier F., Minnemann T., Zimmermann H., Zimmermann U., Weber M. M. Long-term graft function of adult rat and human islets encapsulated in novel alginate-based microcapsules after transplantation in immunocompetent diabetic mice. Diabetes 2005; 54: 687–693
- Shapiro A. M., Ricordi C., Hering B. J., Auchincloss H., Lindblad R., Robertson R. P., Secchi A., Brendel M. D., Berney T., Brennan D. C., Cagliero E., Alejandro R., Ryan E. A., DiMercurio B., Morel P., Polonsky K. S., Reems J. A., Bretzel R. G., Bertuzzi F., Froud T., Kandaswamy R., Sutherland D. E., Eisenbarth G., Segal M., Preiksaitis J., Korbutt G. S., Barton F. B., Viviano L., Seyfert-Margolis V., Bluestone J., Lakey J. R. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355: 1318–1330
- Soon-Shiong P., Feldman E., Nelson R., Heintz R., Yao Q., Yao Z., Zheng T., Merideth N., Skjak-Braek G., Espevik T., et al. Long-term reversal of diabetes by the injection of immunoprotected islets. Proc Natl Acad Sci U S A 1993; 90: 5843–5847
- Soon-Shiong P., Heintz R. E., Merideth N., Yao Q. X., Yao Z., Zheng T., Murphy M., Moloney M. K., Schmehl M., Harris M., et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet 1994; 343: 950–951
- Soria, B. , Roche, E. , Reig, J. A. and Martin, F. 2005. Generation of insulin-producing cells from stem cells. Novartis Found Symp 265: 158–167; discussion 167-173, 204-111.
- Strand B. L., Gaserod O., Kulseng B., Espevik T., Skjak-Baek G. Alginate-polylysine-alginate microcapsules: effect of size reduction on capsule properties. J Microencapsul 2002; 19: 615–630
- Strand B. L., Ryan T. L., In't Veld P., Kulseng B., Rokstad A. M., Skjak-Brek G., Espevik T. Poly-L-Lysine induces fibrosis on alginate microcapsules via the induction of cytokines. Cell Transplant 2001; 10: 263–275
- Sun Y., Chen L., Hou X. G., Hou W. K., Dong J. J., Sun L., Tang K. X., Wang B., Song J., Li H., Wang K. X. Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chin Med J (Engl) 2007; 120: 771–776
- Sun Y., Ma X., Zhou D., Vacek I., Sun A. M. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest 1996; 98: 1417–1422
- Ta M., Choi Y., Atouf F., Park C. H., Lumelsky N. The defined combination of growth factors controls generation of long-term-replicating islet progenitor-like cells from cultures of adult mouse pancreas. Stem Cells 2006; 24: 1738–1749
- Tamarina N. A., Wang Y., Mariotto L., Kuznetsov A., Bond C., Adelman J., Philipson L. H. Small-conductance calcium-activated K+ channels are expressed in pancreatic islets and regulate glucose responses. Diabetes 2003; 52: 2000–2006
- Thompson, D. M., Fung, M. A. and Warnock, G. L. 2007. Islet transplantation. N Engl J Med 356: 964; author reply 964–965.
- Thu B., Gaserod O., Paus D., Mikkelsen A., Skjak-Braek G., Toffanin R., Vittur F., Rizzo R. Inhomogeneous alginate gel spheres: an assessment of the polymer gradients by synchrotron radiation-induced X-ray emission, magnetic resonance microimaging, and mathematical modeling. Biopolymers 2000; 53: 60–71
- Trivedi N., Keegan M., Steil G. M., Hollister-Lock J., Hasenkamp W. M., Colton C. K., Bonner-Weir S., Weir G. C. Islets in alginate macrobeads reverse diabetes despite minimal acute insulin secretory responses. Transplantation 2001; 71: 203–211
- Tsujimura T., Kuroda Y., Kin T., Avila J. G., Rajotte R. V., Korbutt G. S., Ryan E. A., Shapiro A. M., Lakey J. R. Human islet transplantation from pancreases with prolonged cold ischemia using additional preservation by the two-layer (UW solution/perfluorochemical) cold-storage method. Transplantation 2002; 74: 1687–1691
- van de Linde P., Boog P. J., Tysma O. M., Elliott J. F., Roelen D. L., Claas F. H., de Fijter J. W., Roep B. O. Selective unresponsiveness to beta cell autoantigens after induction immunosuppression in pancreas transplantation with anti-interleukin-2 receptor antibody versus anti-thymocyte globulin. Clin Exp Immunol 2007; 149: 56–62
- Vandenbossche G. M., Bracke M. E., Cuvelier C. A., Bortier H. E., Mareel M. M., Remon J. P. Host reaction against alginate-polylysine microcapsules containing living cells. J Pharm Pharmacol 1993; 45: 121–125
- Weber C., Ayres-Price J., Costanzo M., Becker A., Stall A. NOD mouse peritoneal cellular response to poly-L-lysine-alginate microencapsulated rat islets. Transplant Proc 1994; 26: 1116–1119
- Xu, B. Y., Yang, H., Serreze, D. V., MacIntosh, R., Yu, W. and Wright, J. R., Jr. 2005. Rapid destruction of encapsulated islet xenografts by NOD mice is CD4-dependent and facilitated by B-cells: innate immunity and autoimmunity do not play significant roles. Transplantation 80: 402–409.
- Yang H., Acker J., Chen A., McGann L. In situ assessment of cell viability. Cell Transplant 1998; 7: 443–451