Abstract
The glutathione-s-transferases are a family of multifunctional enzymes involved in the detoxification of electrophilic xenobiotics primarily through conjugation to reduce glutathione. A form of the enzyme, designated GSH-S transferase ρ, was purified chicken erythrocyte by acetone precipitation, ethanol-chloroform treatment, DEAE-Cellulose, Q-Sepharose, Sephadex G-100 chromatography. The molecular weight of GST purified from chicken erythrocyte was estimated as 47,500 Da by gel filtration. The subunit molecular weight of chicken erythrocyte GST as determined by electrophoresis in the presence of sodium dodecyl sulfate was predicted as 24,000 Da. The specific activity was found to be 20.39 U/mg. The km for CDNB calculated from Lineweaver-Burk plot was 0.71 mM. Optimum temperature of maximum GST activity was 28°C for CDNB. The maximal activity of the enzyme was observed at pH 7.5. The activity of purified GST is inhibited by DDT, urea, CDNB, Triton X-100, DTNB.
INTRODUCTION
Glutathione-S-transferase (GST, EC 2.5.1.18) are a family of multifunctional proteins that are presumed to be involved in the detoxification of a multitude of xenobiotics through their catalytic and noncatalitic activities Citation[1], Citation[2]. These enzymes catalyse reactions in which the sulphur atom of glutathione provides electrons for nucleophilic attack on second electrophilic substrate Citation[3]. Thus, GSTs play an important role in protecting tissues from oxidative damage and oxidative stress Citation[4].
Several isoenzymes of GST have been isolated from the cytosol fraction of mammalian tissues, and three classes of the enzyme have been referred to as basic (α-ε), near-neutral (µ), and acidic (ρ-π). The members of the three classes have clearly different substrate specificities, indicating distinct roles of the various enzymes in detoxication reactions Citation[5–7]. Multiple forms of this enzyme have been found in human liver, brain, lung, pancreas, Japanese gagil, mucor muced, Chiranomi, gnoxia-tolerant turtle, quines pig liver, blue crub Citation[8–17]. However in human placenta and erythrocytes only one form each have been reported Citation[18], Citation[19].
Nishinaka (1992) also showed that class π GST in human placenta is sensitive to biological disulphides Citation[20]. Awasthi and co-workers (1980) have reported the presence of class ρ GST in human erythrocytes has enzyme activity towards CDNB as substrate Citation[21]. The physiologic role of this enzyme is not known. It has been suggested that the location of the enzyme in erythrocytes is ideal for the removal of circulating xenobiotics. One might also consider that GST occurs in erythrocytes primarily for the protection of erythrocytes against electrophilic compounds, rather than serving a general protective function in the body Citation[22], Citation[23].
In the study we described the purification and characterization of GST from chicken erythrocyte. In addition, the inhibition effect of some chemical on the chicken erythrocyte GST activity was examined.
Material and Methods
Bovine serum albumin, chloroform, ammonium sulphate, ethanol, methanol were purchased from Merck, Germany. DEAE cellulose, Q Sepharose, Sephadex G-100, β-mercaptoethanol, 1-chloro-2,4-dinitrobenzene, TEMED, bromo phenol blue, molecular weight standards for gel exclusion and SDS/PAGE were purchased from Sigma Chemical Company, USA. Other reagents were all analytical grade.
Enzyme Assay
The GST activity in the hemolysate and various enzyme preparations were determined according to the method of Habig and colleagues (1974) Citation[24]. The reaction mixture contained in a total volume of 1 ml, 1mM GSH and 1mM 1 chloro-2,4-dinitrobenzene and 0.1 M phosphate buffer (pH 6.5). One unit of the GST activity corresponds to 1µmol of the substrate conjugated to GSH per min at 25°C.
Protein Determination
Determination of protein content of extracts was determined by the dye-binding method of Bradford using bovine serum albumin (BSA) as a standard Citation[25].
Purification Procedures
All experimental procedures were carried out at 4°C.
Hemolysis of Blood
Fresh venous chicken blood with 2.5% (v/v) citric acid solution as an anticoagulant was used throughout. Venous blood was centrifuged (4000×g, 10 min), plasma was removed and the erythrocytes were washed three times with cold 0.9% saline by centrifugation. The packed cells were homolyzed by adding three volumes of cold distilled water with stirring for 20 min and freeze-thaw.
Acetone Treatment
Chilled acetone (30%) was added to the hemolysate and the protein was precipitated at centrifugation at 14000×g for 20 min. The precipitate was suspended in 0.05 M potassium phosphate buffer pH 7.5. The samples was stirred for 15 min and the insoluble material removed by centrifugation (14000×g) for 15 min.
Ethanol-Chloroform Treatment
To each ml of resultant solution 1 ml of an ethanol-chloroform mixture (3 parts of chloroform to 1 part of ethanol) was added while stirring vigorously for about 1 min. The denaturated hemoglobin is removed by centrifugation. The ethanol-chloroform is then evaporated in vacuo.
DEAE-Cellulose Chromatography
The ethanol-chloroform fractions were applied onto a DEAE-cellulose column (1.5×40cm) previously equilibrated with 10 mM Tris-chloride, pH 7.5, containing 0.1 mM EDTA and 0.1 mM GSH. The enzyme was eluted with 10 mM Tris-chloride, pH 7.5, containing 0.1 mM EDTA, 1 mM GSH and (0.1–1 M) NaCl. Fractions of 1.5 ml each were collected at a flow rate of 0.4 ml/min. The active fractions were pooled and concentrated by 10,000 NMWC ultrafilter.
Q-Sepharose Column Chromatography
The concentrated fractions were applied to a Q-Sepharose column (1.5×20 cm), equilibrated with 10 mM Tris-chloride, pH 7.5, containing 0.1 mM EDTA and 0.1 mM GSH. Elution was carried out stepwise with 10 mM Tris-chloride, pH 7.5, containing 0.1 mM EDTA, 1 mM GSH and 0.5 M NaCl at a flow rate of 0.2 ml/min. Fractions of 1.5 ml were collected and the fractions containing GST activity were appropriately combined and concentrated by 10,000 NMWC ultrafilter.
Sephadex G-100 Gel Filtration
The concentration GST solution was adsorbed to a Sephadex G-100 column (1×90 cm) that had been in equilibration with 10 mM phosphate buffer pH 7.0, containing 0.1 mM EDTA, and GST were eluted with 10 mM phosphate buffer pH 7.0, containing 0.1 mM EDTA and 0.5 M NaCl at a flow rate 0.2 ml/min. The fractions of 2 ml were collected.
Molecular Weight Determination
The molecular weight of native enzyme was determined by size exclusion on a Sephadex G-100 column previously equilibrated in 10 mM phosphate buffer pH 7.0, containing 0.1 mM EDTA.
Sitocrom c (12,400 Da), carbonic anhidrase (29,000 Da), bovine serum albumin (66,000 Da), alcohol dehydrogenase (150,000 Da), β-amilase (200,000 Da) were used as molecular weight calibration markers.
Polyacrylamide Gel Electrophoresis
SDS/PAGE electrophoresis was performed using the buffer system described by Laemmli (1970) Citation[26]. The stacking and resolving gels containing 4% (w/v) and 12% (w/v) of acrylamide, respectively. Bovine serum albumin (66,000 Da), ovalbumin (45,000 Da), glyceraldehyde-3-phosphate dehydrogenase (36,000 Da), carbonic anhydrase (29,000 Da), tripsinogen (24,000 Da), tripsin inhibitör (20,100 Da), α-lactalbumin (14,200 Da) were used as reference proteins.
Effect of pH
The effect of pH on GST activity was determined under the assay conditions described using various buffers at a concentration 0.1 M sodium acetate (pH 3.0–6.0), phosphate (6.0–8.0), and Tris-HCl (8.0–10.0) at 25°C.
Effect of Temperature
The chicken erythrocyte GST activity as a function of temperature was determined at various temperatures from 10–70°C. The heat stability of the enzyme was determined by adding 1.0°ml of the enzyme solution into performed test tube set-up in a water bath. After one hour heating at 10–70°C, the enzyme solution was rapidly cooled in ice and remaining activity was assayed at 28°C.
Enzyme Kinetics
The Michalis-Menten constant (Km) was calculated from the Linewear-Burk plots using CDNB over varying concentrations (between 0.25 mM and 4 mM) at 28°C Citation[27]. The substrate concentration range used in all cases was 0.25–4 mM.
Storage Stability
Storage stability of chicken erythrocyte GST was determined of −15, 4, and 28°C CDNB as substrate. The activity was determined daily. Purified samples stored at −15°C were kept in separate vials for each observation to avoid repeated freeze/thaw effect on the enzyme.
Effect of Some Compounds on the Enzyme Activity
To determine the effect of inhibitors on chicken erythrocyte GST activity, the purified enzyme was preincubated with the various concentration inhibitors in 0.1 M phosphate buffer pH 6.5 at 28°C. Residual GST activity was measured under standard assay conditions. To determine the inhibitor concentration that reduced the enzyme activity by 50% (I50) regression analysis graphs were drawn by using percent inhibition values. I50 values were determined from graphs.
RESULTS AND dISCUSSION
GST from chicken erythrocytes was purified using acetone fractionation, ethanol-chloroform treatment, DEAE cellulose chromatography, Q sepharose chromatography, and gel filtration Sephadex G-100. The purification procedure for GST chicken erythrocyte is summarized in . The first purification step of GST from initial extract was achieved by fractional precipitation of proteins by using (NH4)2SO4, polyethylen glycol (PEG), ethanol, and acetone methods. Ammonium sulphate, PEG, and ethanol precipitation of chicken erythrocyte GST was not performed due to resulting partial inactivation of the enzyme. The highest recovered activity was obtained as 0.21 U/mg for 30% acetone precipitation. Acetone precipitation of the initial extract followed by ethanol-chloroform treatment step resulted in 11.0-fold purification of GST. As can be seen from and , the degrees of purification of GST were 56-fold after DEAE cellulose chromatography, 122.8-fold after Q sepharose. The specific activity of GST fraction after DEAE cellulose and Q sepharose ion-exchange chromatography was 3.37 U/mg and 7.37 U/mg, respectively. The purified enzyme gave a single major peak after Sephadex G-100 column chromatography and specific activity was 20.39 U/mg (). The specific activity of the purified chicken erythrocyte GST was lower than that for purified GST from human erythrocyte. The specific activity of human erythrocyte GST have been reported 66U/mg Citation[28], Citation[29].
Figure 1. DEAE cellulose chromatography of chicken erythrocyte GST. (…•…) GST activity, (—o—) absorbance at 280 nm.
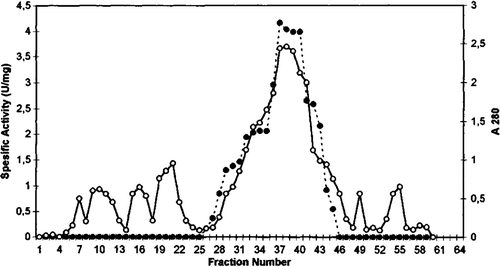
Figure 2. Q sepharose chromatography of chicken erythrocyte GST. (…•…) GST activity, (—o—) absorbance at 280 nm.
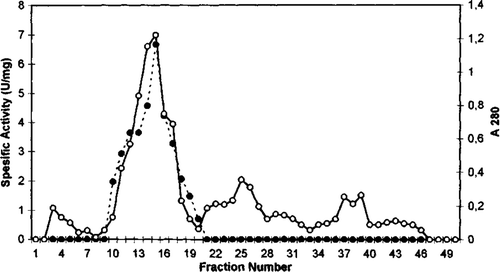
Figure 3. Sephadex G-100 gel filtration chromatography of chicken erythrocyte GST. (…•…) GST activity, (—o—) absorbance at 280 nm.
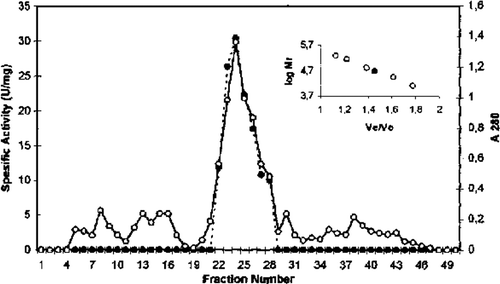
Table 1. Purification steps for chicken erythrocyte GST
The molecular weight of the purified enzyme was estimated by gel-filtration to be approximately 47,500 Da. This value corresponds roughly to those from purified GST ρ from human erythrocyte Citation[28]. The molecular weight GST bovine plasenta, rat liver and dog lens have been reported as 42,000, 45,000, and 50,000 Da, respectively Citation[30], Citation[31]. The subunit molecular weight as determined by electrophoresis in the presence of sodium dodecyl sulphate and comparison with proteins of known molecular weight was estimated as 24,000 Da ().
Figure 4. SDS-polyacrylamide gel electrophoresis of chicken erythrocyte GST (a) protein standards; (b) purified chicken erythrocyte GST.
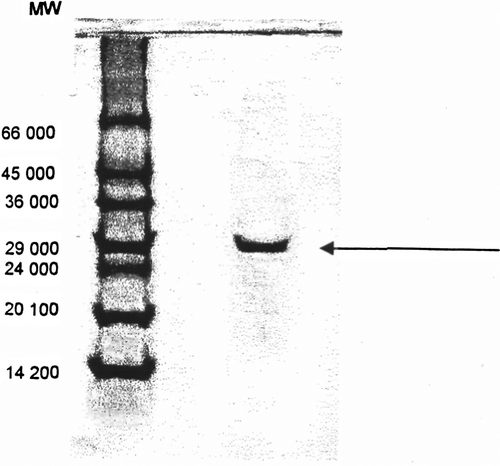
GST isolated from human erythrocyte have molecular weights of 24,000 Da and contain two equally sized subunits Citation[32].
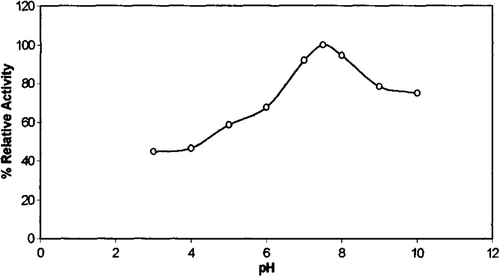
Activity profiles of chicken erythrocyte GST were investigated at 28°C with CDNB as substrate and at different pH values in 100 mM sodium acetate, pH 3.0–6.0, and 100 mM phosphate, pH 6.0–8.0, and 100 mM tris-HCl, pH 8.0–10.0. As can be seen from , the optimum pH value was determined to be 7.5 with CDNB as substrate. This result is similar to those observed for the human erythrocyte GST Citation[33].
Optimum pH values for the enzyme from different sources and different substrates have been reported: Frear and Swenson reported pH optimum between pH 6.6 and 6.8 for activity in a semicrude protein extract from maize assayed with atrazine as a substrate Citation[34].
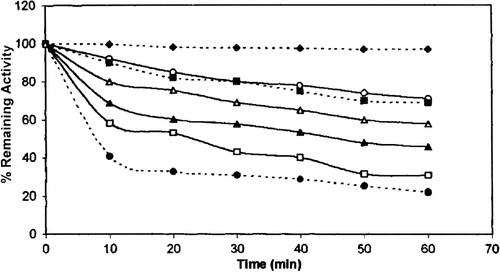
As can be seen chicken erythrocyte GST was stable for 60 min in the pH 7.5 at 28 °C. Incubation of the enzyme for 60 min. at pH 4.0 and 9.0 caused 78% and 29% loss of activity, respectively. The stability of the purified enzyme in acidic media was lower than that in basic media.
Figure 6. pH stability of chicken erythrocyte GST. pH 7.5(… ♦ …), pH 7 (—○—), pH 8 (…□…), pH 9 (—Δ—), pH 6 (—▴—), pH 5 (— □___), pH 4 (…•…).
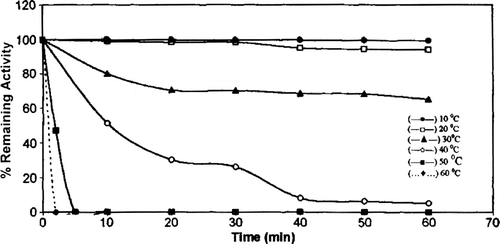
Heat stability of chicken erythrocyte GST was investigated between 10°C and 60°C over a 1-hour period (). The enzyme was stable at lower temperatures. A decrease in GST activity of 35% and 95% was found at 30 and 40°C, respectively. Chicken erythrocyte GST was completely inactivated after 5 min. at 50°C.
Figure 7. Heat stability of chicken erythrocyte GST. 10 °C (—•—), 20 °C (—□—), 30 °C (—▴—), 40 °C (—○—), 50 °C (— •—), 60 °C (… •…).
The crude enzyme preparation was unstable. On storage at 28°C and 4°C the initial extract lost its activity after 2 days and 3 days, respectively. The purified enzyme lost its activity after 5 days at 28°C; however, it was observed to be much more stable at −15°C and only lost 10% activity on storage for 5 days. These results are consistent with results reported in the literature by others Citation[35].
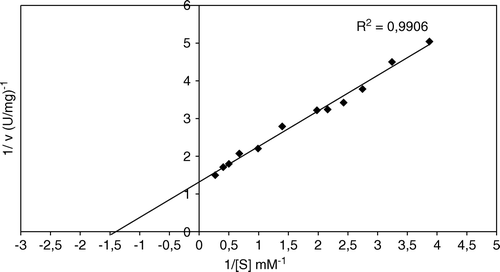
The Km value for chicken erythrocyte GST ρ with CDNB was 0.71 mM as determined by a Lineweaver-Burk plot (). Schröder et al. (1994) reported a Km of 0.5 mM for enzyme from human erythrocyte Citation[36].
Figure 8. Effect of substrate (CDNB) concentration on chicken erythrocytes GST (Lineweaver-Burk plot).
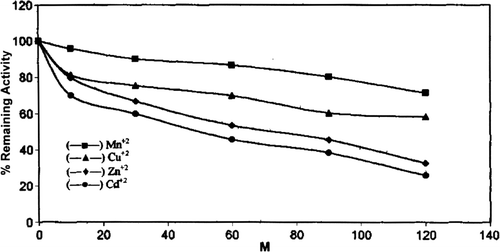
The effect of different divalent cations Zn+2, Cu+2, Mn+2, Cd+2 was studied on chicken erythrocyte GST activity. Purified enzyme was incubated with different concentration of divalent cations for 20 minutes in phosphate buffer (28°C, pH 7.5) (). All the cations had an inhibitory effect on chicken erythrocyte GST activity, but of all the cations Cd was found to be the most effective inhibitor of GST from chicken erythrocyte. 50% inhibition was observed at 50 mM Cd concentration. Divalent cation inhibition of GST has been established by the early workers Citation[37].
The treatment of chicken erythrocyte GST with 2-mercaptoethanol and DTT caused a gradual reduction in the activity () and 50% inhibition was observed at 4 mM and 10 mM concentration of respective thiols after 60 minutes incubation. The decrease in enzyme activity depends on the incubation time and the nature of the thiol compounds; 2-mercaptoethanol was the most potent reagent for reduction of the enzyme activity. Thiol inhibition of enzymes has been established by the early workers Citation[38].
Table 2. Effect of 2-mercaptoethanol and dithiothreitol on chicken erythrocyte GST after l hours incubation
50% inhibition was observed at 2.8.10−7 M for mercury acetate. This metabolite was a more potent reagent for inhibition of the enzyme activity. Certain heavy metal ions, including Hg+2 (Mercury II), are examples of non-competitive inhibitors. The toxicity of these common environmental agents is primarily a consequence of their interactions with sulphydryl groups in numerous proteins, including many enzymes Citation[39].
The time-depend of changes in the activity of purified enzyme was investigated in various concentrations of SDS. When incubation for 30 min. in the presence of 0.01, 0.02, 0.03 SDS, the enzyme lost 74.7%, 84.2%, 100% of its activity, respectively. SDS binds rapidly to proteins composed of a single polypeptide chain, whereas it binds slowly to proteins consisting of subunits causing irreversible dissociation of proteins into subunits Citation[40].
Incubation of the enzyme in the presence of 1.9 mM urea for 30 min., respectively, caused 50% inhibition (). In the presence of urea, similar enzyme activity losses were reported for enzymes in erythrocyte Citation[41]. According to some authors a complete loss the helical content with time was observed Citation[39].
Table 3. Effect of some chemicals on chicken erythrocyte GST after 1 hours incubation
The enzyme was incubated in the presence of 0.5 mM CDNB for 10 min. When incubation for 10 minutes in the presence of 0.5 mM CDNB the purified GST lost 92% of its activity. 50% inhibition was observed at 0.5 mM CDNB for 1 min. The sequence of addition of reactants in the GST assay was determined to be important that the enzyme is rapidly inactivated by CDNB in the absence of GSH. For this reason, GSH was mixed the buffer containing CDNB immediately before enzyme addition.
Conclusions
Glutathione S transferase represent a group of multifunctional proteins that catalyze the conjugation of reduced glutathione to electrophilic ligands generated by the metabolism of various xenobiotics. Glutathione S transferase from chicken erythrocyte has been purified using acetone precipitation, ethanol-chloroform treatment, DEAE cellulose, Q Sepharose, and Sephadeks G-100 chromatography.
The specific activity of GST fraction after DEAE cellulose and Q Sepharose ion change chromatography was 3.37 U/mg and 7.37 U/mg, respectively.
Acknowledgements
The authors acknowledge the financial support of the Celal Bayar University Grants Commission for this project (1999 C.B.Ü.A.F:99/12).
References
- Jakoby W.B., Habig W.H. Glutathione transferase. In Enzymatic Basis of Detoxication 2. Academic Press, New York 1980; 63–94
- Anderson C., Mosialoy E., Weinander R., Mongenstern K. Enzymology of microsonal GST. Adv Pharmacol 1994; 27: 15–35
- Itai-ling Y., Qing-Ying Z., Lijia N., Shang-geng H., Yim Van Z. Purification, and characterization of novel GST from Atactodea striada guoa. Biochem. Biophy Research Comminication 2003; 307: 626–631
- Chang L.H., Chuang L.F., Tsai C.P., Tu C.P., Tam M.F. Characterization of glutathione-S-transferases from day-old chick liver. Biochemistr 1990; 29: 744–750
- Matthev C.J., Wilce M., Parker W. Structure and function of GST. Biochim. Biophysica. Acta 1994; 1205: 1–18
- Thomas H.R., Pickett C.B. GST structure, regulation and therapeutic implications. T J Biological Chem 1993; 268: 11475–11478
- Kunze T. Purification and characterization of class alpha and Mu GST from porcine liver. Comparative Biochem. Physiol. 1997; 116 B: 397–406
- Partridge C.A., Dao D.D., Awasthi Y.C. The effect of t butylated hydroxytoluene on glutathione linked detoxification mechanisims in rat. Arch Tox 1983; 53: 41–48
- Singh S.V., Dao D.D., Srivastava S.K., Awasthi Y.C. Purification and characterization of GST of human kidney. Biochem J 1987; 246: 179–186
- Tsuchida S., Sato K. Glutathione-S-transferase and cancer. LRC Crit Rev Biochem Anal Biol 1992; 27: 337–384
- Sharad S.S., Snju G., Manju S., Rajandra S., Hassan A., Ansari G.A.S., Awasthi Y.C. Purification and characterization of GST from rat pancreas. Biochim. Biophysica Acta 1991; 1079: 285–292
- Dai H., Edens F.W., Roe K.M. Glutathione-S-transferases in the Japanese gagil: tissue distrubition and purification of the liver iszymes. J. Biochem.Toxicol 1996; 11: 85–96
- Hamed R.R., Abu-Shady M.R., El-Beih F.M., Abdalla A.M., Afifi G.M. Purification, and characterization of glutathione-S-transferases from mucor muced. Pol. J.Microbiol 2005; 54: 153–160
- Wai, Kung, Y., Jhon, W., Ho 1997. Purification, and characterization of multiple GST isozymes from Chiranomi. Comperative Biochem. Physiol., 16: 397–406.
- Wilmare W.G., Storey K.B. Purification, and characterization of glutathione-S-transferases from the gnoxia-tolerant turtle, Trachemys scripta elegans. FEBS J. 2005; 272: 3602–3614
- Oshino K., Kamei K., Nishioka M., Shin M. Purification, and characterization of glutathione-S-transferases from quines pig liver. J. Biochem (Tokyo) 1990; 107: 105–110
- Sim G.O., Robyn D., Jurna T.A. Isolation of multiple GST from blue crab. Biochem. Mol. Biol. Int. 1993; 24: 875–880
- Polidoro G., Di Ilio C., Del Boccio G., Zulli P., Federici G. Molecular and catalytic properties of purified GST from human placenta. Biochemical Med 1981; 25: 247–259
- Juronen E., Tesa G., Uskulan U., Pooga M., Mikelsaar A.V. Purification, characterization and tissue distribution of human class thela GST T 1–1. Biochem Mol. Biol. Int. 1996; 39: 21–29
- Nishmaka T., Kodaka R., Nanjo H., Terada T. GST isoenzymes in rat lens. Bochem Int 1992; 26: 35–141
- Awashi Y.C., Dao D.D., Saneto R.P. Inter relationship between anionic on cationic from of GST of human liver. Biochem J 1980; 191: 1–10
- Warholm A.K., Alexandrie J., Högberg K., Siguardsson A., Rannug A. Molecular and catalytic properties of GST from human liver. An enzyme efficiently conjugtingepoxide. Pharmogogenetics 1994; 4: 307–311
- Schroder K., Hallier E., Peter H., Bolt H.M. Biochem. Pharmacol. 1992; 43: 1671–1674
- Habig W.H., Pabst M.J., Jakoby W.B. GST: The first enzymatic step in mercapturic acid formation. J Biol Chem 1974; 249: 7130–7139
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–255
- Laemmli U.K. Cleavage of structural protein during the assembly of the head of bacteriognage. Nature 1970; 227: 680–685
- Lineweaver H., Burk D. The determination of enzyme dissociation constants. J Am. Chem Soc 1934; 56: 658–663
- Warlhom M., Guthenberg C., Mannervik B. Molecular and catalytic properties of GST from human liver. An enzyme efficiently conjugating epoxide. Biochem 1983; 22: 3610–3617
- Marcus C.J., Habig W.H., Jakoby W.B. Glutathione transferase from human blood. Arch Biochem Biophys. 1978; 188: 287–293
- Schaffer J., Gallay O., Ladenstein R. Glutathione-S-transferase from bowine placenta. J Biol Chem. 1988; 25: 17405–17411
- Prohaska J.R., Ganther H.E. Glutathione peroxidase activity of GST purified from rat liver. Biochem Biophys Research Commun. 1977; 76: 437–445
- Nishmaka T., Kodaka R., Nanjo H., Terada T. Inactivation of human placenta GST by SH/SS exchange reaction with biological disulfides. Biochem Biophys Res Commun 1991; 174: 580–585
- Meyer D.J., Coles B., Pemble S.E., Gilmore K.S., Fraser G.M., Kettere B. Theta a class of glutathione transferases purified from rat and man. Biochem J. 1991; 274: 409–414
- Frear D.S., Swansoom H.R. Purification and characterization of GST from maize. Phytochem. 1970; 9: 2123–2129
- Aydemir T., Tarhan L. Purification and partially characterization of superoxide dismutase from chicken erythrocytes. Turk J Chem. 2001; 25: 451–459
- Schröder K., Hallier E., Meyer D.J., Bolt H.M. Metabolism of dichloromethane to formaldehiyde in human erthrocytes: influence of polmorphism of glutathione transferase theta. Arch Toxicol. 1994; 68: 423–427
- Sidhu M., Prasad R., Gill K.D., Nath R. Alterations in isoforms of GST in liver and kidney of cadmium exposed rhesus monkeys: purification and kinetic characterization. Mol Cell Biochem. 1997; 166: 55–63
- Ritter P. Biochemistry: A Foundation. The Brooks/Cole Publishing Company, Pacific Grove, California 1996
- Miyahara T., Takeda A., Hachimori A., Samejima T. On the heterogeneily of catalase from goat liver. J Biochem. 1978; 84: 1267–1276
- Takeda A., Hirano K., Shiroya Y., Samejima T. On the denaturation of porcine erythrocytes catalase with alkali urea and guanidine hydrochloride in relation to it's subunit structure. J. Biochem. 1983; 93: 967–975
- Wasserman B.P., Hultin H.O. Effect of deglycosylation on the stability of Aspergillus niger catalase. Arch Biochem Biophys. 1981; 212: 385–392