Abstract
This work reports for the first time the expression of a soluble B2 globin chain that is part of the extracellular hexagonal-bilayer haemoglobin from Arenicola marina. Two recombinant B2 globins were produced, one fused with gluthatione S-tranferase (B2-GST) and the other without a fusion tag (RecB2) and requiring a different purification procedure. We also describe a new method for the expression of globin that uses Studier's auto-induction medium together with the heme precursor δ-aminolevulinic acid. Media supplementation with the heme precursor δ-aminolevulinic acid in the culture increased heme synthesis by E. coli leading to the expression of the recombinant B2 globins in their active form. RecB2 and B2-GST were expressed with a yield of up to 105 mg/l of E. coli culture. Our approach is rapid and requires only one chromatographic purification step for B2-GST and three purification steps for RecB2. The overall results on RecB2 and B2-GST show that the recombinant globins exhibit similar properties to those of Arenicola marina native HBL-Hb with a great stability and a strong oxygen binding. The results and methodologies described in this paper are the beginning of a work aiming at reconstituting a recombinant HBL-Hb by genetic engineering in order to produce an innovative oxygen carrier for therapeutic applications.
INTRODUCTION
The polychaete Arenicola marina inhabits the interdidal zone and forms large populations on sandy shores in Brittany. This lugworm possesses a giant, extracellular, hexagonal bilayer hemoglobin (HBL-Hb), typical of annelids, and circulating in the vascular system. A model of the quaternary structure of Arenicola marina HBL-Hb has been proposed by Zal et al. based on electrospray ionization mass spectrometry (ESI-MS) analysis and Multi- Angle-Laser-Ligth-Scattering (MALLS) measurements Citation[1]. These authors reported that this macromolecule was composed of eight different globin chains and two structural linker chains. Recently, five globin and one linker cDNAs have been sequenced by Zal's group, encoding for A2a, A2b, A2c, B1, B2, and L2 chains, and in agreement with the masses determined by ESI-MS Citation[2]. Royer et al. highlighted a remarkable hierarchical organization of A. marina HBL-Hb by crystallography Citation[3], following that of Lumbricus terrestris, another annelid Citation[4]. According to this model, the HBL-Hb has a molecular mass of~3.6 MDa, and is made up of two hexagonal layers comprising a total of 144 globin subunits (15–17 kDa) and 36 structural linker chains (24–27 kDa). The various globin subunits are arranged into one or several types of trimers, and with monomeric chains form 12 dodecamers. These latter assemble onto a central core formed by linker subunits. Consequently, this giant HBL-Hb could bind up to 144 oxygen molecules on a single molecule. Interestingly, this HBL-Hb has recently been proposed as a new class of oxygen carriers for use as a red blood cell substitute Citation[5]. Indeed, this molecule possesses interesting properties, placing this biopolymer in the second generation of blood substitutes. This macromolecule is reported to be resistant to auto-oxidation and subunit dissociation under physiological conditions. During pre-clinical trials, no apparent signs of toxicity were observed after the infusion of HBL-Hb to mice and rats Citation[5].
Another interesting property is the property of reassociation of the subunits after dissociation by chemical reagents of A. marina HBL-Hb, similarly to L. terrestris HBL-Hb Citation[5], Citation[6]. Consequently, one of the possible ways of HBL-Hb production in order to use it as a blood substitute would be to assemble a recombinant molecule by genetic engineering using this special self-assembling property on individually expressed subunits.
With recent advances in recombinant DNA technologies, native or specifically modified hemoglobins have been produced by microorganisms, transgenic plants, or animals Citation[7], Citation[8]. Many studies described the expression or coexpression of human intracellular α- and β-globin in Escherichia coli. Different experiments showed the difficulty of expressing the monomers α-and β-globin in bacteria. Expression of fusion α-globin alone involves the formation of unstable proteins and inclusion bodies in the bacterial cytoplasm Citation[9]. It has recently been shown that the coexpression of fusion α-globin with α-hemoglobin-stabilizing protein (AHSP) allows the production of recombinant soluble α-globin that is stable and functional Citation[10]. Others methods, such as the co-expression with methionine aminopeptidase (MAP), allows also the production of soluble α-globin or α/β-globin Citation[11], Citation[12]. For most of the culture media or the expression systems, the yields of recombinant proteins are moderate or low and require many purification steps.
In marine annelid polychaetes, only the intracellular haemoglobin of the Glycera dibranchiata has been expressed in bacteria Citation[13]. Recently, Léon et al. expressed an invertebrate hemoglobin from Lucina pectinata (HbI) in fusion with a His tag Citation[14]. This fusion HbI was soluble, stable, and produced at a high yield of proteins without the need for the coexpression of MAP or molecular chaperone.
In this paper, we describe, for the first time, the expression of a soluble Arenicola marina B2 globin chain using an E. coli expression system, the first step toward the production of an artificial HBL-Hb by genetic engineering. Two recombinant B2 globins were produced, one fused with gluthatione S-tranferase (GST) and the other without a fusion tag and requiring a different purification procedure. This study also reports a new method for the expression of globin, using the Studier's auto-induction medium Citation[15], together with the heme precursor δ-aminolevulinic acid, allowing the production of soluble B2 globin with a high yield.
MATERIALS AND METHODS
Construction of the pGEX-B2 and pET-Duet-B2 Plasmids, and E. coli Transformation
The coding sequence of Arenicola marina B2 globin cDNA was cloned into the prokaryotic expression vectors pET-Duet 1 and pGEX-5X3 (Novagen) to produce a recombinant B2 globin. For the construction in pGEX-5X3, the desired sequence was amplified by PCR with two primers, 5′-CGGGATCCCGATGGACGACTGTTGTACCACCGAG-3′ and 5′-TCCCCCGGGCTAATCCTCGAGGAGGGAAGCAAT-3′, which are complementary with the 5′- and 3′- end sequence of the A. marina B2 globin cDNA and contain a BamHI and a SmaI site, respectively.
For construction in pET-Duet1 site 1, the desired sequence was amplified by PCR with two primers, 5′-CATGCCATGGACGACTGTTGTACCACC-3′ and 5′-CCCAAGCTTAATCCTCGAGGAGGGAAGCA-3′, which are complementary with the 5′- and 3′- end sequence of the A. marina B2 globin cDNA and contain a NcoI and a HindIII site, respectively. The PCR products were cloned using the TOPO®-TA cloning kit (Invitrogen), following the manufacturer's instructions. The positive recombinant clones were isolated and plasmid DNA was prepared with the Flexiprep kit (Amersham Pharmacia Biotech). Purified plasmids containing the B2 globin insert were used in a dye-primer cycle sequencing reaction to check that no mutations were introduced. The sequencing was performed with the universal primers T3 or T7, and the Big Dye® Terminator V3.1 Cycle Sequencing Kit (AppliedBiosystems). PCR products were subsequently run on 31000 Genetic Analyser (Applied Biosystems).
The positive B2 globin recombinant clones and the pET-Duet 1 and pGEX-5X3 vectors were doubly digested with endonucleases NocI/HindIII or BamHI/SmaI in separate reactions. Digestion products were gel-purified using the Qiaquick gel purification kit. The A. marina B2 globin insert and the pET-Duet and 1 pGEX-5X3 vectors were ligated using T4 DNA ligase in duplicate, and these mixtures were used to transform competent TOP10 E. coli cells. Positive clones were used for minipreps in order to obtain enough plasmid to transform the expression bacteria (E. coli BL21/pGEX-B2 or Origami B/ pET-Duet-B2).
Expression and Solubilization of Different B2 Globin Proteins
E. coli cells, containing the various plamids, were grown overnight at 37°C in 4 ml Luria-Bertani medium supplemented with 100µg/ml ampicillin. ZYP-50-52 auto-induction medium Citation[15] used for the expression of the B2 globins contained 1X ZY (10 g tryptone, 5 g yeast extract, 925 ml water), 20X NPS (1M Na2HPO4, 1M KH2PO4, 500 mM (NH4)2SO4), 50X 5052 (25% glycerol, 2.5% glucose, 10% α-lactose monohydrate), and 2 mM MgSO4.
These cultures were supplemented with ampicillin (100µg/ml), kanamycin (30µg/ml), tetracycline (25µg/ml), and with 1 mM 5-aminolevulinic acid (ALA, haem precursor) Citation[16]. The cells were grown 3–4 days in 2-L flasks containing 500ml ZYP-50-52 at 32°C until the optical density at 600 nm reached 10. Expression of B2 globins was induced automatically by α-lactose monohydrate present in the media after 2–3 days of growth. There was then a clear change in the medium's color to red. The bacterial cells were harvested by centrifugation at room temperature.
The cell pellets were resuspended in lysis buffer (sucrose 20%; glycerol 10%; Tris-HCL (pH 8.0) 50 mM, Na2S2O5 0.2 mM, MgCl2 2 mM, anti-protease cocktail and DNase I), lysed in a French press (Constant Cell Disruption Systems) at 1.6 Kbar. The cell debris was removed again by centrifugation at 4°C for 40 min at 10 000 rpm (Eppendorf) and the soluble fractions were saved.
Purification of Fusion B2-GST and In Vitro Binding Assays
The blood-red supernatant containing the GST-B2 globin was mixed with glutathion-Sepharose 4B beads (Amersham Biosciences). The beads were first washed by 10 (bead) volumes of PBS. The beads were then washed by 3 (bead) volumes of NaCl 1M and the beads were finally washed by 10 (bead) volumes of PBS. The blood-red beads were stored frozen at −20°C or eluted by the addition of five bead volumes of elution buffer (50 mM Tris-HCl pH 8.0; reduced glutathione 10 mM). The GST fusion protein elute was concentrated by centrifugation on an Amicon Ultra-30 concentrator, cut-off molecular weight 30 kDa (Millipore, Billerica, Massachusetts). In a second experiment, the B2 globin was cleaved from the GST proteins by addition of factor Xa (50 U/mg protein), and the incubation was carried out for 7 hours at room temperature. The cleavage mix was concentrated by centrifugation on an Amicon Ultra-10 concentrator and was purified by HPLC on a Superdex 75 (Amersham Biosciences) equilibrated with 25 mM Tris-HCl pH 7.5 at room temperature.
Purification of “Native” B2 Globin
The blood-red supernatant containing the B2 “native” globin was then fractionated with ammonium sulphate at 60% saturation. The supernatant was collected by centrifugation at 10000 g for 20 min and dialysed against 25 mM Tris-HCl pH 7.5 overnight at 4°C.
The dialysate was then loaded onto a 2 cm X 10 cm column of DEAE-Sepharose (Pharmacia), equilibrated with 25 mM Tris-HCl pH 7.5 at room temperature. The column was washed with the same buffer until the A280nm was less than 0.001. The “native” B2 globin was then eluted with a linear NaCl gradient of 0 mM at 500 mM NaCl. The fraction containing the “native” B2 globin, identified and collected by measuring the absorbance at 414 nm and 280 nm, were concentrated by centrifugation on an Amicon Ultra-10 concentrator. The proteins were then separated through a 10/300 Superdex 75 gel-filtration column (Pharmacia) equilibrated with 25 mM Tris-HCl pH 7.5 at room temperature. The colored fractions containing the “native” B2 globin, identified and collected by measuring the absorbance at 414 nm and 280 nm, were concentrated by centrifugation on an Amicon concentrator as described above.
The different recombinants globins (B2-GST or “native” B2) were washed by centrifugation on an Amicon Ultra-10 concentrator and stored in a specific saline buffer developed in the laboratory (400 mMol l−1 NaCl, 2.95 mMol l−1 KCl, 32 mMol l−1 MgSO4, 11 mMol l−1 CaCl2 and 50 mMol l−1 Tris-HCl at pH 7.0, Citation[17]).
Protein Analysis
Different aliquots were withdrawn during the expression, solubilization, and purification steps of the expression of the recombinant proteins. The samples were analyzed by SDS/PAGE (Ready Gels Tris-HCl Glycine 4–20% BioRad). The gels were stained with Coomassie brilliant blue R-250 and dried onto Whatman 3MM paper.
Protein concentrations of “native” B2 (hereafter referred to as Rec-B2) or B2-GST were determined using haem concentration as a proxy with Drabkin's method Citation[18] using Drabkin's reagent (Sigma). In this method all the hemoglobin in the sample is converted into methemoglobin and then converted into cyanomethemoglobin. The absorbance of cyanomethemoglobin is then measured in a spectrophotometer at 540 nm.
Spectrophotometric Studies
UV and visible spectral measurements were carried out in a UVmc2 spectrophotometer (SAFAS, Monaco).
The spectra of the oxidized and deoxy- forms of the rec B2 and B2-GST were measured at 25°C in 100 mM potassium phosphate buffer at pH 7.0. Deoxygenation of the B2 recombinant globins was achieved by adding an excess of sodium dithionite (=hydrosulfite). The B2 recombinant globins were oxidized in the presence of 1 mM potassium ferricyanide at pH 7.0 for 20 min resulting in metB2 recombinant globin spectra.
Measurement of Auto-oxidation
Auto-oxidation rate constants were measured at 37°C in 100 mM potassium phosphate buffer pH 7.0, with 1 mM EDTA. An excess of sodium dithionite was added to obtain the ferrous protein. The solution of reduced protein was immediately loaded onto a small G-25-medium gel filtration column and eluted quickly with 100 mM potassium phosphate pH 7.0, with 1 mM EDTA to eliminate the sodium dithionite. The protein spontaneously bound oxygen from the atmosphere. The different solutions were then added to a sample cuvette and the auto-oxidation kinetics was followed by measuring the complete absorption spectra between 500 and 620 nm.
Oxygen Binding Properties
Oxygen equilibrium curves were determined on 3-µl samples of HbA (human adult Hb), AmHb (Arenicola marina HBL-Hb), B2-GST, and Rec-B2 using a thermostated diffusion chamber linked to a mass-flow meter gas mixing system Citation[19]. The diffusion chamber was placed in the light path of a diode array spectrophotometer (Optic Ocean) and the absorbance was measured at 430 nm. Oxygenation data based on at least six equilibrium steps between 0.3 and 0.7 fractional saturation (Y) were converted to Hill plots {log [Y/(1 – Y)] against log PO2, (where PO2 is the oxygen partial pressure) for the estimation of the half-saturation oxygen partial pressure (P50) and Hill's cooperativity coefficient at half-saturation (n50) Citation[20].
Intact Protein Mass Determination
Mass determination was performed on the purified proteins. Electrospray (ESI) mass-spectrometry data were acquired on a LCQ-Advantage ESI-ion trap system (Thermo-Fisher), scanning over the m/z range 500-2000. Samples were desalted by ultrafiltration on an Amicon-5kDa against 32 mL of MilliQ water, at 4°C prior to analysis. A solution of protein (0.1 mg.mL−1) in acetonitrile/water (1:1, v/v) containing 0.1 % (v/v) formic acid was introduced into the electrospray source at 5 µl.min−1.
Protein Identification by LC-MS/MS Analysis
In-gel digestion
After separation on SDS/PAGE, the band corresponding to the Mw of globin was excised and in-gel digested with Trypsin, essentially as described elsewhere Citation[21]. Briefly, the gel band was washed with 100 µL of 25 mM NH4HCO3, followed by 100 µL of 50% acetonitrile in 25mM NH4HCO3. Proteins were reduced by incubation with 10 mM DTT (1 h at 57 °C), and alkylated with 100 µL of 55 mM iodoacetamide (45 min at room temperature). Gel spots were further washed and dried in a vacuum centrifuge. The proteins were digested overnight at 37°C by the addition of 10 µL of Trypsin (12.5 ng/µL in 25mM NH4HCO3; modified Trypsin purchased from Promega,Madison, WI).
LC-MS/MS analysis
Nanoscale capillary liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses of the digested proteins were performed using a Switchos-Ultimate II capillary LC system (LC Packings/Dionex, Amsterdam, the Netherlands) coupled to a hybrid quadrupole, orthogonal, acceleration time-of-flight mass spectrometer (Q-TOF Global, Waters, Manchester, UK). Chromatographic separation was conducted on a reverse-phase capillary column (Pepmap C18, 75 mm i.d., 15 cm length, LC Packings) at a flow rate of 200 nL.min−1. The gradient consisted of a linear increase from 2% to 40% of acetonitrile in 50 min, followed by a rapid increase to 50% of acetonitrile within 10 min.
Mass data acquisitions were piloted by the Mass Lynx software (Waters) using a data dependent acquisition mode: MS data were recorded for 1 sec on the mass-to-charge (m/z) range 400–1500, after which the three most intense ions (doubly or triply charged ions) were selected and fragmented in the collision cell (MS/MS measurements).
Protein identification
Collected data were searched against the TrEMBL database (release 35) using both MASCOT 2.2 (Matrix Science, London, http://www.matrixscience.com) and Protein Lynx Global Server (version 2.1, Waters). Mass accuracy was set to 150 ppm for parent ions and 0.3 Da for MS/MS fragments. One missed trypsin cleavage was allowed per peptide. This led to the unambiguous identification of Q53164 entry. In order to increase the sequence coverage, a de novo sequencing of the remaining peptides was performed and the sequence stretches were aligned against Q53164. Results were validated and exported in an analytical report using the OVNIp software developed by INRA Nantes (http://wwwappli.nantes.inra.fr:8180/OVNIp).
RESULTS AND DISCUSSION
Expression and Purification of Recombinant B2-GST and RecB2 Globins
The sequence coding for A. marina B2 globin was cloned from purified cDNA as described in the Materials and Methods. The globins B2-GST and RecB2 were expressed in auto-induction medium in E. coli BL21 (DE3). Association of two methods, auto-induction medium Citation[15] and addition of ALA, allowed us to obtain soluble B2-GST and RecB2 globins.
In a first experiment, the A. marina B2 globin was expressed as a fusion protein with GST under the control of a tac promoter (). The pGEX-B2 construct was derived from the pGEX-5X3 expression system. The B2-GST recombinant protein was expressed at a high-level in E. coli BL21 (DE3). The B2-GST recombinant protein allows a purification of the protein in a single step. The SDS-PAGE analysis (a) shows the different steps of purification of recombinant B2-GST. The recombinant protein was expressed at a high level and obtained in the soluble fraction (a, lane 2). After purification by affinity chromatography on glutathione Sepharose 4B and elution by addition of reduced gluthatione, the SDS-PAGE revealed the presence of a single band corresponding to B2-GST (a, lane 3). Gel permeation chromatography on Superdex 75 10/300 revealed the presence of single peak pure at 98.7% (data not shown) and a concentration of 105.6 mg/l of culture according to the Drabkin's method.
Figure 1. Construction of plasmids pGEX and pET-Duet 1 containing Arenicola marina B2 globin cDNA for the production in BL21 E. coli. Map of pGEX-B2 (a) and pET DUET-1 B2 (b) plasmids.
Figure 2. Expression and purification of recombinant B2-GST globin. (a) Analysis of the purified B2-GST globin by SDS/PAGE on a gradient 4–20% polyacrilamide gel after Coomassie blue staining. Lane 1, molecular weight markers (kDa shown on the left); lane 2, total fraction of soluble proteins from cells containing the pGEX-B2 plasmid; lane 3, soluble B2-GST after purification and elution from the bound glutathione; lane 4, cleavage B2-GST by factor Xa; lane 5, purification by gel filtration Superdex 75. (b) Elution profile of the B2-GST globin on a superdex 75 10/300GL column after cleavage by factor Xa. The elution was followed by the absorbance at 280 nm (solid line) and 414 nm (dashed line).
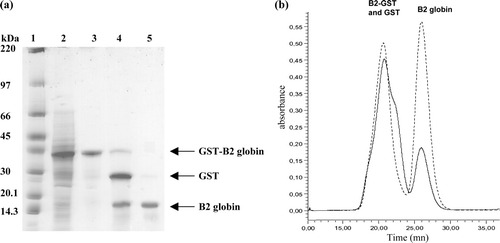
After Factor Xa cleavage of the fusion protein B2-GST, SDS-PAGE revealed the presence of three bands (a, lane 4). The first band of low intensity represents the uncleaved fusion B2-GST protein (44 kDa), the second band corresponds at the GST subunit only (27 kDa), and the last band to the B2 globin (17 kDa). Purification of the cleavage mix by gel filtration on Superdex 75 10/300 (b) showed the presence of two peaks. The analysis of the second fraction by SDS-PAGE (a, lane 5) revealed the presence of a single band at 17 kDa corresponding at the overexpressed B2 globin. This protein is pure at 95% and at a yield of 43 mg/l of culture (). The first fraction of the gel filtration (b) contains the uncleaved B2-GST protein and the subunit GST (verified by SDS-PAGE).
Table 1. Comparison of the recombinant globins yield
These first results show the solubility and stability of A. marina B2 globin as a GST fusion protein. In addition, this protein was produced at a higher yield () than those obtained when GST-αHb was co-expressed with GST-ASHP Citation[10] and for His-rHbI from Lucina pectinataCitation[14].
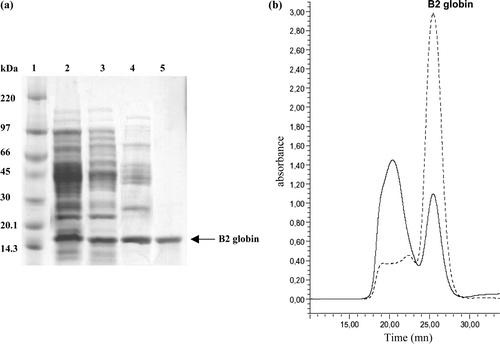
In a second experiment, the A. marina B2 globin was without a fusion protein. The recombinant B2 globin (RecB2) was produced when the cloned gene was placed under the control of the T7 RNA polymerase promoter in the pET-Duet-1 expression vector (b). The recombinant RecB2 was purified to near homogeneity by a three-step procedure, whose progress was followed by SDS-PAGE (a). The association of auto-induction media with ALA for the culture allowed us to obtain a high-level expression in the soluble fraction (a, lane 2). After the first steps of purification (ammonium sulphate fractionation 60% and DEAE-sepharose anion-exchange chromatography), the SDS-PAGE showed a decrease of contaminant protein (a, lanes 3 and 4). The purification of the DEAE fraction by gel filtration on Superdex 75 10/300 (b) showed the presence of two peaks. The analysis of the second fraction by SDS-PAGE (a, lane 5) revealed the presence of a single band at 17 kDa corresponding to the overexpressed B2 globin. After a second analysis by gel filtration (a, b), this protein was found to be pure at 96.8% and a yield of 11mg/l of culture using Drabkin's method ().
Figure 3. Expression and purification of recombinant RecB2 globin. (a) Analysis of the purified RecB2 globin by SDS/PAGE on a gradient 4–20% polyacrilamide gel after Coomassie blue staining (1–2 µg proteins/lane). Lane 1, molecular weight markers (kDa shown on the left); lane 2, total fraction of soluble proteins from cells containing the pET-Duet-B2 plasmid; lane 3, supernatant fraction obtained after ammonium sulfate fractionation 60%; lane 4, first purification by DEAE-Sepharose; lane 5, second purification by gel filtration Superdex 75. (b) Elution profile of the RecB2 globin on a superdex 75 10/300GL column after purification on DEAE Sepharose. The elution was followed by the absorbance at 280 nm (solid line) and 414 nm (dashed line).
These results also indicate the solubility and stability of recombinant Arenicola marina B2 globin. In addition, this protein was produced with a higher yield than those obtained when α-Hb was co-expressed with methionine aminopeptidase Citation[11] or Chloroplastic Hb with ALA Citation[22] ().
Analysis of Proteins by Mass Spectrometry
The goal of the ESI-MS analysis of the intact purified protein was to determine its mass and compare it to the 17033 Da previously reported by Zal et al. Citation[1], and to the calculated mass based on its primary sequence Citation[2]. The raw multicharged spectrum (a) and the deconvoluted mass spectrum (b) show the presence of an abundant protein with an Mw of 17257.55±0.78 (relative abundance of 75%) and adducts of +18 Da, which could be attributed to water molecules. This is 93.5 Da higher than the expected 17164 Da for the intact protein (17033 Da without the initiator methionine (M) of 131 Da).
Figure 4. ESI-MS of overexpressed B2 globin. (a) Smoothed, multiply charged ESI mass spectrum. (b) Corresponding deconvoluted spectrum. (c) List of the peptide sequences identified using the Mascot search engine in Arenicola marina globin B2 (Q53I64) (from the LC-MS/MS analysis of the digested 1D-SDS PAGE band). (d) Product ion spectra of the doubly charged precursor ion at 706.25 m/z (the spectrum is deconvoluted into (M + H)+ species). The proposed amino acid sequence for the N-terminus peptide (including a modified Met having a side chain mass of 169 Da) and the expected yn (C-terminus ions) and bn (N-terminus ions) fragment-ions are represented on the spectrum.
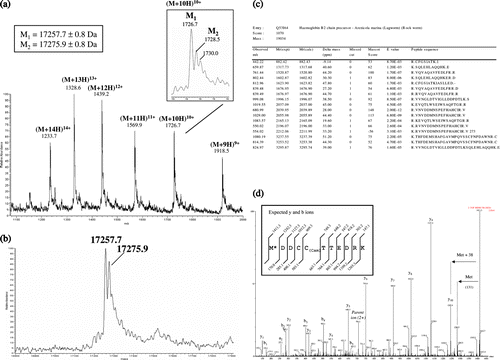
An LC-MS/MS analysis was performed on the tryptic digest of the excised SDS-PAGE band corresponding to the protein of interest in order to confirm that it corresponds to the B2 globin. The mass data (c) were confronted to the TrEMBL database (release 35) using the MASCOT and PLGS2 search engines. An unequivocal assignment could be made to haemoglobin B2 of Arenicola marina (Q53I64), the identified peptides leading to a sequence coverage of 83 % (c).
One peptide at 1410.6 Da (doubly charged at 706.3 m/z) remained non-identified after the MASCOT search and was submitted to de novo sequencing. It could be assigned to the N-terminal fragment “MDDCCTTEDRK” (carrying the initiator methionine) without any ambiguity (d). However, the measured mass for this peptide (1410.6 Da) together with the MS/MS spectrum suggest that the N-terminus methionine is modified (it displays an extra mass of 38 Da). We failed to determine more precisely the nature of this modification. However, it may contribute to part of the delta mass observed for the intact protein (mass difference of + 93.5 Da compared to the expected mass, taking into account the initiator methionine).
Spectral Characterization of Recombinant Proteins B2-GST and RecB2 Globins
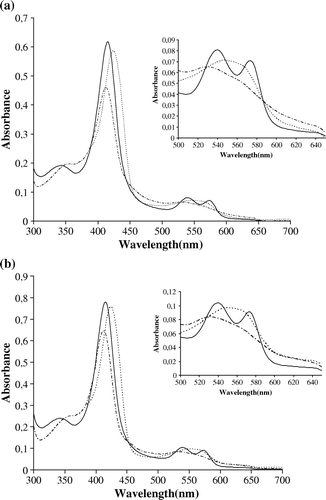
RecB2 and B2-GST were characterized by their light-absorption spectra. Qualitative comparisons show that the spectra of Rec-B2 and B2-GST measured between 300 and 700nm (a, b) are very similar to each other, and this for different ligand and iron states. The oxy-B2 globin has a characteristic Soret band at 418 nm, with α and β bands at 572 nm and 540 nm, respectively. When the solution is treated with sodium dithionite, the α and β bands disappear and are replaced by a single broad band with a peak at 558 nm, while the Soret band shifts towards the visible region at 426 nm. This shift in the Soret band indicates that after the treatment with sodium hydrosulfite there is no ligand on the heme and the iron is now penta-coordinated. The ferric form of the recombinant globins was obtained after treatment with potassium ferricyanide. The α and β peaks disappear and the Soret band shifts to 414 nm. These results show that the Rec-B2 and B2-GST globins have spectral features characteristic of a typical globin. These results were similar to those observed for the recombinant globin of Lucina pectinataCitation[14].
Auto-oxidation and Functionality of B2-GST and RecB2 Globins
The auto-oxidation kinetic of the RecB2 and B2-GST globins in the oxygenated form was compared to those obtained for HbA and HbAm. illustrates the time courses of auto-oxidation. The transition from the oxy- to the met- spectrum of RecB2, B2-GST, HbA and HbAm exhibits well-defined isosbetic points at 522 and 593 nm (b). The auto-oxidation mechanism for haemoproteins developed by Weiss involves the dissociation of the superoxide anion O2 and the formation of the ferric ion Citation[23]. All auto-oxidation curves could be simulated as a monoexponential process (a). The data are summarized in a table (c). RecB2 and B2-GST globins have a kox of 0.008 h−1 and 0.004 h−1, respectively, with a half-life (t1/2) of 78 h and 148 h at 37°C, and pH 7.0 (c). The auto-oxidation of RecB2 seems faster than for B2-GST, which could be explained by the fusion GST protein thus avoiding the auto-oxidation of this recombinant globin. These values of half-life were two or three times greater than the value for HbA obtained under the same conditions as reported in the literature.
Figure 6. Auto-oxidation kinetics of RecB2 (♦) and B2-GST (•), compared with those of Hba (▴) and HBL-Hb (▪). The transition of the ferrous oxy to ferric hexacoordinated form is shown on the graph (b). Experimental conditions were 100 mM potassium phosphate buffer pH 7.0, with 1 mM EDTA.
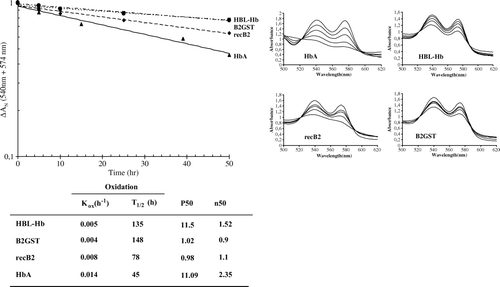
The functional properties of HbA, AmHb, B2-GST, and RecB2 were determined using a thermostated diffusion chamber linked to a mass-flow meter gas mixing system. Oxygen binding molecules are usually compared by means of their oxygen dissociation curve, which gives access to characteristic parameters such as the O2 affinity (P50, the partial pressure of oxygen necessary to saturate half of the oxygen binding sites and consequently the higher the P50, the weaker the oxygen is bound) and cooperativity (n50) (). Under the same conditions, AmHb and HbA (purified) have P50 similar (11.5 mmHg and 11.09 mmHg) and the recombinant monomeric form RecB2 and B2-GST globins have P50 of 0.98 mmHg and 1.02 mmHg. These results show, as reported in the literature, that recombinant monomeric forms have a greater oxygen affinity that native Hb.
Table 2. Functional properties
Conclusion
We describe the first expression of a soluble Arenicola marina B2 globin chain using an E. coli expression system. Two recombinant B2 globins were produced: one fused with gluthatione S-tranferase (GST), and the other one without a fusion tag (and requiring a different purification procedure). In addition, we describe a new method for the expression of globin that involves Studier's auto-induction medium Citation[15], and the heme precursor δ-aminolevulinic acid. Media supplementation with the heme precursor δ-aminolevulinic acid in the culture increased the ability of E. coli to produce heme, leading to the expression of the recombinant B2 globins in the active form. High culture densities and high yield of recombinant B2 globins were obtained in the bioreactor or scale with Studier's auto-induction medium. Furthermore, RecB2 or B2-GST fusion proteins were expressed alone in bacteria without the need for the coexpression of MAP or of a molecular chaperone. With these methods RecB2 and B2-GST were expressed yielding up to 105 mg/l of E. coli culture. Our procedure is rapid and requires only one chromatographic purification step for B2-GST and three purifications steps for RecB2. The expression system described here may be especially interesting for the study of recombinant globin, stable or not. UV-Vis spectral analysis of RecB2 and B2-GST showed absorption maxima identical to those of the recombinant globin His-HbI of Lucina pectinataCitation[14]. The overall results on RecB2 and B2-GST show that the recombinant globins exhibit similar properties to those of Arenicola marina native HBL-Hb with a great stability and a strong oxygen binding. The results and methodologies described in this paper are the beginning of a work to reconstitute a recombinant HBL-Hb by genetic engineering using the auto-assembling property of HBL-Hb already demonstrated Citation[24]. The second step will be to produce a recombinant dimer globin B2-B1 or B2-A2b in the expression vector pET-Duet 1. The polycistronic expression system pST39 kindly provided by Dr. Song Tan could be used for overexpressing recombinant trimer or dodecamer with the production of three or four globins in the same bacteria Citation[25]. The production of GST fusion globin such as B2-GST may be useful in vitro to study the property of reassociation of HBL-Hb by GST pull-down approach. This work will be pursued in the laboratory using the polycistronic vectors in order to express several globins of Arenicola marina. This approach associated with the techniques of production presented in this paper would allow us to produce a synthetic HBL-Hb.
Acknowledgements
The authors would like to thank the valorization department from CNRS that allowed us to carry out this specific research. This work was supported by CNRS, UPMC, European grant (FEDER no. Presage 3814), and the Conseil Régional de Bretagne (contract no. 809) (FZ). TH is a postdoctoral fellow of the CNRS (Fund from DAE). We would also like to thank T. Burmester for his initial advice regarding the use of δ-aminolevulinic acid for the expression of heme-containing proteins. We thank Audrey Geairon (INRA UR1268 BIA, Nantes, BIBS Platform) for excellent technical assistance in mass spectrometry analyses. We would like to thanks Stéphane Hourdez for his useful comments on this manuscript.
References
- Zal F., Green B.N., Lallier F.H., Vinogradov S.N., Toulmond A. Quaternary structure of the extracellular haemoglobin of the lugworm Arenicola marina: a multi-angle-laser-light-scattering and electrospray-ionisation-mass-spectrometry analysis. Eur J Biochem 1997; 243: 85–92
- Chabasse C., Bailly X., Rousselot M., Zal F. The multigenic family of the extracellular hemoglobin from the annelid polychaete Arenicola marina. Comp Biochem Physiol B Biochem Mol Biol. 2006; 144: 319–25
- Royer W.E., Omartian M.N., Knapp J.E. Low resolution crystal structure of Arenicola erythrocruorin: Influence of coiled coils on the architecture of a megadalton respiratory protein. J Mol Biol. 2007; 365: 226–36
- Royer W.E., Strand K., van Heel M., Hendrickson W.A. Structural hierarchy in erythrocruorin, the giant respiratory assemblage of annelids. Proc Natl Acad Sci U S A 2000; 97: 7107–7111
- Rousselot M., Delpy E., Drieu La Rochelle C., Lagente V., Pirow R., Rees J-F., Hagege A., Le Guen D., Hourdez S., Zal F. Arenicola marina extracellular hemoglobin: a new promising blood substitute. Biotechnol. J. 2006a; 1: 333–345
- Sharma, P.K., Kuchumov, A.R., Chottard, G., Martin, P.D., Wall, J.S., Vinogradov, S.N. 1996. The role of the dodecamer subunit in the dissociation and reassembly of the hexagonal bilayer structure of Lumbricus terrestris hemoglobin. J Biol Chem.271: 8754–8762.
- Chang, T.M.S. 1997. Blood Substitutes: Principles, Methods, Products and Clinical Trials. 1, Karger, Basel.
- Tsai A., Vandegriff K., Intaglietaa M., Winslow R. Targeted oxygen delivery by cell-free Hb: a new basis for oxtgen therapeutics. Am. J. Physiol. 2003; 285: 1411–1419
- Jessen T.H., Komiyama N.H., Tame J., Pagnier J., Shih D., Luisi B., Fermi G., Nagai K. Production of human hemoglobin in Escherichia coli using cleavable fusion protein expression vector. Methods Enzymol. 1994; 231: 347–364
- Vasseur-Godbillon C., Hamdane D., Marden M. C., Baudin-Creuza V. High-yield expression in Escherichia coli of soluble human alpha-hemoglobin complexed with its molecular chaperone. Protein. Eng. Des Sel. 2006; 19: 91–97
- Adachi K., Yamaguchi T., Yang Y., Konitzer P.T., Pang J., Reddy K.S., Ivanova M., Ferrone F., Surrey S. Expression of functional soluble human (-globin chains of hemoglobin in bacteria. Prot. Expr. Purif. 2000; 20: 37–44
- Shen T.J, Ho N.T., Zou M., Sun D.P., Cottam P.F., Simplaceanu V., Tam M.F., Bell D.A., Ho C. Production of human normal adult and fetal hemoglobins in Escherichia coli. Protein Eng. 1997; 10: 1085–1097
- Zafar R.S., Weber R.E., Sharma P.K., Vinogradov S.N., Walz D.A. Purification and characterization of recombinant polymeric hemoglobin P1 of Glycera dibranchiate. Protein Expr Purif. 1993; 4: 547–51
- Léon R.G., Munier-Lehmann H., Baudin-Creuza V., Pietri R., López-Garriga J., Cadilla C.L. High-level production of recombinant sulfide-reactive hemoglobin I from Lucina pectinata in Escherichia coli. High yields of fully functional holoprotein synthesis in the BLi5 E. coli strain. Protein Expr Purif. 2004; 38: 184–95
- Studier F.W. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif. 2005; 41: 207–34
- Pesce A, Nardini M, Dewilde S, Ascenzi P, Burmester T, Hankeln T, Moens L, Bolognesi M. Human neuroglobin: crystals and preliminary X-ray diffraction analysis. Acta Crystallogr D Biol Crystallogr. 2002; 58: 1848–50
- Zal F., Lallier F.H., Wall J.S., Vinogradov S.N., Toulmond A. The multi-hemoglobin system of the hydrothermal vent tube worm Riftia pachyptila: I- Reexamination of the number and masses of its constituents. J. Biol. Chem. 1996; 271: 8869–8874
- Drabkin D.L., Austin J.H. Spectrophotometric studies. II. Preparations from washed blood cells; nitric oxide haemoglobin and sulfhemoglobin. J. Biol. Chem. 1935; 112: 51
- Sick H., Gersonde K. Method of continuous registration of O2 binding curves of hemoproteins by means of a diffusion chamber. Anal Biochem 1969; 32: 362–376
- Weber R.E. Cationic control of O2 affinity in lugworm erythrocruorin. Nature 1981; 292: 386–387
- Devouge V., Rogniaux H., Nesi N., Tessier D., Gueguen J., Larre C. Differential proteomic analysis of four near-isogenic Brassica napus varieties bred for their erucic acid and glucosinolate contents. J Proteome Res. 2007; 6: 1342–1353
- Couture M., Guertin M. Purification and spectroscopic characterization of a recombinant chloroplastic hemoglobin from the green unicellular alga Chlamydomonas eugametos. Eur J Biochem. 1996; 242: 779–87
- Weiss J.J. Nature of the iron–oxygen bond in oxyhæmoglobin. Nature 1964; 202: 83–84
- Rousselot M., Le Guen D., Zal F. Novel dissociation mechanism of a polychaetous annelid extracellular haemoglobin. Febs J. 2006b; 273: 1582–96
- Tan S. A modular polycistronic expression system for overexpressing protein complexes in Escherichia coli. Prot. Expr. Purif. 2001; 21: 224–234