Abstract
Background: Peak oxygen uptake (VO2peak) established during progressive cardiopulmonary exercise testing (CPET) is the “gold-standard” for cardiorespiratory fitness. However, CPET measurements may be limited in patients with aneurysmal subarachnoid hemorrhage (a-SAH) by disease-related complaints, such as cardiovascular health-risks or anxiety. Furthermore, CPET with gas-exchange analyses require specialized knowledge and infrastructure with limited availability in most rehabilitation facilities.
Objectives: To determine whether an easy-to-administer six-minute walk test (6MWT) is a valid clinical alternative to progressive CPET in order to predict VO2peak in individuals with a-SAH.
Methods: Twenty-seven patients performed the 6MWT and CPET with gas-exchange analyses on a cycle ergometer. Univariate and multivariate regression models were made to investigate the predictability of VO2peak from the six-minute walk distance (6MWD).
Results: Univariate regression showed that the 6MWD was strongly related to VO2peak (r = 0.75, p < 0.001), with an explained variance of 56% and a prediction error of 4.12 ml/kg/min, representing 18% of mean VO2peak. Adding age and sex to an extended multivariate regression model improved this relationship (r = 0.82, p < 0.001), with an explained variance of 67% and a prediction error of 3.67 ml/kg/min corresponding to 16% of mean VO2peak.
Conclusions: The 6MWT is an easy-to-administer submaximal exercise test that can be selected to estimate cardiorespiratory fitness at an aggregated level, in groups of patients with a-SAH, which may help to evaluate interventions in a clinical or research setting. However, the relatively large prediction error does not allow for an accurate prediction in individual patients.
Introduction
An aneurysmal subarachnoid hemorrhage (a-SAH) is a subtype of stroke caused by a ruptured intracranial aneurysm.Citation1 The incidence rates have remained stable over decades ranging from 6 to 8 per 100,000 person-years.Citation1,2It is reported that a-SAH has long-term consequences such as fatigue, depressive symptoms and problems in cognitive functioning that may persist four years post-onset.Citation3,4 Furthermore, half of the patients experience problems in resuming previous activities, 64% report on one or more participation restrictions, and only one-third is able to fully resume their previous occupation.Citation4–7
Previously, we showed that the cardiorespiratory fitness was approximately 30% lower in patients with a-SAH compared to control subjects.Citation8 The beneficial health effects of improved cardiorespiratory fitness in individuals with stroke, not caused by a-SAH, are well-recognized and exercise training has become an integral component of stroke rehabilitation.Citation9–11 However, the benefits of improved cardiorespiratory fitness remain to be established in individuals with a-SAH. The assessment of cardiorespiratory fitness may help to target and improve rehabilitation programs for individuals with a-SAH.
The gold standard for measuring cardiorespiratory fitness is peak oxygen uptake (VO2peak) obtained with indirect calorimetry during progressive cardiopulmonary exercise testing (CPET).Citation12 However, disease-related complaints, such as cardiovascular health risks or anxiety, may limit the use of progressive CPET in individuals with a-SAH.Citation13,14 Moreover, progressive CPET measurements with gas-exchange analyses require specialized knowledge and infrastructure, with limited availability in most rehabilitation facilities.
The six-minute walk test (6MWT) is an easy-to-administer submaximal exercise test, in which the covered distance walked within six minutes is measured.Citation15 The six-minute walk distance (6MWD) is found to be predictive of VO2peak in patients with cardiopulmonary disorders.Citation16–18 In patients with stroke, not caused by a-SAH, the 6MWD seems to be less predictive of VO2peak. Associations between 6MWD and VO2peak range from 0.34 to 0.74.Citation19 The 6MWD seems to be determined more by impaired walking capacity, than by limited VO2peak.Citation19–22 Because patients with a-SAH usually do not suffer from impairments that directly affect walking capacity,Citation23,24 the 6MWD may be more indicative of VO2peak in these patients. If this is so, the 6MWT may be an easy-to-use instrument to assess cardiorespiratory fitness after a-SAH and will be of great value in daily clinical practice.
The present study investigates the relationship between the 6MWD and VO2peak, and examines whether the 6MWD can predict VO2peak in individuals with a-SAH. We hypothesize that the 6MWD is a predictive measure of VO2peak in individuals with a-SAH.
Methods
Setting and participants
This cross-sectional study, entitled HIPS-Rehab, was part of the longitudinal observational study: “Hypopituitarism In Patients after Subarachnoid hemorrhage study (HIPS)”.Citation25 The present study describes measures of physical fitness that were obtained six months after a-SAH. The study was approved by the Medical Ethics Committee of the Erasmus University Medical Center, and all participants gave written informed consent.
Individuals with a-SAH admitted to the department of Neurology of the Erasmus Medical Center between June 2009 and June 2012 were eligible for inclusion when they were discharged from the Intensive Care Unit and aged ≥18 years. Diagnosis of a-SAH was confirmed by computerized tomography (CT) of the brain and, in cases with negative CT, by lumbar puncture. Presence and location of the aneurysm was determined by CT angiography and/or a digital subtraction angiography.
Excluded from the study were patients meeting any of the following criteria: (1) hypothalamic or pituitary disease diagnosed prior to a-SAH; (2) history of cranial irradiation; (3) trauma capitis prior to a-SAH; (4) other intracranial lesion apart from a-SAH; or (5) other medical or psychiatric condition or laboratory abnormality that may interfere with the outcome of the study. Additional exclusion criteria regarding the CPET measurements were: aged ≥70 years, and absolute contra-indication for CPET.
Procedure
Contra-indications and health risks for physical exercise testing were examined by the treating physician using the guidelines for exercise testing and prescription, established by the American College of Sports Medicine (ACSM)Citation26 and the Physical Activity Readiness Questionnaire (PAR-Q).Citation27 Hereafter, the 6MWT and progressive CPET were performed sequentially in this order. Sufficient resting periods were provided between the tests. A sports physician served as an emergency back-up during the progressive CPET.
Six-minute walk test
The 6MWT is an easy-to-administer submaximal exercise test and was applied as described by the American Thoracic Society.Citation28 The 6MWT showed good to excellent test–retest reliability after stroke.Citation29 Participants were instructed to walk as far as they could along a 30-m indoor, continuous track with a hard surface during a 6-min period. The 6MWT was not practiced beforehand, since this resembles clinical practice. Consistent encouragement was provided after each minute. Participants were allowed to take rest during the test, but were instructed to resume walking as soon as they were able to do so.Citation28 The 6MWD (m) was registered at the end of the test. Heart rate (HR) was recorded using a HR monitor.
Cardiopulmonary exercise test
Progressive CPET was performed on an electronically braked cycle ergometer, which is considered feasible and safe in individuals with stroke who underwent pre-test medical screening.Citation30 A ramp protocol was implemented which was preceded by a four-min warm-up. Hereafter, the resistance increased every 10 s (for females: 12 W/min, for males: 16 W/min) to ensure that volitional exhaustion was reached within 8–14 min. The participants were instructed to pedal at a rate of 60–70 revolutions/min. CPET was terminated when participants voluntary stopped or were unable to maintain the target pedal rate. CPET could also be terminated because of increased health risks, as prescribed in the guidelines of the ACSM.Citation31
During progressive CPET, blood pressure was measured for safety reasons using an automatic system, and heart function was monitored continuously with a 12-lead electrocardiogram. Gas-exchange analyses were applied by indirect calorimetry using a breath-by-breath oximetry analysing system. Before each measurement, volume and gas calibrations were performed. VO2peak was defined as the highest mean oxygen uptake during 30 s of exercise and was expressed in absolute VO2peak (mL/min) and VO2peak per kg body mass (mL/kg/min).
The following criteria were used to objectively determine the performance of maximal exercise testing: (1) respiratory exchange ratio (RER) > 1.0,Citation32 or (2) peak heart rate (HRpeak) within 10 beats per minute (bpm) of the age-predicted maximum heart rate (HRmax), calculated from the formula of Tanaka et al.: HRmax = 208−[0.7 × age ].Citation33 As β-blocker medication reduces HRmax by 25–30%, the equation was adjusted for those with β-blocker medication: HRmax = 0.7 [208−(0.7 × age)].Citation30
Clinical characteristics
The following clinical characteristics were collected to describe the study population: World Federation of Neurologic Surgeons (WFNS) grade,Citation34 Glasgow Coma Scale (GCS) score,Citation35 location of aneurysm, treatment modality of the aneurysm, and presence of hypopituitarism (yes/no) or hydrocephalus (yes/no). Additionally, neurologic comorbidity (paresis or spasticity) was examined and body mass index (BMI) was calculated from height and body mass (kg/m²).
Statistical analyses
Participants that met the objective criteria for maximal exercise testing were included in the final analyses. All data are expressed as mean (SD) unless otherwise indicated. The assumptions for normality and linear regression analysis were met. In order to compare clinical characteristics of participants versus non-participants, independent t-tests were used for continuous data and chi-square tests for categorical data.
A univariate linear regression model was performed using VO2peak as dependent variable and 6MWD (m) as independent variable. In a multivariate linear regression model age and sex were added using step-wise regression with variables being added in a forward model. The explained variance (r2) and the correlation coefficient (r) were analyzed. Although there are no hard rules to describe correlational strength, we considered a correlation coefficient of r > 0.70 as a strong correlation, representing a good estimation of VO2peak from 6MWD at an aggregated group level.Citation36 Furthermore, the standard error of the estimate (SEE), as a percentage of mean VO2peak was calculated to analyze the magnitude of the prediction error which reflects the prediction accuracy at an individual level.
Additionally, paired samples t-test was used to compare the 6MWD with normative values which were calculated from the formula established by Enright et al.Citation37 All analyses were performed using IBM SPSS Statistics, version 20, and a probability value of p < 0.05 was considered statistically significant.
Results
Between June 2009 and June 2012, 241 patients were admitted to the ICU with a diagnosis of a-SAH, 84 were included in HIPS from which 52 volunteered to participate in HIPS-Rehab. Participants in HIPS-Rehab (n = 52) did not differ from non-participants (n = 32) with regard to: sex (p = 0.291), age (p = 0.996), WFNS-grade (p = 0.505) and GCS score at admission (p = 0.136), the location of the aneurysm (p = 0.469), the administered treatment modality (p = 0.489), and the presence of hypopituitarism (p = 0.353) or hydrocephalus (p = 0.559).
In total, 27 successful measurements of both 6MWT and CPET were analyzed (52%). Of the original sample nine patients were aged >70 years, seven could not perform CPET because of logistic reasons, five showed absolute contraindications to CPET, two did not meet the objective criteria for maximal physical exertion, one was not able to perform CPET because of an additional injury and another one could not perform 6MWT because of visual impairment. Although participants included in the analyses were younger (mean age difference 6.8 years, 95% CI −12.94 to −0.057, p = 0.033), there were no significant differences compared to those who were excluded. Table presents characteristics of the participants. The majority had a ruptured aneurysm in the anterior circulation (63%), most underwent endovascular coiling (78%), and 23 (85%) were graded to WFNS I or II with a mean GCS score of 13.7 (2.3). Participants did not show neuro–motor deficits such as paresis or spasticity.
Table 1. Clinical characteristics.
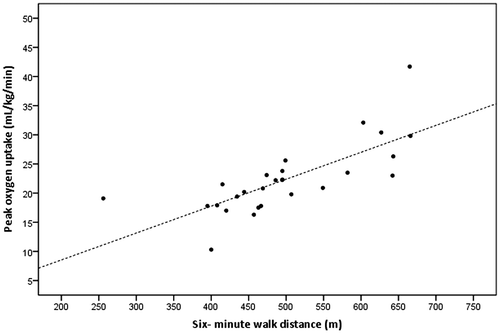
Although 21 participants (78%) had an increased health risk for physical exercise, there were no serious unexpected side-effects observed during or after the 6MWT or the progressive CPET. Outcome measures of 6MWT and CPET are presented in Table . Mean 6MWD was 498 (98) m, which is significantly lower than the calculated mean norm values (=557 (70) m; 95% CI for the difference = −99.1 to −18.6, p = 0.006). HR at the end of 6MWT was 114 (20) bpm, reflecting 67% of predicted maximum HR (= 171 (6) bpm). Results for CPET measurement showed a mean VO2peak of 22.3 (6.0) mL/kg/min, with a mean HRpeak of 152 (24) bpm, reflecting 89% of predicted HRmax (= 171 (6) bpm). The univariate linear regression model, with VO2peak as dependent variable and 6MWD as independent variable revealed a strong relationship (r = 0.75, B = 0.05, 95% CI for B = 0.03–0.06, p < 0.001), with an explained variance of 56% (Table ). SEE was 4.12 ml/kg/min, representing 18% of mean VO2peak. Figure shows the relationship between VO2peak and 6MWD.
Table 2. Results of the six-min walk test and cardiopulmonary exercise test.
Table 3. Univariate linear regression analysis with VO2peak (ml/kg/min) as dependent variable and the six-min walk distance (6MWD) as independent variable.
The extended multivariate linear regression model was significant (p < 0.001), with an explained variance of 67% and a SEE of 3.67 ml/kg/min, representing 16% of mean VO2peak (Table ). In addition to 6MWD (B = 0.04, 95% CI for B = 0.03–0.06, p < 0.001), age significantly contributed to the prediction of VO2peak (B = −0.21, 95% CI for B = −0.38 to −0.04, p = 0.017), whereas sex did not (B = 1.52, 95% CI for B = −1.76 to 4.80, p = 0.349).
Table 4. Multivariate linear regression analysis with VO2peak (ml/kg/min) as dependent variable and the six-min walk distance (m), corrected for sex and age, as independent variable.
Discussion
The present study examined whether the 6MWT is a valid alternative to progressive CPET to predict the cardiorespiratory fitness after a-SAH. A significant, strong correlation was found between the 6MWD and VO2peak (r = 0.82), with a prediction error representing 16% of mean VO2peak. Since post-stroke exercise programs improve cardiorespiratory fitness by 9–23%,Citation38 we consider the prediction error of 16% too large to accurately predict VO2peak at an individual level. However, the 6MWT can be used to predict cardiorespiratory fitness at an aggregated level in groups of patients with a-SAH in clinical and research settings.
According to Outermans et al., correlation coefficients between VO2peak and 6MWD in patients with stroke, not caused by a-SAH, range from 0.34 to 0.74.Citation19 They showed that the relationship is more determined by balance problems and neuro-motor impairments, which affect the walking ability, than by a limited VO2peak.Citation19,20 Patterson et al. found that the variance in 6MWD in individuals with stroke was explained by VO2peak for those who walked more quickly, and by balance for those who walked more slowly.Citation39 In the present study, the relatively strong relationship between 6MWD and VO2peak can be explained by the fact that individuals with a-SAH have relatively mild neurologic morbidity that did not affect the walking ability.
The present findings are in line with studies in patients with cardiopulmonary disorders. Studies reported correlation coefficients for the relationship between 6MWD and VO2peak that ranges from 0.68 to 0.82.Citation40–42 Ross et al.Citation17 studied the predictability of VO2peak from the 6MWD by analyzing the magnitude of the SEE across 10 different studies, including 1,083 cardiopulmonary patients. They recognized moderate-to-strong relationships and reported on poor prediction accuracies as well; they concluded that the 6MWT can be used to estimate mean VO2peak at an aggregated level, but that the 6MWT cannot predict VO2peak at an individual level.
In patients with a-SAH, the mean 6MWD was found to be 498 m, which is higher compared to individuals with other types of stroke (6MWD ranging from 216 to 401 m).Citation19 However, the mean 6MWD is 25% lower compared to that of sex and age-matched norm values, suggesting compromised cardiorespiratory fitness.Citation37
Five participants had absolute contra-indications for progressive CPET and the majority had increased health risks for physical exercise. This emphasizes the need for safe and valid alternatives to progressive CPET after a-SAH. Since the 6MWT cannot accurately predict VO2peak at an individual level, studies are warranted to investigate other options. Future research may consider the use of submaximal cycle ergometer protocols. A recently introduced submaximal cycle ergometer test by Ekblom-Bak et al. seems to predict peak oxygen uptake with a very small prediction error that represents only 9.3% of mean VO2peak.Citation43 However, its validity needs to be determined in patient categories.
Study limitations
Since prediction models often require large study samples, our relatively small sample size can be considered a limitation. However, considering the invasive CPET measurement and the rather low prevalence rate of a-SAH, the present sample size is worth mentioning in the stroke literature. Another limitation is that selection bias may have occurred toward individuals who are willing to perform exercise until voluntary exhaustion, which may have affected the external validity of the present study sample. However, apart from age, participants did not significantly differ from non-participants (also on WFNS grade and GCS score at admission). Finally, for pragmatic reasons, the 6MWT and the CPET were performed on a single day. Although we provided ample resting time, ideally the tests should be performed on separate days to provide sufficient rest between the two tests.
Conclusion
The 6MWD was strongly related to VO2peak in patients with a-SAH. Therefore, the 6MWT can be used to predict mean VO2peak at an aggregated group level. This is relevant for the evaluation of therapy programs in the clinical setting and for research purposes. However, the prediction error was too large to accurately predict VO2peak in individual patients. Since the importance of cardiorespiratory fitness is well-recognized in stroke rehabilitation, easy-to-administer submaximal exercise tests need to be identified, to target and improve post-acute rehabilitation programs for individuals with a-SAH.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Dutch Brain Foundation [grant number 15F07.06].
Notes on contributors
Wouter J. Harmsen is a PhD candidate in the Department of Rotterdam Neurorehabilitation Research; the author has a strong interest in exercise rehabilitation, physical behavior, and epidemiological studies in patients with acquired brain injury.
Gerard M. Ribbers is a professor in neurorehabilitation in the Department of Rotterdam Neurorehabilitation Research; the author has a strong interest in rehabilitation medicine, neural plasticity, motor recovery, neuro-engineering, assistive technology, and patient-related outcome measures after acquired brain injury.
Jorrit Slaman is a senior researcher in the Department of Rotterdam Neurorehabilitation Research; the author has an interest in patient related outcome measures, exercise rehabilitation, healthy lifestyles, and physical behavior in patients with neurologic conditions.
Majanka H. Heijenbrok-Kal is a senior epidemiologist in the Department of Rotterdam Neurorehabilitation Research; the author has a strong interest in patient related outcome measures after acquired brain injury, and extensive expertise in epidemiology, biostatistics, and data sciences.
Ladbon Khajeh is a PhD candidate in the Department of Neurology; the author has a strong interest in neurological diseases and studies the health consequences of pituitary dysfunction after aneurysmal subarachnoid hemorrhage.
Fop van Kooten is a neurologist and a senior researcher in the Department of Neurology and a member of the neurovascular research and training group; the author has an interest in neurological diseases.
Sebastiaan J. C. M. M. Neggers is an internist and endocrinologist in the Department of Endocrinology; the author has an interest in the effects of neuroendocrine dysfunction on health-related outcome.
Rita J. van den Berg-Emons is the study director of the research group MoveFit; the author has a strong interest in exercise rehabilitation, healthy lifestyles, physical fitness, and physical behavior in patients with chronic conditions.
References
- van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318.10.1016/S0140-6736(07)60153-6
- Connolly ES Jr., Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2012;43:1711–1737.
- Kutlubaev MA, Barugh AJ, Mead GE. Fatigue after subarachnoid haemorrhage: A systematic review. J Psychosom Res. 2012;72:305–310.10.1016/j.jpsychores.2011.12.008
- Boerboom W, Heijenbrok-Kal MH, Khajeh L, van Kooten F, Ribbers GM. Long-term functioning of patients with aneurysmal subarachnoid hemorrhage: A 4-yr follow-up study. Am J Phys Med Rehabil. 2016;95:112–120.10.1097/PHM.0000000000000353
- Buunk AM, Groen RJ, Veenstra WS, Spikman JM. Leisure and social participation in patients 4–10 years after aneurysmal subarachnoid haemorrhage. Brain Inj. 2015;29:1589–1596.10.3109/02699052.2015.1073789
- Passier PE, Visser-Meily JM, Rinkel GJ, Lindeman E, Post MW. Life satisfaction and return to work after aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2011;20:324–329.10.1016/j.jstrokecerebrovasdis.2010.02.001
- Huenges Wajer IM, Visser-Meily JM, Greebe P, Post MW, Rinkel GJ, van Zandvoort MJ. Restrictions and satisfaction with participation in patients who are adl-independent after an aneurysmal subarachnoid hemorrhage. Top Stroke Rehabil. 2016;1–8. [Epub ahead of print]
- Harmsen WJ, Ribbers GM, Zegers B, Sneekes EM, Heijenbrok-Kal MH, Khajeh L, van den Berg-Emons HJ. Impaired cardiorespiratory fitness after aneurysmal subarachnoid hemorrhage. J Rehabil Med. 2016;48:769–775.
- Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical activity and exercise recommendations for stroke survivors: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2014;45:2532–2553.
- Sandberg K, Kleist M, Falk L, Enthoven P. Effects of twice-weekly intense aerobic exercise in early subacute stroke: A randomized controlled trial. Arch Phys Med Rehabil. 2016;97:1244–1253.
- Al-Jarrah M, Shaheen S, Harries N, Kissani N, Molteni F, Bar Haim S, et al. Individualized treadmill and strength training for chronic stroke rehabilitation: Effects of imbalance. Top Stroke Rehabil. 2014;21(Suppl 1):S25–S32.10.1310/tsr21S1-S25
- Ross RM. Ats/accp statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:1451–1452.
- Visser-Meily JM, Rinkel GJ, Vergouwen MD, Passier PE, van Zandvoort MJ, Post MW. Post-traumatic stress disorder in patients 3 years after aneurysmal subarachnoid haemorrhage. Cerebrovasc Dis. 2013;36:126–130.10.1159/000353642
- van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: Diagnosis, causes and management. Brain. 2001;124:249–278.10.1093/brain/124.2.249
- Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, et al. The 6-min walk: A new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923.
- McGavin CR, Artvinli M, Naoe H, McHardy GJ. Dyspnoea, disability, and distance walked: Comparison of estimates of exercise performance in respiratory disease. BMJ. 1978;2:241–243.10.1136/bmj.2.6132.241
- Ross RM, Murthy JN, Wollak ID, Jackson AS. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm Med. 2010;10:31.10.1186/1471-2466-10-31
- Cavalheri V, Hernandes NA, Camillo CA, Probst VS, Ramos D, Pitta F. Estimation of maximal work rate based on the 6-min walk test and fat-free mass in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91:1626–1628.10.1016/j.apmr.2010.07.002
- Outermans J, van de Port I, Wittink H, de Groot J, Kwakkel G. How strongly is aerobic capacity correlated with walking speed and distance after stroke? Systematic review and meta-analysis. Phys Ther. 2015;95:835–853.10.2522/ptj.20140081
- Slaman J, Dallmeijer A, Stam H, Russchen H, Roebroeck M, van den Berg-Emons R, et al. The six-minute walk test cannot predict peak cardiopulmonary fitness in ambulatory adolescents and young adults with cerebral palsy. Arch Phys Med Rehabil. 2013;94:2227–2233.10.1016/j.apmr.2013.05.023
- Awad LN, Reisman DS, Wright TR, Roos MA, Binder-Macleod SA. Maximum walking speed is a key determinant of long distance walking function after stroke. Top Stroke Rehabil. 2014;21:502–509.10.1310/tsr2106-502
- Muren MA, Hutler M, Hooper J. Functional capacity and health-related quality of life in individuals post stroke. Top Stroke Rehabil. 2008;15:51–58.10.1310/tsr1501-51
- Patel MD, Tilling K, Lawrence E, Rudd AG, Wolfe CDA, McKevitt C. Relationships between long-term stroke disability, handicap and health-related quality of life. Age Ageing. 2006;35:273–279.10.1093/ageing/afj074
- Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: A systematic review. Stroke. 1997;28:660–664.10.1161/01.STR.28.3.660
- Khajeh L, Blijdorp K, Heijenbrok-Kal MH, Sneekes EM, van den Berg-Emons HJ, van der Lely AJ, et al. Pituitary dysfunction after aneurysmal subarachnoid haemorrhage: Course and clinical predictors-the hips study. J Neurol Neurosurg Psychiatry. 2015;86:905–910.
- Thompson PD, Arena R, Riebe D, Pescatello LS. American College of Sports M. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep. 2013;12:215–21710.1249/JSR.0b013e31829a68cf
- physiology CSfe. Physical activity readiness questionaire (par q) – (revised 2002). 2012
- Laboratories ATSCoPSfCPF. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117
- Eng JJ, Dawson AS, Chu KS. Submaximal exercise in persons with stroke: Test–retest reliability and concurrent validity with maximal oxygen consumption. Arch Phys Med Rehabil. 2004;85:113–118.10.1016/S0003-9993(03)00436-2
- Tang A, Sibley KM, Thomas SG, McIlroy WE, Brooks D. Maximal exercise test results in subacute stroke. Arch Phys Med Rehabil. 2006;87:1100–1105.10.1016/j.apmr.2006.04.016
- Thompson PD, Arena R, Riebe D, Pescatello LS. American College of Sports M. Acsm’s new preparticipation health screening recommendations from acsm’s guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep. 2013;12:215–217.
- Edvardsen E, Hem E, Anderssen SA. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: A cross-sectional study. PLoS One. 2014;9:e85276.10.1371/journal.pone.0085276
- Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156.10.1016/S0735-1097(00)01054-8
- Teasdale GM, Drake CG, Hunt W, Kassell N, Sano K, Pertuiset B, et al. A universal subarachnoid hemorrhage scale: Report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry. 1988;51:1457.10.1136/jnnp.51.11.1457
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84.
- Gerstman B-G. Basic biostatistics. Burlington, MA: Jones & Barlett; 2009.
- Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387.10.1164/ajrccm.158.5.9710086
- Ivey FM, Hafer-Macko CE, Macko RF. Exercise rehabilitation after stroke. NeuroRX. 2006;3:439–450.10.1016/j.nurx.2006.07.011
- Patterson SL, Forrester LW, Rodgers MM, Ryan AS, Ivey FM, Sorkin JD, et al. Determinants of walking function after stroke: Differences by deficit severity. Arch Phys Med Rehabil. 2007;88:115–119.10.1016/j.apmr.2006.10.025
- Zugck C, Kruger C, Durr S, Gerber SH, Haunstetter A, Hornig K, et al. Is the 6-min walk test a reliable substitute for peak oxygen uptake in patients with dilated cardiomyopathy? Eur Heart J. 2000;21:540–549.10.1053/euhj.1999.1861
- Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000;161:487–492.10.1164/ajrccm.161.2.9906015
- Zapico AG, Fuentes D, Rojo-Tirado MA, Calderón FJ, Rosenzweig EB, Garofano RP. Predicting peak oxygen uptake from the 6-min walk test in patients with pulmonary hypertension. J Cardiopulm Rehabil Prev. 2016;36:203–208.10.1097/HCR.0000000000000174
- Ekblom-Bak E, Björkman F, Hellenius ML, Ekblom B. A new submaximal cycle ergometer test for prediction of VO2max. Scand J Med Sci Sports. 2014;24:319–326.10.1111/sms.2014.24.issue-2