ABSTRACT
Background: Little is known about physical activity (PA) in people with stroke living in low-income areas. The aim of this study was to characterize and contrast the levels and patterns of PA between stroke survivors with different ambulation status living in low-income areas in Cape Town, South Africa.
Methods: In this cross-sectional study, 45 community-dwelling stroke survivors living in low-income areas in Cape Town participated. Accelerometers (Actigraph wGT3X-BT) were used to assess PA levels (vector magnitude counts and number of steps) and time spent sedentary, in light and moderate-to-vigorous PA (MVPA). Total daily PA and within-day activity patterns were compared between limited community ambulators (gait speed: <0.8 m/s) and community ambulators (gait speed: ≥0.8 m/s).
Results: Limited community ambulators (n = 23) took fewer steps per day (1091 vs. 3524 steps, P < .001), spent more time sedentary (80% vs 68%, P = .002) and less time in light PA (18% vs 25%, P = .008) and MVPA (1% vs 5%, P < .001) than community ambulators (n = 22). The limited ambulation group had a consistent pattern of PA across the day without any significant variations in PA levels or intensity, whereas the unlimited ambulating group was most active in the morning followed by a gradual reduction in PA throughout the day.
Conclusions: Community ambulating stroke survivors showed greater PA levels and a more variable diurnal pattern in contrast to the limited ambulation group. Different interventions may be required to assist the different groups to start engaging in health-enhancing PA.
Introduction
Stroke is one of the leading causes of disability worldwide today, and approximately 90% of the disease burden is attributable to modifiable risk factors.Citation1 Low physical activity (PA) is a well-known modifiable risk factor for strokeCitation2 and tailored PA is important for functional recoveryCitation3 and risk reduction of recurrent strokes.Citation4 As risk profiles, disease characteristics and etiology of stroke, as well as access to care and rehabilitation, differ between low-to-middle and high-income countries,Citation5,Citation6 it is important to establish information about PA behavior after stroke in different contexts.
A recent systematic review on PA after stroke shows that chronic stroke survivors, on average, take 4078 steps/day, which is substantially lower than healthy persons (8338 steps/day)Citation7 and recommendations of ≥6500 steps/day for persons with disability.Citation8 Although these studies have provided significant information about the total daily PA after stroke, important limitations to the existing body of research exist. First, most previous studies on objectively measured PA in stroke survivors are limited to developed countries, limiting our understanding of the role of context, particularly resources, on the impact of stroke.Citation7,Citation9 Second, little is known about how stroke survivors accumulate PA in different intensities across the day, such as time in sedentary (e.g. sitting and lying) or in moderate-to-vigorous physical activity (MVPA),Citation7 of which both are important for cardiovascular health.Citation10,Citation11 Third, as studies have established a strong association between ambulation status and PA after stroke,Citation12 it is likely that the total daily PA and within-day PA patterns differ between survivors with varying abilities to ambulate. Still, most previous studies have explored PA among stroke survivors with mild mobility impairments.Citation7,Citation13 Understanding the behavior of PA of stroke survivors with varying ability to ambulate in more detail (e.g. how the pattern of PA changes within a day) would provide a stronger foundation on which to develop a sensitive and contextualized program for PA promotion after stroke.Citation14
In South Africa, stroke is a major concern with high economical costs,Citation15 and an increasing incidence and mortality rate is evident.Citation16 Furthermore, a systematic approach to stroke management has not fully been implemented in the public health-care sector of South Africa and limited resources hinder all survivors of stroke to receive appropriate acute care and rehabilitation,Citation17 including the availability of assistive technology.Citation18 We have previously demonstrated low levels of PA in community dwelling stroke survivors living in areas of poverty, high levels of crimes and limited resources for rehabilitation in Cape Town, South Africa.Citation19 In order to inform intervention strategies for stroke survivors with varied levels of function in this context, we aimed to conduct a detailed secondary analysis exploring PA behavior in survivors of stroke with different ambulation status living in low-income areas in Cape Town, South Africa. Specific aims were to characterize and contrast: 1) the total volume and intensity of daily PA and 2) the patterns of PA over the course of a day between stroke survivors with a self-selected gait speed reflecting limited community ambulation (<0.8 m/s) and community ambulation (≥0.8 m/s).Citation20
Materials and methods
Study participants
Inclusion criteria for this cross-sectional study were ≥18 years of age, stroke ≥6 months prior to enrollment, and being able to walk short distances with/without a walking aid independently. Exclusion criteria were cognitive impairment and global aphasia affecting the ability to provide informed consent and other medical conditions affecting mobility substantially. Participants were recruited between March and August 2015 from social support groups in low-income areas of Cape Town, South Africa. The protocol was approved by the University of the Western Cape’s institutional review boards (Number 15/6/82). All participants provided written informed consent and the study was performed in accordance with the STROBE guidelines.
Data collection
Data collection covered three steps. First, demographic data and stroke-related variables; years since stroke onset, number of recurrent strokes, number of falls the last 3 months, use of a walking aid, and provision of in- and outpatient rehabilitation were collected using structured interviews. Inpatient rehabilitation was defined as receiving therapeutic interventions at a rehabilitation center, i.e. being institutionalized, within the first 3 months after stroke onset, and outpatient rehabilitation was defined as therapeutic interventions from either/or a physiotherapist or occupational therapist in the community after ≥3 months of stroke onset. The level of independence in activities of daily living was assessed with the Barthel IndexCitation21 and participants who reported dependency in one or more items were classified as dependent in activities of daily living. Fear of falling was assessed with the Falls Efficacy Scale-International (FES-I)Citation22 and depressive symptoms with the Hospital Anxiety and Depression Scale (HADS).Citation23 These composite self-report measures were presented as a sum-score where a higher score indicates a greater degree of fear of falling and anxiety/depression. The FES-I has shown to be valid in elderly peopleCitation24 and HADS is valid and reliable in people with stroke.Citation25
Second, performance-based tests were used to assess balance and gait. Balance was assessed with the Mini Balance Evaluation Systems Test, which is a 14-item clinical test covering different components of balance control, each item is scored on a three-level ordinal scale from 0 (unable or requiring help) to 2 (normal), and the result is summarized as a total score (maximum score of 28 points).Citation26 Self-selected gait speed was measured using the Six Meter Walk Test, where participants were instructed to walk in their comfortable pace without assistance for 10 m.Citation27 In order to ensure steady-state gait (i.e. neglecting the acceleration and deceleration phase), we calculated the time it took participants to walk the middle 6 m of the 10-m distance. The mean gait speed (m/s) of two trials was used for analysis. To explore the relationship between mobility status and PA, participants were classified using the following gait speed categories: limited community ambulation (<0.8 m/s) and community ambulation (≥0.8 m/s).Citation20
Third, PA was measured at 30 Hz using triaxial accelerometers (Actigraph GT3X)a during free-living conditions.Citation28 Participants were instructed to wear the accelerometer for ≥5 consecutive days around the hip of their non-paretic side, attached above the iliac crest with an elastic band. Participants were instructed to only remove the device when showering, bathing, and at night, and to fill in a diary in order to keep track of the times the device was worn. PA data were downloaded and processed with ActiLife 6 softwarea and episodes of ≥90 min of consecutive zeroes were considered non-wear time and not included in the analysis.Citation29 Participants with ≥3 days of valid PA data (≥8 h of wear time) were included in the analysis.Citation30,Citation31 The total vector magnitude counts (sum of triaxial vector counts) and the number of steps per day were used to reflect the volume of PA, while the following cutoff points were used for intensity categories: sedentary (0–99 counts/min), light intensity PA (100–1041 counts/min), and MVPA (≥1042 counts/min).Citation32,Citation33 The proportion of participants meeting the recommended PA levels of 6500 steps per day for populations with disability was also reported.Citation8
Data management and statistical analysis
Statistical analyses were carried out using IBM SPSS, version 23.0 (SPSS Inc., Chicago, Illinois, USA). Descriptive statistics, median (interquartile range (IQR)) and numbers (percentages), were used to present demographics, stroke-related, balance, and gait variables. These variables were compared between community ambulators and limited community ambulators using the Mann–Whitney U-test, χ2, and Fisher’s exact test.
There were no differences between weekdays and weekend days for total daily PA and within-day patterns of PA. Thus, weekdays and weekend days were merged in all analyses. For the first aim of this study (i.e. accumulation of total daily PA), the volume of PA and time spent in different PA intensities were non-normally distributed and therefore contrasted between the community ambulating and limited community ambulating group using the Mann–Whitney U-test. For the second aim (i.e. within-day patterns of PA), we identified hours with complete PA data (i.e. 60 min of wear time) and calculated individual average PA profiles for a 14-h time period: morning (8 am to 12 am), afternoon (1 pm to 5 pm), and evening (5 pm to 9 pm). This time period was used for analyses as a majority of the participants had valid data for this period. Linear mixed-model analysis was used to investigate differences between groups (unlimited community ambulators vs. limited community ambulators) and time (8 am to 21 pm) for vector magnitude counts, time spent sedentary, light PA, and MVPA. In case of a significant interaction effect (i.e. group x time), post hoc tests with Bonferroni corrections were applied. The significance level was set at P ≤.05 for all analyses.
Results
Participants’ characteristics
Of the 45 participants with stroke taking part in this study, 23 were classified as limited community ambulators and 22 as community ambulators (see for participants’ characteristics). Irrespective of ambulation group, median time since stroke was approximately 2.5 years and a vast majority was not working (unemployed: 61–68% or retired: 23–39%) and had ≥2 chronic diseases (95–96%). Compared to the community ambulation group, a larger proportion in the limited community ambulating group had experienced a fall in the last 3 months (43% vs 14%), used a walking aid (78% vs 32%) and indicated dependency in activities of daily living (87% vs 45%). The limited community ambulating group also demonstrated a more severe disability profile for fear of falling, depressive symptoms, gait, and balance.
Table 1. Participants’ characteristics of stroke survivors presenting with a self-selected walking speed reflecting limited community ambulation (<0.8 m/s) and community ambulation (≥0.8 m/s).
Volume and intensity of daily physical activity
The limited community group demonstrated lower vector magnitude counts (203 987 vs 342 740 counts, P = .001), fewer steps per day (1091 vs. 3524 steps, P ≤ 0.001) as well as spent more time sedentary (80% vs 68%, P = .002) and less time in light PA (18% vs 25%, P = .008) and MVPA (1% vs 5%, P < .001), compared to the community ambulation group (see ). Very few participants took ≥6500 steps per day; 6 (27%) in the community ambulation group and 1 (4%) in the limited community ambulation group.
Table 2. Daily volume and intensity of physical activity of stroke survivors presenting with a self-selected walking speed reflecting limited community ambulation (<0.8 m/s) and community ambulation (≥0.8 m/s).
Within-day patterns of physical activity
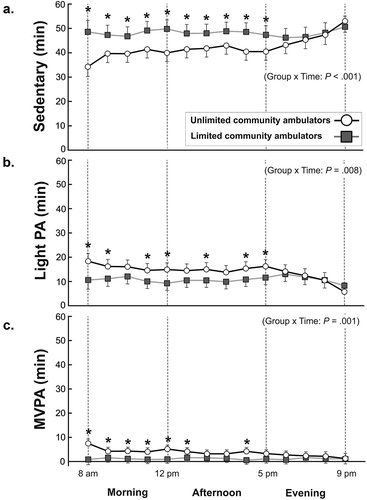
As illustrated in and ), the within-day patterns for vector magnitude counts, time in sedentary, light PA, and MVPA were different for the community ambulation and limited ambulation group (group x time: P < .001). While the limited ambulation group had a consistent pattern across the day without any significant variations neither in PA volume or intensity, the community ambulation group was most active in the morning followed by a gradual reduction in PA throughout the day. The most evident between-group difference in PA profiles was observed for sedentary time (), where the limited ambulation group spent 7–14 min and 6–8 min more time sedentary in morning (P ≤ .008) and afternoon hours (P < .028), respectively, compared to the community ambulation group. Similarly, the limited ambulation group spent more time in light PA for most of the morning (diff: 6–8 min, P ≤ .028) and afternoon hours (diff: 4 min, P ≤ .029) than the community ambulation group. In contrast, the community ambulation group predominantly spent greater time in MPVA in the morning hours than the limited ambulation group (diff: 3–7 min, P ≤ .034). Irrespective of the PA domain, activity in the evening was similar between groups.
Figure 1. Physical activity volume. Vector magnitude counts for the unlimited community ambulating group and the limited community ambulating group across the day. Data are plotted as the average (95% confidence intervals) physical activity for morning (8 am to 12 am), afternoon (1 pm to 5 pm), and evening hours (5 pm to 9 pm). * Significant between-group differences (P ≤ .05).
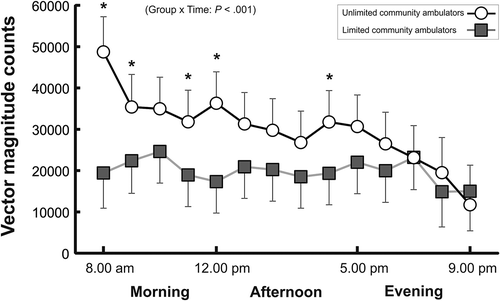
Figure 2. Physical activity intensity. (a) Time spent sedentary, (b) light physical activity and (c) in moderate-to-vigorous activity (MVPA) for the unlimited community ambulating group and the limited community ambulating group across the day. Data are plotted as the average (95% confidence intervals) physical activity for morning (8 am to 12 am), afternoon (1 pm to 5 pm), and evening hours (5 pm to 9 pm). * Significant between-group differences (P ≤.05).
Discussion
To our knowledge, this is the first study to conduct a detailed investigation of PA behavior in stroke survivors with different ambulation status living in low-income areas. Our findings revealed that stroke survivors with more limited capacity to ambulate not only had lower levels of PA and more sedentary time but also accumulated their PA evenly throughout the day. In contrast, the volume and intensity of PA varied across the day in the community ambulation group but despite their ability to independently ambulate outside their home, their PA was insufficient to obtain cardiovascular health benefits. Altogether, our findings highlight that rehabilitation programs should caution against a “one size fits all” approach to PA promotion and should rather tailor interventions that consider functioning profiles and needs of survivors.
In line with our findings, previous literature affirms that physical factors, gait and balance control, impact on stroke survivors’ ability to participate in PA.Citation12 Our findings corroborate with previous studies in the sub-acute phase demonstrating that stroke survivors with a gait speed of approximately ≥0.9 m/s took more steps per day and spent more time in MVPA than survivors with more severe walking limitations.Citation34,Citation35 We further deducted that even the community ambulation group in our study had a lower PA level (3524 steps/day) compared to previous observations of chronic stroke survivors in more developed countries (4078 steps/day).Citation7 We believe that the contextual setting where the present study was situated, areas of poverty and limited resources for rehabilitation in Cape Town,Citation17,Citation18 could have negatively impacted on the ability to engage in PA following stroke. The low PA levels, combined with pronounced sedentary behavior in both ambulation groups (68–80% of the day), highlight the need of developing targeted interventions to increase PA and improve cardiovascular health in this vulnerable population. There is also a need to future investigate how PA changes over time after stroke in relation to functional recovery in light of the provision of acute and rehabilitation services after the onset of stroke in this context.
The community ambulation group demonstrated higher levels and intensity of PA in the morning and afternoon than the limited ambulation group, whereas both groups were equally sedentary in the evenings (see and ). While stroke survivors with limited capacity to ambulate demonstrated a more constant diurnal PA pattern, the community ambulation group showed a gradual reduction in PA throughout the day. In line with our findings, Tieges et al. Citation36 previously reported sedentary time of 35–40 min/hour in the morning followed by a continuous increase in sedentary time to 50–55 min/hour in the evening in a community ambulating stroke cohort.Citation36 As suggested by Tieges et al. Citation36, this pattern might be related to energy depletion in the morning resulting in afternoon fatigue.Citation36 The daily PA pattern observed in community ambulating stroke survivors also resembles the patterns found in persons with Parkinson’s diseaseCitation37,Citation38 but differs from the two peaks PA pattern (mid-morning and afternoon) observed in healthy older adults.Citation37 Differences in diurnal PA patterns between the ambulation group in our study could be due to the greater disability of mobility, balance performance, and psychological-related symptoms (e.g. fear of falling, anxiety, and depression)Citation39 manifesting in the limited ambulation group.
The present findings highlight the need to tailor rehabilitation programs for PA promotion based on the stroke survivor´s ambulation status. The levels of PA of stroke survivors with a self-selected gait speed <0.8 m/s may be addressed by enhancing functional capacity through gait and balance exercises.Citation40 Upscaling of such rehabilitation services would be of particular importance in South Africa where these services are not routinely provided.Citation17,Citation18 Recent findings have also shown that breaking up prolong sedentary time with short bouts of light PA leads to clinically relevant improvements in systolic blood pressure (3–4 mmHg) in stroke survivors.Citation41 These findings are encouraging since this approach could be achievable for stroke survivors with limited capacity to ambulate who will find it difficult to reach recommended levels of MVPA. On the other hand, given the potential of engaging in PA among community ambulating stroke survivors, it is quintessential to understand barriers to exercise, specifically related to socio-cultural environments, values, and norms. Understanding the role of society and its provision of services to promote PA could also assist in the development of secondary stroke prevention programs suitable for the contextual setting of South Africa.Citation42,Citation43
The assessment of PA at one time point is considered a limitation, in that we are limited in our understanding of the direction of the relationship between ambulation status and PA behavior. Secondly, the recruitment from social support groups could have restricted the generalization of the study findings, since individuals from such settings may be more active and integrated in society compared to those not taking part in social groups. Thirdly, we have not accounted for a comprehensive assessment of cognition and fatigue, which may be linked to PA volume and intensities.Citation12 Finally, the MVPA cutoff used in this study was developed for healthy older adults and corresponds to a walking speed of 0.9 m/s,Citation32 which is similar to the median self-selected walking speed in the community ambulation group (0.85 m/s) but substantially higher than the gait speed in the limited ambulating group (0.33 m/s). Accordingly, due to higher energy costs during basic activities after stroke,Citation44 walking in a slower pace could reflect MVPA for stroke survivors with greater disability and our results might underestimate the intensity of PA. To improve the accuracy of PA assessment in the stroke population, it is important to develop PA intensity cutoffs for stroke survivors with different ambulation status.
To conclude, while community ambulating stroke survivors showed greater PA levels and a more variable diurnal PA pattern, highlighting their functional capacity to engage in PA, the lower levels of PA and stable PA pattern in the limited ambulation group likely reflect marked functioning problems. Still, regardless of ambulation status, both groups were well below the recommendations of PA necessary for the enhancement of cardiovascular health. In order to support stroke survivors to engage in PA, our findings suggest the need to tailor programs for stroke survivors based on their ambulation status, which will most likely manifest in different rehabilitation content.
Acknowledgments
The authors would like to thank all the participants who contributed to this work and Isa Lawal for his support with data collection.
Additional information
Funding
References
- Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet Neurol. 2016;15(9):913–924. doi:10.1016/S1474-4422(16)30073-4.
- O’Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388(10046):761–775. doi:10.1016/S0140-6736(16)30506-2.
- Veerbeek JM, van Wegen E, van Peppen R, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One. 2014;9(2):e87987.
- Gallanagh S, Quinn TJ, Alexander J, Walters MR. Physical activity in the prevention and treatment of stroke. ISRN Neurol. 2011;2011:953818. doi:10.5402/2011/953818.
- Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global burden of disease study 2010. Lancet. 2014;383(9913):245–254. doi:10.1016/S0140-6736(13)61953-4.
- Platz T. Evidence-based guidelines and clinical pathways in stroke rehabilitation: an international perspective. Front Neurol. 2019;10:200. doi:10.3389/fneur.2019.00200.
- Fini NA, Holland AE, Keating J, Simek J, Bernhardt J. How physically active are people following stroke? Systematic review and quantitative synthesis. Phys Ther. 2017;97(7):707–717. doi:10.1093/ptj/pzx038.
- Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80. doi:10.1186/1479-5868-8-80.
- English C, Manns PJ, Tucak C, Bernhardt J. Physical activity and sedentary behaviors in people with stroke living in the community: a systematic review. Phys Ther. 2014;94(2):185–196. doi:10.2522/ptj.20130175.
- Biddle SJ, Bennie JA, Bauman AE, et al. Too much sitting and all-cause mortality: is there a causal link? BMC Public Health. 2016;16(1):635. doi:10.1186/s12889-016-3307-3.
- Jefferis BJ, Parsons TJ, Sartini C, et al. Does total volume of physical activity matter more than pattern for onset of CVD? A prospective cohort study of older British men. Int J Cardiol. 2019;278:267–272. doi:10.1016/j.ijcard.2018.12.024.
- Thilarajah S, Mentiplay BF, Bower KJ, et al. Factors associated with post-stroke physical activity: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2018;99(9):1876–1889. doi:10.1016/j.apmr.2017.09.117.
- Field MJ, Gebruers N, Sundaram TS, Nicholson S, Mead G. Physical activity after stroke: a systematic review and meta-analysis. ISRN Stroke. 2013;2013:13. doi:10.1155/2013/464176. Article ID 464176.
- van Wijck F, Bernhardt J, Billinger SA, et al. Improving life after stroke needs global efforts to implement evidence-based physical activity pathways. Int J Stroke. 2019;14(5):457–459. doi:10.1177/1747493019840930.
- Maredza M, Chola L. Economic burden of stroke in a rural South African setting. eNeurologicalSci. 2016;3:26–32. doi:10.1016/j.ensci.2016.01.001.
- Maredza M, Bertram MY, Tollman SM. Disease burden of stroke in rural South Africa: an estimate of incidence, mortality and disability adjusted life years. BMC Neurol. 2015;15:54. doi: 10.1186/s12883-015-0311-7.
- Rhoda A, Smith M, Putman K, Mpofu R, DeWeerdt W, Dewit L. Motor and functional recovery after stroke: a comparison between rehabilitation settings in a developed versus a developing country. BMC Health Serv Res. 2014;14:82. doi: 10.1186/1472-6963-14-82
- Rhoda A, Cunningham N, Azaria S, Urimubenshi G. Provision of inpatient rehabilitation and challenges experienced with participation post discharge: quantitative and qualitative inquiry of African stroke patients. BMC Health Serv Res. 2015;15:423. doi:10.1186/s12913-015-1057-z.
- Joseph C, Conradsson D, Hagstromer M, Lawal I, Rhoda A. Objectively assessed physical activity and associated factors of sedentary behavior among survivors of stroke living in Cape Town, South Africa. Disabil Rehabil. 2018;;40(21):2509–2515. doi: 10.1080/09638288.2017
- Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26(6):982–989. doi:10.1161/01.STR.26.6.982.
- Hsueh IP, Lee MM, Hsieh CL. Psychometric characteristics of the Barthel activities of daily living index in stroke patients. J Formos Med Assoc. 2001;100:526–532.
- Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the falls efficacy scale-international (FES-I). Age Ageing. 2005;34(6):614–619. doi:10.1093/ageing/afi196.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/acp.1983.67.issue-6.
- Delbaere K, Close JC, Mikolaizak AS, Sachdev PS, Brodaty H, Lord SR. The falls efficacy scale international (FES-I). A comprehensive longitudinal validation study. Age Ageing. 2010;39(2):210–216. doi:10.1093/ageing/afp225.
- Aben I, Verhey F, Lousberg R, Lodder J, Honig A. Validity of the beck depression inventory, hospital anxiety and depression scale, SCL-90, and hamilton depression rating scale as screening instruments for depression in stroke patients. Psychosomatics. 2002;43:386–393.
- Tsang CS, Liao LR, Chung RC, Pang MY. Psychometric properties of the mini-balance evaluation systems test (Mini-BESTest) in community-dwelling individuals with chronic stroke. Phys Ther. 2013;93(8):1102–1115. doi:10.2522/ptj.20120454.
- Lam HS, Lau FW, Chan GK, Sykes K. The validity and reliability of a 6-metre timed walk for the functional assessment of patients with stroke. Physiother Theory Pract. 2010;26(4):251–255. doi:10.3109/09593980903015235.
- John D, Freedson P. ActiGraph and actical physical activity monitors: a peek under the hood. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S86–89. doi:10.1249/MSS.0b013e3182399f5e.
- Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–364. doi:10.1249/MSS.0b013e3181ed61a3.
- Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62. doi:10.1186/1479-5868-8-62.
- Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37(11 Suppl):S531–543. doi:10.1249/01.mss.0000185657.86065.98.
- Copeland JL, Esliger DW. Accelerometer assessment of physical activity in active, healthy older adults. J Aging Phys Act. 2009;17:17–30.
- Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37(11 Suppl):S512–522. doi:10.1249/01.mss.0000185659.11982.3d.
- Shimizu N, Hashidate H, Ota T, Suzuki T, Yatsunami M. Characteristics of intensity-based physical activity according to gait ability in people hospitalized with subacute stroke: a cross-sectional study. Phys Ther Res. 2019;22(1):17–25. doi:10.1298/ptr.E9971.
- Joseph C, Stromback B, Hagstromer M, Conradsson D. Accelerometry: A feasible method to monitor physical activity during sub-acute rehabilitation of persons with stroke. J Rehabil Med. 2018;50:429–434.
- Tieges Z, Mead G, Allerhand M, et al. Sedentary behavior in the first year after stroke: a longitudinal cohort study with objective measures. Arch Phys Med Rehab. 2015;96(1):15–23. doi:10.1016/j.apmr.2014.08.015.
- Rochester L, Jones D, Hetherington V, et al. Gait and gait-related activities and fatigue in Parkinson’s disease: what is the relationship? Disabil Rehabil. 2006;28(22):1365–1371. doi:10.1080/09638280600638034.
- Wallen MB, Franzen E, Nero H, Hagstromer M. Levels and patterns of physical activity and sedentary behavior in elderly people with mild to moderate parkinson disease. Phys Ther. 2015;95(8):1135–1141. doi:10.2522/ptj.20140374.
- Hendrickx W, Riveros C, Askim T, et al. Identifying factors associated with sedentary time after stroke. Secondary analysis of pooled data from nine primary studies. Top Stroke Rehabil. 2019;26(5):327–334. doi:10.1080/10749357.2019.1601419.
- English C, Hillier SL, Lynch EA. Circuit class therapy for improving mobility after stroke. Cochrane Database Syst Rev. 2017;2;6:CD007513. doi: 10.1002/14651858.CD007513.pub3.
- English C, Janssen H, Crowfoot G, et al. Frequent, short bouts of light-intensity exercises while standing decreases systolic blood pressure: breaking up sitting time after stroke (BUST-Stroke) trial. Int J Stroke. 2018;13(9):932–940. doi:10.1177/1747493018798535.
- Billinger Sandra A, Arena R, Bernhardt J, et al. Physical activity and exercise recommendations for stroke survivors. Stroke. 2014;45(8):2532–2553. doi:10.1161/STR.0000000000000022.
- Pang MYC, Charlesworth SA, Lau RWK, Chung RCK. Using aerobic exercise to improve health outcomes and quality of life in stroke: evidence-based exercise prescription recommendations. Cerebrovascular Dis. 2013;35(1):7–22. doi:10.1159/000346075.
- Kramer SF, Churilov L, Kroeders R, Pang MY, Bernhardt J. Changes in activity levels in the first month after stroke. J Phys Ther Sci. 2013;25(5):599–604. doi:10.1589/jpts.25.599.
