ABSTRACT
Background
Frailty in older individuals is an underappreciated condition that affects the incidence and/or prognosis of stroke.
Objectives
We evaluated the prevalence of pre-onset frailty in patients with acute first-onset and recurrent strokes and association between pre-onset frailty and functional disability at hospital discharge.
Methods
This prospective cohort study included 210 acute stroke patients admitted to the Stroke Unit of Nippon Medical School Hospital during November 2021–June 2022. The mean participant age was 79.2 ± 7.4 years. Age, sex, pre-onset frailty, body mass index (BMI), stroke type, medical history, and National Institutes of Health Stroke Scale (NIHSS) score at admission were evaluated. Frailty was defined as a clinical frailty scale (CFS) score ≥ 5. Frailty prevalence was calculated for all patients, and scores of functional disabilities at discharge were evaluated using modified Rankin scale.
Results
Overall frailty prevalence was 31% in all stroke patients, with 24% and 47% of first-onset and recurrent strokes, respectively. Pre-onset frailty, NIHSS score at admission, age, stroke type, previous stroke, sex, BMI, dyslipidemia, and atrial fibrillation were significantly associated with functional disability at discharge. Logistic regression analysis revealed that CFS score, NIHSS score at admission, and previous stroke were independent predictors of functional disability at discharge.
Conclusions
Approximately one-fourth of patients with first-onset stroke had pre-onset frailty; the rate doubled in recurrent stroke; these rates appear to be much larger than rate in healthy individuals. Pre-onset frailty, a negative independent factor affecting functional disability at discharge, is important for pre-onset frailty evaluation and rehabilitation intervention in acute stroke patients.
Introduction
Stroke is one of the most common noninfectious disease causing death and disability worldwide; the fourth leading cause of death; and the second leading cause of long-term care in Japan.Citation1 In the 1970s, the median age for stroke onset was in the 50s, but it is now in the 70s (70 and 77 years for men and women, respectively) in Japan.Citation2,Citation3 Additionally, the aging of patients with stroke and their survival rates are expected to continue to increase due to demographic changes, advances in treatment, and prolonged life expectancy.Citation4
Frailty, defined as a state of decreased physiological reserve and increased vulnerability to various stresses, is a clinical condition with reversible characteristics.Citation5,Citation6 The prevalence of frailty among community-dwelling older individuals aged ≥65 years is 7–10%.Citation7–9 Although frailty is associated with physical inactivity and lifestyle-related diseases, information on association between frailty and incidence of stroke is limited. In a previous meta-analysis, the prevalence of frailty in patients with chronic stroke was 22% (95% confidence interval [CI]: 16–27%).Citation10 Although there are some reports on frailty in patients with chronic stroke, few studies have focused on pre-onset frailty in patients with acute stroke, and reports that distinguish between first-onset and recurrent stroke or studies in Asian population are scarce.
Furthermore, few prospective studies have been conducted to date on the presence or absence of pre-onset frailty, which may influence functional recovery and outcomes following acute stroke.Citation11,Citation12 The prevalence of pre-onset frailty was 54% in patients with acute stroke aged ≥70 years, and frailty was independently associated with 28-day mortality after onset, with mortality being higher in frail individuals than in non-frail individuals (16.7 versus 5%, p < 0.01).Citation11 In another study, the frailty and pre-frailty groups had reduced quality of life at 3 and 18 months after stroke onset (p < 0.01).Citation12 The rate of pre-onset frailty can be higher in patients with stroke than in community-dwelling older adults. Moreover, no studies have compared the incidence of pre-onset frailty between patients with first-onset stroke and those with recurrent stroke. Furthermore, the impact of pre-onset frailty on short-term functional outcomes has not been prospectively investigated. It is important to evaluate the presence of pre-onset frailty in patients with first-onset and recurrent strokeCitation4 and investigate its effects on post-onset limitations in activity of daily living (ADL) and functional outcomes in planning rehabilitation therapy. The clinical frailty scale (CFS)Citation13 is a frailty assessment tool with established reliability and validityCitation14,Citation15 that can evaluate frailty using simple illustrations and text. CFS can be used to assess pre-onset frailty even after stroke as it is not affected by physical disability caused by stroke.Citation11 Considering this gap in literature, we aimed to investigate the prevalence of frailty in patients with acute stroke and association between frailty and functional disability at discharge.
Materials and methods
Participants
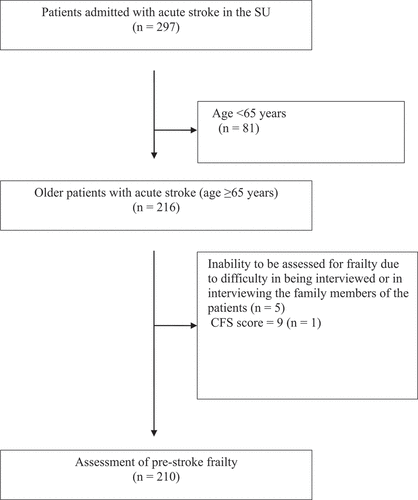
We recruited consecutive patients with acute stroke admitted to the Stroke Unit (SU) of Nippon Medical School Hospital between 1 November 2021 and 30 June 2022. Patients aged <65 years, those who disagreed to participate in the study, and those who could not be assessed for frailty due to difficulty in interviewing them or their family members were excluded. This study was approved by the ethics committee of Nippon Medical School (B-2020-302). All participants or their family members provided written informed consent before participating in the study. This study conforms to the STROBE guidelines.
Clinical characteristics
Data on age, sex, body mass index (BMI), medical history of stroke, hypertension, dyslipidemia, diabetes mellitus, smoking, ischemic heart disease, and atrial fibrillation were recorded from a registry database. Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) score on admission, on day 7, and at discharge. Data on stroke type (infarct or intracerebral hemorrhage), modified Rankin scale (mRS) score at discharge, length of hospitalization, and discharge from hospital were also recorded.
Frailty
CFS is scored on a scale of 1–9 based on several factors, including (1) overall fitness level, (2) degree of active diseases and symptoms, (3) ADL impairment, and (4) cognitive level. The CFS score just before stroke onset was evaluated by examiners by collecting information from the patient verbally on admission. In case the patient had difficulty in providing information for pre-onset CFS score owing to the poor consciousness, possible poor cognitive status, or language ability, the information for pre-onset CFS was obtained from the medical record. Examiners were provided the guidance needed to use the rating scale in advance. The Japanese version of the scale was used with permission from the developer.Citation16 Pre-onset CFS score ≥ 5 was defined as frailty,Citation17 and patients with a pre-onset CFS score of 9, corresponding to terminal illness, were excluded from the study, as previously reported.Citation11,Citation17 The percentage of each pre-onset CFS score and prevalence of frailty were calculated.
Statistical analyses
The Mann – Whitney U test was used to compare the prevalence of frailty between patients with first onset and those with recurrent disease. The Mann – Whitney U test and chi-square test were used to examine differences in variables depending on the presence or absence of frailty and functional disability at discharge. Multivariable logistic regression analysis was used to identify independent factors associated with functional disability (mRS score ≤ 2 or ≥ 3) at discharge. Variables with p values < 0.05 in the univariate analyses were incorporated into the logistic regression analysis. Odds ratios (ORs) are presented with corresponding 95% CIs. All analyses were performed using SPSS version 27 statistical software (IBM Corp., Armonk, NY, USA), and p values < 0.05 were considered statistically significant.
For univariate analysis, an effect size of 0.8 and a significance level of α = 0.05% with a power of 80% required 134 cases to be analyzed.
Results
During the study period, 297 consecutive patients with acute stroke were admitted to the SU of Nippon Medical School Hospital. Among them, 81 patients aged <65 years, five patients who could not be assessed for frailty due to difficulty in interviewing them or their family members, and one patient with a CFS score of 9 were excluded. Finally, 210 patients were included (). The mean age of the participants was 79.2 ± 7.4 years, and 121 (58%) participants were men.
presents the clinical characteristics of participants and differences in each survey item according to the presence or absence of frailty. Significant differences were observed for age, BMI, previous stroke, mRS score at discharge, atrial fibrillation (p < 0.001), sex, and NIHSS score (admission, day 7, and discharge) (p < 0.01).
Table 1. Comparison of clinical characteristics between the non-frail and frail groups.
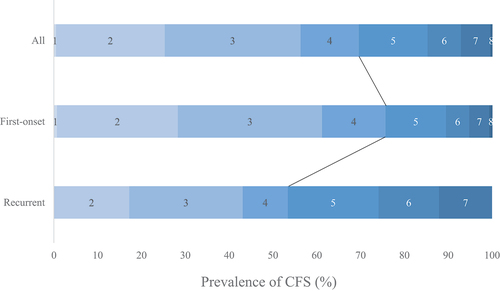
The distribution of CFS scores is presented in . A peak was observed at a CFS score of 3 in the all, first-onset, and recurrent stroke patients. The prevalence of frailty was 31%, 24%, and 47% in overall, first-onset, and recurrent stroke patients, respectively, with a statistically significant difference between the first-onset and recurrent stroke patients (p < 0.01).
Figure 2. Distribution of pre-onset CFS scores. the prevalence of frailty (CFS score ≥ 5) is 31, 24, and 47% in the overall, first-onset, and recurrent groups, respectively. The distribution peak is at a CFS score of 3.
presents the differences in each survey item according to the presence or absence of functional disabilities at discharge. Pre-onset frailty and NIHSS score at admission (p < 0.001); age and stroke type (p < 0.01); sex, BMI, dyslipidemia, and atrial fibrillation (p < 0.05) significantly affected functional disability at discharge.
Table 2. Comparison of clinical background characteristics by functional disability at discharge.
presents the results of the multivariate analysis of the effect of frailty on functional disability at discharge. Logistic regression analysis with functional disability at discharge (mRS score ≥ 3) as the dependent variable and age, sex, BMI, CFS score, NIHSS score on admission, stroke type, and medical history (stroke, dyslipidemia, and atrial fibrillation) as independent variables revealed that CFS score (OR: 1.486, 95% CI: 1.128–1.958, p < 0.01), NIHSS score (OR, 1.210; 95% CI: 1.119–1.307, p < 0.001), and history of stroke (OR, 2.575; 95% CI: 1.086–6.107, p < 0.05) were significant independent variables.
Table 3. Logistic regression analysis of predictors of poor discharge outcome (mRS ≥3).
Discussion
In the study, the data of 210 patients with acute stroke admitted to a Japanese SU were extracted from the prospective stroke registry. We found that the overall prevalence of pre-onset frailty in patients with stroke was 31%, which was significantly higher than the 7–10% prevalence of frailty among community-dwelling older adults.Citation7–9 A novel finding of this study was that 24% patients with first-onset stroke had frailty, and the rate doubled in patients with recurrent stroke. Multiple logistic regression analysis demonstrated that the CFS score was an independent factor associated with poor outcome even after adjusting for the effects of stroke severity and comorbidities.
Arteriosclerosis risk factors such as hypertension,Citation18 diabetes mellitus,Citation19 and dyslipidemiaCitation20 are strongly associated with the development of stroke. Lifestyle issues such as diet and exercise are also significantly associated with stroke. Moreover, adult men and women who performed physical activity in their leisure time had a significantly lower risk of stroke.Citation21,Citation22 Furthermore, a study on the effect of physical activity on stroke recurrence revealed that patients with recurrent stroke had significantly higher levels of visceral fat and lower levels of physical activity.Citation23 Individuals with hypertension, diabetes, and heart disease were shown to have significantly lower physical activity, lower VO2 max, and lower frequency of instrumental ADL practices.Citation24–27 In the current study, the peak CFS score was 3, with few patients having moderate-to-high-intensity exercise habits. In previous studies involving community-dwelling older individuals, >30% participants had a CFS score of 1–2.Citation8,Citation9 A CFS score of 2 was defined as “I exercise regularly,” and a CFS score of 3 was defined as “I do nothing more than walking on a daily basis.” Patients with stroke are already functionally impaired before strokeCitation28 and may have lower activity levels than community-dwelling older individuals. The American Stroke Association recommends 40 min of moderate aerobic exercise three to four times a week in physically active patients with stroke to prevent stroke recurrence.Citation29 In adults with frailty, assessment and improvement of frailty, in addition to pharmacotherapy, are important for the prevention of stroke onset and recurrence. Further prospective studies are needed to determine if frailty is an independent risk factor for stroke onset.
In the current study, pre-onset frailty, NIHSS score on admission, and history of stroke were identified as independent factors negatively affecting functional disability at discharge. The presence of pre-stroke frailty is associated with poor functional outcomesCitation30,Citation31 and is not related to age and sex.Citation32 The presence of pre-stroke frailty had a negative effect on the improvement in NIHSS scores after thrombolysisCitation11 and was negatively associated with mRS scores after 3 months of endovascular treatment.Citation33 Therefore, rehabilitation program should be implemented considering frailty, leading to decreased walking speed, grip strength, physical activity, balance ability, weight loss, and exhaustion due to a decrease in physiological reserve capacity.Citation34,Citation35
This study had some limitations. First, it was a single-center study. Second, because this study did not use a control group, there is limited interpretation of whether frailty itself influences stroke onset. In addition, there is a lack of information on psychological and social frailty. Third, previous studies have revealed that cognitive function before stroke onset is associated with short-term outcomes.Citation36,Citation37 Furthermore, the severity and outcome of ischemic stroke differ by the subtype.Citation38 Thus, the effect frailty on functional disability at discharge needs to be investigated. In addition, it is necessary to investigate the association between pre-onset frailty and impaired consciousness and delirium after stroke onset and long-term ADL limitations.
Despite these limitations, this study was prospective and included consecutive patients, which was useful in understanding the characteristics of frailty in patients with stroke in Japan. The study revealed the difference in frailty between the first-onset and recurrent groups and the effect of frailty on functional disability at the time of hospital discharge after adjusting for the effects of initial stroke severity and comorbidities. Furthermore, because this was a single-center study, there were no differences in the amount or methods of rehabilitation evaluation and therapy, which may be a strength, unlike in multicenter studies.
Overall, 24% of patients with first-onset stroke had frailty, and the rate doubled in patients with recurrent stroke. These frequencies were higher than those in community-dwelling older individuals. Frailty was an independent factor negatively affecting the mRS score at hospital discharge, suggesting the importance of early rehabilitation evaluation and treatment for patients with acute stroke and frailty. Further studies are needed on patients with stroke with frailty to determine the impact of frailty on long-term outcomes and the effectiveness of the intervention in such patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- https://www8.cao.go.jp/kourei/whitepaper/w-2019/html/zenbun/s1_2_2.html (in Japanese)
- Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, Gotoh S, et al. Secular trends in cardiovascular disease and its risk factors in Japanese: half-century data from the hisayama study (1961-2009). Circulation. 2013;128(11):1198–1205. doi:10.1161/CIRCULATIONAHA.113.002424.
- Toyoda K, Yoshimura S, Nakai M, Koga M, Sasahara Y, Sonoda K, et al. Twenty-year change in severity and outcome of ischemic and hemorrhagic strokes. JAMA Neurol. 2022;79(1):61–69. doi:10.1001/jamaneurol.2021.4346.
- Toyoda K, Inoue M, Koga M. Small but steady steps in stroke medicine in Japan. J Am Heart Assoc. 2019;8(16):e013306. doi:10.1161/JAHA.119.013306.
- Linda P, Fried CMT, Walston J, et al. Frailty in older adults- evidence for a phenotype. J Gerontol Med Sci. 2001;56A(3):N0:M146–M156. doi:10.1093/gerona/56.3.M146.
- Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi:10.1016/j.jamda.2013.03.022.
- Kojima G, Iliffe S, Taniguchi Y, Shimada H, Rakugi H, Walters K. Prevalence of frailty in Japan: a systematic review and meta-analysis. J Epidemiol. 2017;27(8):347–353. doi:10.1016/j.je.2016.09.008.
- O’halloran AM, Hartley P, Moloney D, McGarrigle C, Kenny RA, Romero-Ortuno R. Informing patterns of health and social care utilisation in Irish older people according to the clinical frailty scale. HRB Open Res. 2021;4:54. doi:10.12688/hrbopenres.13301.1.
- Chang CI, Chan DC, Kuo KN, Hsiung CA, Chen CY. Prevalence and correlates of geriatric frailty in a Northern Taiwan community. J Formos Med Assoc. 2011;110(4):247–257. doi:10.1016/S0929-6646(11)60037-5.
- Palmer K, Vetrano DL, Padua L, Romano V, Rivoiro C, Scelfo B, et al. Frailty syndromes in persons with cerebrovascular disease: a systematic review and meta-analysis. Front Neurol. 2019;10:1255. doi:10.3389/fneur.2019.01255.
- Evans NR, Wall J, To B, Wallis SJ, Romero-Ortuno R, Warburton EA. Clinical frailty independently predicts early mortality after ischaemic stroke. Age Ageing. 2020;49(4):588–591. doi:10.1093/ageing/afaa004.
- Wæhler IS, Saltvedt I, Lydersen S, Fure B, Askim T, Einstad MS, et al. Association between in-hospital frailty and health-related quality of life after stroke: the nor-COAST study. BMC Neurol. 2021;21(1):100. doi:10.1186/s12883-021-02128-5.
- Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi:10.1503/cmaj.050051.
- Shears M, Takaoka A, Rochwerg B, Bagshaw SM, Johnstone J, Holding A, et al. Assessing frailty in the intensive care unit: a reliability and validity study. J Crit Care. 2018;45:197–203. doi:10.1016/j.jcrc.2018.02.004.
- Gregorevic KJ, Hubbard RE, Lim WK, Katz B. The clinical frailty scale predicts functional decline and mortality when used by junior medical staff: a prospective cohort study. BMC Geriatr. 2016;16(1):117. doi:10.1186/s12877-016-0292-4.
- https://www.dal.ca/sites/gmr/our-tools/clinical-frailty-scale/cfs-guidance.html
- Church S, Rogers E, Rockwood K, Theou O. A scoping review of the clinical frailty scale. BMC Geriatr. 2020;20(1):393. doi:10.1186/s12877-020-01801-7.
- Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808. doi:10.1016/S0140-6736(19)32008-2.
- Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. The Lancet. 2010;375(9733):2215–2222. doi:10.1016/S0140-6736(10)60484-9.
- Zhang X, Patel A, Horibe H, Wu Z, Barzi F, Rodgers A, et al. Cholesterol, coronary heart disease, and stroke in the Asia pacific region. Int J Epidemiol. 2003;32:563–572. doi:10.1093/ije/dyg106.
- Kelley GA, Kelley KS. Leisure time physical activity reduces the risk for stroke in adults: a reanalysis of a meta-analysis using the inverse-heterogeneity model. Stroke Res Treat. 2019;2019:8264502. doi:10.1155/2019/8264502.
- Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34(10):2475–2481. doi:10.1161/01.STR.0000091843.02517.9D.
- Ushio M, Kanaoka M, Kinoshita Y, Maeno S, Fujita K. Moderate-to-vigorous physical activity and the risk of stroke recurrence in patients with a history of minor ischemic stroke in Japan: a retrospective analysis. Top Stroke Rehabil. 2018;25(8):591–598. doi:10.1080/10749357.2018.1507309.
- Ribisl PM, Lang W, Jaramillo SA, Jakicic JM, Stewart KJ, Bahnson J, et al. Exercise capacity and cardiovascular/metabolic characteristics of overweight and obese individuals with type 2 diabetes: the look AHEAD clinical trial. Diabetes Care. 2007;30(10):2679–2684. doi:10.2337/dc06-2487.
- Carbone S, Del Buono MG, Ozemek C, Lavie CJ. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog Cardiovasc Dis. 2019;62(4):327–333. doi:10.1016/j.pcad.2019.08.004.
- Fukui S, Kawakami M, Otaka Y, Ishikawa A, Yashima F, Hayashida K, et al. Activities of daily living among elderly persons with severe aortic stenosis. Disabil Rehabil. 2021;43(3):338–344. doi:10.1080/09638288.2019.1624838.
- Iwasawa T, Fukui S, Kawakami M, Kawakami T, Kataoka M, Yuasa S, et al. Factors related to instrumental activities of daily living in persons with chronic thromboembolic pulmonary hypertension. Chron Respir Dis. 2021;18:14799731211046634. doi:10.1177/14799731211046634.
- Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40(4):1032–1037. doi:10.1161/STROKEAHA.108.542894.
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American college of Sports Medicine and the American heart association. Circulation. 2007;116:1081–1093. doi:10.1161/CIRCULATIONAHA.107.185649.
- Pinho J, Küppers C, Nikoubashman O, Wiesmann M, Schulz JB, Reich A, et al. Frailty is an outcome predictor in patients with acute ischemic stroke receiving endovascular treatment. Age Ageing. 2021;50(5):1785–1791. doi:10.1093/ageing/afab092.
- Schnieder M, Bähr M, Kirsch M, Maier I, Behme D, Riedel CH, et al. Analysis of frailty in geriatric patients as a prognostic factor in endovascular treated patients with large vessel occlusion strokes. J Clin Med. 2021;10(10):2171. doi:10.3390/jcm10102171.
- Joyce N, Atkinson T, McGuire K, Wiggam MI, Gordon PL, Kerr EL, et al. Frailty and stroke thrombectomy outcomes—an observational cohort study. Age Ageing. 2022;51(2):51. doi:10.1093/ageing/afab260.
- Tan BYQ, JSY H, Leow AS, Chia MLJ, Sia CH, Koh YY, et al. Effect of frailty on outcomes of endovascular treatment for acute ischaemic stroke in older patients. Age Ageing. 2022;51(4):51. doi:10.1093/ageing/afac096.
- Fried LP, Watson J Frailty and failure to thrive. In: Blass JP EWJ, et al. ed. Hazzard’s Principles of Geriatric Medicine and Gerontology. New York: McGraw-Hill; 1998:pp. 1387–1402
- Mori E, Aoyagi Y, Kono Y, Asai H, Tomita H, Izawa H. Exploring the factors associated with decreased dynamic balance ability in older patients with heart failure. Heart & Lung. 2023;58:139–143. doi:10.1016/j.hrtlng.2022.11.016.
- Suda S, Nishimura T, Ishiwata A, Muraga K, Aoki J, Kanamaru T, et al. Early cognitive impairment after minor stroke: associated factors and functional outcome. J Stroke Cerebrovasc Dis. 2020;29(5):104749. doi:10.1016/j.jstrokecerebrovasdis.2020.104749.
- Kanamaru T, Suda S, Muraga K, Ishiwata A, Aoki J, Suzuki K, et al. Pre-stroke cognitive impairment in acute ischemic stroke patients predicts poor functional outcome after mechanical thrombectomy. Neurol Sci. 2021;42(11):4629–4635. doi:10.1007/s10072-021-05158-6.
- Kimura K, Kazui S, Minematsu K, Yamaguchi T. Japan multicenter stroke investigators C. Hospital-based prospective registration of acute ischemic stroke and transient ischemic attack in Japan. J Stroke Cerebrovasc Dis. 2004;13:1–11. doi:10.1016/j.jstrokecerebrovasdis.2003.11.025.