Abstract
Objective: To assess incidence of urinary tract infection (UTI) among patients with recent spinal cord injury (SCI) who initiated intermittent catheterization (IC).
Design: Retrospective chart review.
Setting: Two European SCI rehabilitation centers.
Participants: Seventy-three consecutive patients with recent SCI who initiated IC.
Outcome measures: Incidence of UTI, using six different definitions, each based on microbiology ± symptomatology ± mention of UTI . Rates were expressed in terms of numbers of UTIs per 100 patient-months (PMs). Attention was focused on first-noted UTI during the three-month follow-up, as assessed with each of the six definitions.
Results: Fifty-eight percent of patients (n = 33) met ≥1 definitions for UTI during follow-up (rate: 31.5 UTIs per 100 PMs), ranging from 14% (5.3 per 100 PMs; definition requiring bacteriuria, pyuria, and presence of symptoms) to 45% (22.7 per 100 PMs; definition requiring “mention of UTI”). Ten cases were identified using the definition that required bacteriuria, pyuria, and symptoms, whereas definitions that required bacteriuria and either pyuria or symptoms resulted in the identification of 20–25 cases. Median time to UTI ranged from 42 days (“mention of UTI”) to 81 days (definition requiring bacteriuria and ≥100 leukocytes/mm3).
Conclusion: Depending on definition, 14% to 45% of patients with recent SCI experience UTI within three months of initiating IC. Definitions requiring bacteriuria and either pyuria or symptoms consistently identified about twice as many cases as those that required all three conditions. Standardizing definitions may help improve detection, treatment, and prevention of UTI within this vulnerable population.
Introduction
Spinal cord injury (SCI) is a relatively rare and life-changing event often attributed to trauma (e.g. car accidents, falls, violence), vertebral fracture(s) secondary to other conditions (e.g. osteoporosis, metastatic cancer), infection, or neurodegenerative diseases.Citation1 SCI may negatively impact the ability to eat, dress, wash, stand, and/or walk; depending on severity, bodily functions may be affected, including ability to breathe, digest, and/or evacuate waste. SCI also may limit participation in social and daily activities.Citation2,Citation3
About 80% of patients experience bladder dysfunction.Citation4 For most such patients, intermittent catheterization (IC) is the preferred method for bladder management as it is less likely to damage the relevant area,Citation5 reduces risk of urinary tract infection (UTI),Citation6–9 and helps maintain independence (all vs. indwelling catheter, reflex voiding, or Credé/Valsalva). While integral to bladder management, catheters are not without risk, with almost 20% of SCI patients reporting ≥1 UTIs annually.Citation10 This risk of UTI, coupled with other catheter-related complications (e.g. bladder distention, urinary incontinence)Citation11 collectively contribute to high levels of morbidity and mortality in this population.Citation7
Several definitions are used to identify UTI among SCI patients, each based on microbiological and/or laboratory testing, symptomatology, or some combination thereof.Citation12,Citation13 Consequently, estimates of UTI incidence vary. The lack of a standardized definition compromises the ability to assess studies in this area, and has implications for patients, providers, and the healthcare system, as coverage and reimbursement policies may limit care, possibly including access to certain types of catheters, to those who meet a particular definition for UTI. The ability to accurately identify UTI is important to help optimize care and minimize morbidity and mortality among SCI patients. Accordingly, we examined the impact of different definitions on incidence of UTI among SCI patients who initiated IC.
Methods
Study design
Retrospective chart review, based at two European SCI centers: Neurological Rehabilitation Center Godeshoehe.e.V. (Bonn, Germany) and Sint Maartenskliniek (Ubbergen, The Netherlands). All participants provided written informed consent in accordance with the Declaration of Helsinki. The study was approved by the regional medical ethics committee of Arnhem-Nijmegen (2017-3752) and internal review board of the Sint Maartenskliniek (The Netherlands); and by the University Clinic of Bonn (EK 012-16; Germany).
Sample selection
Seventy-three consecutive patients, who had recently experienced SCI and subsequently initiated IC (50 from the German center; 23 from the Dutch center), were identified. For each selected patient, date of IC initiation was deemed the “index date”, and all relevant information for the three-month period including and following this date (“follow-up”) was abstracted. Time between date of SCI occurrence and index date was deemed the “baseline period” ().
Figure 1 Sample selection criteria. Abbreviations: AIS, American Spinal Injury Association Impairment Scale; IC, intermittent catheterization; SCI, spinal cord injury; UTI, urinary tract infection.
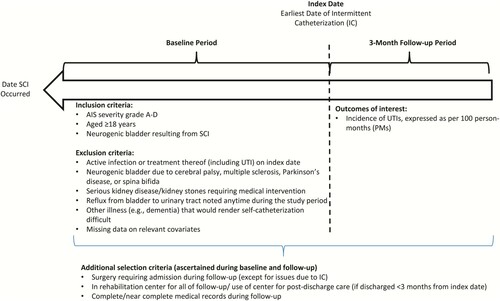
Patients were selected who met all of the following criteria: (a) SCI grade A to D per American Spinal Injury Association (ASIA) Impairment Scale (AIS); (b) neurogenic dysfunction of the lower urinary tract resulting from SCI; (c) evidence of IC use; (d) aged ≥18 years on index date; and (e) stay of ≥3 months at the rehabilitation center where IC was initiated or use of the rehabilitation center for outpatient treatment after discharge (for those with initial stay <3 months).
Patients with evidence of any of the following were excluded: (a) active infection (including but not limited to UTI) or treatment thereof on index date; (b) neurogenic dysfunction of lower urinary tract resulting from cerebral palsy, multiple sclerosis, Parkinson’s disease, or spina bifida; (c) serious kidney disease (defined glomerular filtration rate <40 ml/min/ 1.73 m2) anytime during the study period; (d) kidney stones during the study period for which medical intervention was required; (e) reflux from bladder to urinary tract noted anytime during the study period; (f) other illnesses (e.g. dementia, cognitive function disorder) that would, in the investigator’s opinion, render it difficult to adhere to a self-catheterization schedule or an inability/unwillingness to self-catheterize; (g) ≥1 surgical procedures that required hospitalization during follow-up (excluding procedures due to IC); (h) missing data during follow-up (e.g. transferred to another facility, follow-up at locations other than the study site).
Study measures/data abstraction
Demographic and clinical characteristics
Demographic variables were ascertained using information available during the baseline period. Cause of SCI (accident-related [defined as injury due to car, bike or motorbike accidents, falls, sports, or violence/assault] vs. other causes), location (C1-C4, C5-C8, T1-T12, L1-L5, and S1-S5), and grade (A/B or C/D according to the AIS impairment scale) were ascertained during the baseline period, as was the prevalence of selected comorbidities, smoking status, alcohol use, medication use, and method(s) of voiding (indwelling catheter, IC performed by a family member or caregiver [IFK], or other methods) used between SCI occurrence and index date.
UTIs
A targeted literature review identified five definitions for UTI, including two developed by institutes (i.e. National Institute on Disability and Rehabilitation Research [“NIDRR”]),Citation14 European Association of Urology [“EAU”]Citation15; one developed by a government payer (United States [US] Centers for Medicare and Medicaid Services [“CMS”])Citation16; and two developed by independent researchers (Togan et al.; “Togan”),Citation10 (Burgdörfer et al.; “Burgdörfer”).Citation17 Each of these definitions required bacteriuria, albeit with different “threshold” values. In addition to bacteriuria, the NIDRR definition also required pyuria and the presence of ≥1 relevant symptoms (e.g. cloudy or foul-smelling urine, increased perspiration, lethargy) (all three criteria were required). The other identified definitions required bacteremia plus one additional criterion to establish UTI. Togan required the presence of ≥1 relevant symptoms; both Burgdörfer and EAU required the presence of pyuria; and CMS required the presence of either pyuria or a relevant symptom (). We also developed a sixth definition for UTI, which was mention in the chart of UTI or a related term (this was the only criterion; “ad hoc definition”).
Table 1 Definitions of UTI.
Microbiology and antibiotic use
We examined bacterial etiology of UTIs, using information from cultures drawn between the date UTI was established and the subsequent seven days; the prevalence of specific pathogens was assessed, including but not limited to the “ESKAPE” pathogens (i.e. Enterococcus spp., Staphylococcus spp., Klebsiella spp., Acinetobacter spp., Pseudomonas spp., Enterobacter spp.), which are particularly difficult to treat.Citation18 We classified UTIs as monomicrobial (single pathogen identified) or polymicrobial (≥2 pathogens identified).
Specific antibiotics used to treat UTI were abstracted from patients’ medical records, based on all relevant therapies noted over the same eight-day period used to ascertain etiology.
Statistical analysis
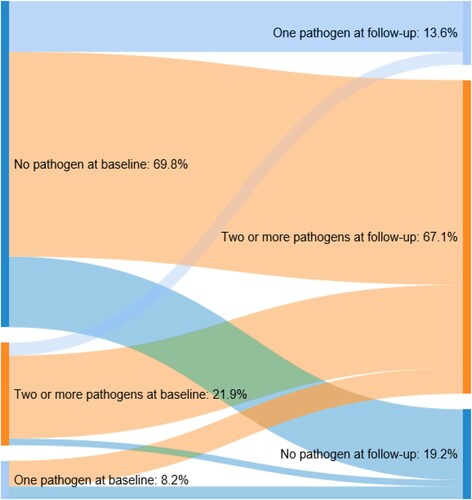
Demographic and clinical characteristics were summarized using descriptive statistics (e.g. frequency and percentages for categorical measures; means [standard deviations], medians [interquartile ranges] for continuous measures). Microbiologic data and antibiotics used to treat UTIs were also summarized descriptively. Sankey diagramsCitation19 were developed to illustrate the prevalence of pathogens (i.e. colonization, asymptomatic bacteriuria, infection) during baseline and follow-up.
Incidence rates for UTIs during follow-up were assessed for each definition described above and expressed as events per 100 patient-months (PMs). Rates were calculated by dividing total number of patients with UTI during follow-up by the corresponding person-time at risk (in months), and multiplying the resulting quotient by 100. Kaplan-–Meier methods were used to examine time to UTI. Attention was focused on the first UTI observed during follow-up.
All data were analyzed using SAS version 9.4.
Results
Demographic and clinical characteristics
Seventy-three patients met selection criteria between April 18, 2016 and May 9, 2018, for Germany (n = 50), and between December 28, 2017 and May 9, 2019, for the Netherlands (n = 23) (time difference due to delays in identifying and enrolling the Dutch site). Most (76.7%) were men; 64% were aged <60 years (). Accidents caused SCI for approximately two-thirds of patients. Forty-nine percent of subjects had SCI at the thoracic nerves (T1 to T12); 64% were AIS grade of A or B.
Table 2 Baseline demographic and clinical characteristics.
Thirty percent were current or former smokers and 28.8% currently or had previously used alcohol. Approximately 41% had a history of urologic disease prior to the initiation of IC, 26% had a history of asymptomatic bacteriuria, and 26% had experienced UTI. Most (86.3%) used indwelling catheters prior to index date; 31.5% had used IC, albeit performed by others. Thirty percent had evidence of colonization (i.e. either a positive bacterial culture without signs or symptoms of infection, or localized UTI) during baseline, most commonly with K. pneumoniae (13.7% of patients), Escherichia coli (10.9% of patients), or Enterococcus sp (9.6% of patients).
Microbiologic data
While 69.8% had no evidence of pathogens during baseline, most (67.1%) had evidence of multiple pathogens during follow-up (). Of those with pathogens during baseline, most had evidence of multiple pathogens during follow-up.
Incidence of UTIs during follow-up
Forty-two patients (57.5%) met criteria for UTI within three months of initiating IC, ranging from 10 (13.7%; the NIDRR definition requiring evidence of bacteriuria, pyuria, and ≥1 symptoms) to 33 (45.2%; ad hoc definition requiring “mention” of UTI; ). The corresponding rate was 31.5 UTIs per 100 PMs, ranging from 5.3 UTIs per 100 PMs (NIDRR definition) to 22.7 per 100 PMs (ad hoc definition). About one-half of patients who developed UTI did so within 41 days of initiating IC ().
Figure 3 Incidence of UTI, by definition. Abbreviations: CMS, Centers for Medicare and Medicaid Services; EAU, European Association of Urology; NIDRR, National Institute on Disability and Rehabilitation Research; US, United States; UTI, urinary tract infection.

Table 3 Incidence of UTI during the three-month follow-up period.
Twenty percent (2 out of 10) of cases identified via NIDRR did not have mention of the term UTI and therefore did not qualify for the ad hoc definition (). All patients identified with the NIDRR definition were also identified with the EAU, CMS, Togan, and Burgdörfer definitions, all of which required bacteriuria and either pyuria or symptoms (NIDRR required both). There was a high degree of agreement between the CMS, Togan, EAU, and Burgdörfer definitions, with the CMS definition identifying the most cases (n = 25), likely due to its requirement of either pyuria or symptoms. Togan, which required symptoms, yielded fewer cases than EAU or Burgdörfer, both of which required pyuria (20 vs. 24 and 21, respectively). All cases identified with Togan, EAU, and Burgdörfer also were identified with CMS. Of the 33 patients identified with our ad hoc definition, 54.5% (n = 18) had bacteriuria and either pyuria or ≥1 symptoms (i.e. met the CMS definition); 24.2% (n = 8) had bacteriuria, pyuria, and ≥1 symptoms (i.e. met the NIDRR definition).
Table 4 Concordance between definitions of UTI.
Infection etiology
Causal pathogens were identified among 97.6% (n = 42) of patients with UTI during follow-up (any definition), most commonly K. pneumoniae (identified in 50.0% of UTIs), E. coli (47.6%), Enterococcus sp (16.7%), and Pseudomonas sp (14.3%) (). About 73.8% of patients with UTI had ≥1 ESKAPE pathogens identified. Most UTIs for which pathogen data were available were polymicrobial (81.0%); 16.7% were monomicrobial.
Table 5 Causal pathogens associated with UTI during follow-up.
Antibiotics used to treat UTIs during follow-up
During follow-up, 90.5% of patients with evidence of UTI received antibiotics, most commonly, ciprofloxacin (54.8%), nitrofurantoin (33.3%), penicillin and its combinations (21.4%), and cephalosporins (19.1%) (). About 12% of patients received carbapenems, and 2.4% received clindamycin. Fifty-seven percent of those treated with antibiotics received polypharmacy; 33.3% received monotherapy.
Table 6 Distribution of antibiotics used to treat UTI during follow-up.
The use of antibiotics varied by definition of UTI employed, ranging from 81.0% of patients, who met the Burgdörfer criteria, to 97.0% of patients who met our ad hoc definition. Ninety-five percent of patients who met Togan criteria received antibiotics, as did 90.0%, 84.0%, and 83.3% of patients who met NIDRR, CMS, and EAU criteria, respectively. Sixty-five percent of patients, who met Togan criteria, received polypharmacy. Patients, who met NIDRR criteria, were nominally most likely to have received carbapenems (20.0% of all such patients received this therapy), colistin (10%), and/or penicillins and combinations thereof (40%). Patients, who met Togan criteria, were nominally most likely to have received ciprofloxacin (70%), cephalosporins (30%), and/or nitrofurantoin (40%).
Discussion
It has been said that “a man with a watch knows what time it is, while one with two watches is never sure”.Citation20 This is applicable to our study, in which we found estimates of UTI within three months of initiation of IC highly dependent on definition used. Estimates ranged from 13.7% (the relatively specific NIDRR definition that required the presence of bacteriuria, symptomatology, and pyuria) to 45.2% (relatively sensitive ad hoc definition that required only mention of UTI or related term[s]). Definitions that included bacteriuria and either pyuria or ≥1 symptom (but not both) performed fairly consistently, with UTI estimates ranging from 27.4% (Togan) to 32.9% (EAU); it was 34.2% with the CMS definition, which explicitly includes bacteriuria and either pyuria or symptoms. We note that even use of the most restrictive NIDRR definition resulted in approximately one in seven patients with evidence of UTI within three months of IC initiation, suggesting that this is an important concern in this population.
Lack of consensus renders the diagnosis of UTI challenging among SCI patients who opt to initiate IC.Citation21 As noted above, incidence of UTI varied substantially based on the definition used, with an approximately three-fold difference between definitions of greatest specificity (NIDRR) and sensitivity (ad hoc). Relative to the NIDRR definition, requiring either pyuria or symptoms, approximately doubled estimates; conversely, about one-third of patients identified by our ad hoc definition did not meet the more stringent criteria of the other five definitions, yet still presumably had characteristics that caused their providers to suspect UTI. Interestingly, antibiotic use was nominally greatest among patients identified with our ad hoc definition that did not require microbiology, pyuria, or symptomatology. While estimates of UTI incidence were fairly consistent among the four definitions that required bacteriuria and either pyuria or symptomatology (range: 27.4% [Togan] to 34.2% [CMS]), antibiotic use was more varied (range: 81.0% [Burgdörfer] to 95.0% [Togan]). The relatively wide range of infection estimates resulting from the use of the six different definitions and discrepancies in antibiotic use is worrisome, as it suggests the true magnitude of UTI in this population is difficult to estimate with certainty.
UTI definitions that required bacteriuria and either pyuria or symptomatology (but not both) performed relatively consistently, “encompassed” all cases identified with the more specific NIDRR definition, and also captured about two-thirds of patients identified with the ad hoc definition. Definitions requiring pyuria resulted in nominally more cases than those requiring symptoms, whereas the CMS definition, which required either symptoms or pyuria, resulted in the most cases. Differences between EAU and Burgdörfer are likely attributable to differences in how bacteriuria (including among asymptomatic patients in the case of the EAU) and pyuria are defined. Moreover, SCI patients may not be able to report UTI-related symptoms due to impaired sensations in their lower urinary tract.Citation22 Conversely, the specific NIDRR definition may be a marker for more complicated/harder to treat infection, based on relatively high levels of use of carbapenems and colistin.
Pathogen distributions, rates of UTI, and antimicrobial resistance vary by geographical location and medical facilityCitation23 (they varied by center in our study [data not shown]).Citation24 However, our findings indicate that irrespective of the definition used, SCI patients are particularly vulnerable to UTI, which may delay rehabilitation, increase morbidity, decrease overall health, and negatively impact quality of life. As UTI is the second-leading cause of death in SCI patients,Citation25 our findings reinforce the critical need to reduce the risk and rate of UTIs in this patient population. We believe a critical initial step is to “harmonize” these competing definitions, which, in turn, should help lead to a better understanding of the true incidence of UTI following initiation of IC. One possible approach may be to use the CMS definition, which mandates the presence of bacteriuria and either pyuria or ≥1 relevant symptoms; while this would likely be sufficient to identify UTI with fairly high confidence, research needs to be conducted to determine the validity of this approach.
Given the impact of SCI on the bladder and its management thereof, UTIs in this patient population tend to be classified as complicated,Citation26,Citation27 and the associated pathogen distribution tends to differ from that of uncomplicated infection. The most commonly implicated pathogens in our study were Klebsiella spp and E. coli (each identified in about one-half of patients), followed by Enterococcus spp and Pseudomonas spp (each identified in 14% to 17%), which is consistent with prior studies conducted in Germany and the Netherlands.Citation28,Citation29 For example, one estimate implicated E. coli in about 35% of UTIs experienced among patients who undergo IC; conversely, K. pneumoniae, P. aeruginosa, and P. mirabilis were identified among 26%, 23%, and 16%, respectively.Citation26 In another study of 145 SCI patients treated at a single rehabilitation center, the most commonly identified isolates among the subgroup of patients who used clean IC (n = 61) were Providencia stuartii (19%), P. mirabilis (16%), E. coli (13%), P.aeruginosa (12%), and K. pneumoniae (8%).Citation30 These data are consistent with guidelines and reviews that note commonly implicated pathogens among patients who undergo IC include E. coli, Proteus spp, Enterococcus spp, Citrobacter spp, Pseudomonas spp, Klebsiella spp, Staphylococcus aureus, Serratia spp, P. stuartii, Acinetobacter spp., S. saprophyticus, and S. faecalis.Citation30–33 Deeper knowledge of likely causal pathogens in this patient population should improve the choice of empiric antimicrobial therapy and ultimately successful and timely UTI resolution. Importantly, our study focused on the time spent in rehabilitation centers, and 86% of patients used indwelling catheters during the baseline period, which may have an increased risk of colonization prior to IC initiation. Risk of bacteriuria is also known to increase with duration of indwelling catheterization.Citation34 It is reasonable to assume that observed pathogen distribution is what is expected from healthcare-associated, as opposed to community-acquired, infection. Further research is needed to better understand the underlying etiology of UTIs in this patient population, accounting for place of residence at the time of infection identification and other possible risk factors (e.g. colonization).
About 73.8% of the patients in our study had ≥1 ESKAPE pathogens, which are particularly difficult to treat and collectively represent a major risk for complicated infections and suboptimal outcomes. Similarly, our finding that approximately eight in 10 UTIs were polymicrobial, coupled with relatively high levels of use of carbapenems (12% of patients received such therapy) and other “salvage” antibiotics, such as colistin, indicate that UTIs experienced by SCI patients are likely complicated and require relatively intensive antibiotic treatment to cover typical and atypical pathogens, and those resistant to commonly used antibiotics. Our findings suggest that UTIs that occur within three months of initiating IC are fairly complex, and of diverse presentation and underlying etiology. Infection prevention also is an important concern, given that the causes of UTI are heterogenous and include physiologic and environmental exposures.Citation35
While it is difficult to directly compare our findings with other estimates due to the use of multiple methodologies and/or UTI definitions,Citation36 they appear consistent with those reported previously, including those of a recent literature review that reported 10% to 68% of SCI patients experience UTI.Citation37 UTI rates from these prior studies range from 12.3 per 100 PMs (originally reported as 0.41 cases per 100 person-days) to 81.0 per 100 PMs (2.7 cases per 100 person-days).Citation38,Citation39
Our study has limitations that merit discussion. First, analyses were limited to information recorded in patients’ charts and subject to variance in quantity and quality of information recorded, with unknowable impacts on our findings. Second, our study represents a convenience sample. Given the relatively small number of SCI patients who met all selection criteria, the generalizability of our findings to other centers in Germany and/or the Netherlands – or in general, for that matter – is unknown. Third, our focus was limited to UTI; however, these patients also are at risk for other catheter-related complications. Accordingly, our study likely underestimates the overall risk of unwanted outcomes among SCI patients who have recently initiated IC. Fourth, we used a relatively short (three months) follow-up period. While our data suggest that the time immediately following initiation of IC is one of relatively high risk for UTI – at least in rehabilitation centers – further work is needed to better understand how different settings, longer “at risk” periods, heightened surveillance, and/or increased patient training may impact these risks. Fifth, all patients were required to contribute three months of follow-up to the extent that patients with stays <3 months in rehabilitation facilities (regardless of reason) differed from those with longer stays, our findings may suffer from immortal time bias. While the impact of this bias is unknowable, this along with the relatively small sample size suggests our findings be interpreted cautiously. Finally, due to data limitations, we could not establish upper vs. lower UTI for all patients in each of the two study centers. Further research is needed to explore the ability of various definitions to accurately and comprehensively identify infection site.
Conclusion
More than one-half of patients, requiring assistance with urine voiding due to SCI, have evidence of UTI within three months of initiating IC. UTI estimates depend greatly on the definition used, ranging from 13.7% (definition requiring bacteriuria, symptomatology, and pyuria) to 45.2% (definition requiring mention of UTI). Definitions that incorporate microbiology and either pyuria or symptomatology perform fairly consistently in estimating UTI incidence, but vary more broadly with respect to the evidence of antibiotic use. Regardless of definition, UTIs experienced by SCI patients are primarily polymicrobial in etiology. Criteria to identify UTI in SCI should be standardized, thereby facilitating improvements in their prevention, detection, and treatment among this vulnerable population.
Disclaimer statements
Contributors None.
Funding This study was funded by Hollister Incorporated (Libertyville, IL, United States), a healthcare company that sponsored this research, and conducted by Evidera (Bethesda, MD, United States), a health economics consultancy with current and ongoing engagements with Hollister Incorporated and other pharmaceutical, biotech, and device companies.
Conflicts of interest Evidera, an HEOR consultancy, was hired to run the study and prepare the manuscript. Authors from Germany and the Netherlands serve as consultants on this and other projects for Hollister Incorporated.
References
- Spinal cord injury facts and figures at a glance. J Spinal Cord Med 2012;35(4):197–8. doi: https://doi.org/10.1179/1079026812Z.00000000063
- Piatt JA, Nagata S, Zahl M, Li J, Rosenbluth JP. Problematic secondary health conditions among adults with spinal cord injury and its impact on social participation and daily life. J Spinal Cord Med 2016;39(6):693–8. doi: https://doi.org/10.1080/10790268.2015.1123845
- Ullrich PM, Spungen AM, Atkinson D, Bombardier CH, Chen Y, Erosa NA, et al. Activity and participation after spinal cord injury: state-of-the-art report. J Rehabil Res Dev 2012;49(1):155–74. doi: https://doi.org/10.1682/JRRD.2010.06.0108
- Taweel WA, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol 2015;7:85–99.
- Consortium for spinal cord M. bladder management for adults with spinal cord injury: a clinical practice guideline for health-care providers. J Spinal Cord Med 2006;29(5):527–73.
- De Ridder DJ, Everaert K, Fernandez LG, Valero JV, Duran AB, Abrisqueta ML, et al. Intermittent catheterisation with hydrophilic-coated catheters (SpeediCath) reduces the risk of clinical urinary tract infection in spinal cord injured patients: a prospective randomised parallel comparative trial. Eur Urol 2005;48(6):991–5. doi: https://doi.org/10.1016/j.eururo.2005.07.018
- Edokpolo LU, Stavris KB, Foster HJ. Intermittent catheterization and recurrent urinary tract infection in spinal cord injury. Top Spinal Cord Inj Rehabil 2012;18(2):187–92. doi: https://doi.org/10.1310/sci1802-187
- Herter R, Kazer MW. Best practices in urinary catheter care. Home Healthc Nurse 2010;28(6):342–9. ; quiz 9-51. doi: https://doi.org/10.1097/NHH.0b013e3181df5d79
- Li L, Ye W, Ruan H, Yang B, Zhang S, Li L. Impact of hydrophilic catheters on urinary tract infections in people with spinal cord injury: systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil 2013;94(4):782–7. doi: https://doi.org/10.1016/j.apmr.2012.11.010
- Togan T, Azap OK, Durukan E, Arslan H. The prevalence, etiologic agents and risk factors for urinary tract infection among spinal cord injury patients. Jundishapur J Microbiol 2014;7(1):e8905. doi: https://doi.org/10.5812/jjm.8905
- Singh R, Rohilla RK, Sangwan K, Siwach R, Magu NK, Sangwan SS. Bladder management methods and urological complications in spinal cord injury patients. Indian J Orthop 2011;45(2):141–7. doi: https://doi.org/10.4103/0019-5413.77134
- Wyndaele JJ, Brauner A, Geerlings SE, Bela K, Peter T, Bjerklund-Johanson TE. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Int 2012;110(11 Pt C):E910–E917. doi: https://doi.org/10.1111/j.1464-410X.2012.11549.x
- Nicolle LE. Urinary tract infections in patients with spinal injuries. Curr Infect Dis Rep 2014;16(1):390. doi: https://doi.org/10.1007/s11908-013-0390-9
- The prevention and management of urinary tract infections among people with spinal cord injuries. National Institute on Disability and rehabilitation Research consensus statement. January 27-29, 1992. SCI Nurs 1993;10(2):49–61.
- Grabe M, Bartoletti R, Bjerklund Johansen TE, Cai T, Cek M, Koves B, et al. Guidelines on Urological Infections 2015 [Available from: https://uroweb.org/wp-content/uploads/19-Urological-infections_LR2.pdf.
- Centers for Medicare and Medicaid Services. Program Memorandum Intermediaries/Carriers 2001. Available from: https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/Downloads/AB01170.pdf.
- Pannek J. Treatment of urinary tract infection in persons with spinal cord injury: guidelines, evidence, and clinical practice. A questionnaire-based survey and review of the literature. J Spinal Cord Med 2011;34(1):11–5. doi: https://doi.org/10.1179/107902610X12886261091839
- Karlowsky JA, Hoban DJ, Hackel MA, Lob SH, Sahm DF. Resistance among Gram-negative ESKAPE pathogens isolated from hospitalized patients with intra-abdominal and urinary tract infections in Latin American countries: SMART 2013-2015. Braz J Infect Dis 2017;21(3):343–8. doi: https://doi.org/10.1016/j.bjid.2017.03.006
- Huang CW, Syed-Abdul S, Jian WS, Iqbal U, Nguyen PA, Lee P, et al. A novel tool for visualizing chronic kidney disease associated polymorbidity: a 13-year cohort study in Taiwan. J Am Med Inform Assoc 2015;22(2):290–8. doi: https://doi.org/10.1093/jamia/ocu044
- Bloch A. Murphy’s Law. New York Perigee; 2003.
- American Urological Association. AUA White paper on catheter-associated urinary tract infections: definitions and significance in the urologic patient 2014. Available from https://www.suna.org/resources/cautiWhitePaper.pdf.
- Kennelly M, Thiruchelvam N, Averbeck MA, Konstatinidis C, Chartier-Kastler E, Trojgaard P, et al. Adult neurogenic lower urinary tract dysfunction and intermittent Catheterisation in a community setting: risk factors model for urinary tract infections. Adv Urol 2019;2019:2757862. doi: https://doi.org/10.1155/2019/2757862
- Khan HA, Baig FK, Mehboob R. Nosocomial infections: epidemiology, prevention, control and surveillance. Asian Pac J Trop Biomed 2017;7(5):478–482. doi: https://doi.org/10.1016/j.apjtb.2017.01.019
- Tandogdu Z, Wagenlehner FM. Global epidemiology of urinary tract infections. Curr Opin Infect Dis 2016;29(1):73–9. doi: https://doi.org/10.1097/QCO.0000000000000228
- Siroky MB. Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med 2002;113(Suppl 1A):67S–79S. doi: https://doi.org/10.1016/S0002-9343(02)01061-6
- Nicolle LE. Committee* ACG. complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol 2005;16(6):349–60. doi: https://doi.org/10.1155/2005/385768
- Yamamoto S. Prevention and treatment of complicated urinary tract infection. Urological Science 2016;38:186–189. doi: https://doi.org/10.1016/j.urols.2016.07.001
- Bischoff S, Walter T, Gerigk M, Ebert M, Vogelmann R. Empiric antibiotic therapy in urinary tract infection in patients with risk factors for antibiotic resistance in a German emergency department. BMC Infect Dis 2018;18(1):56. doi: https://doi.org/10.1186/s12879-018-2960-9
- Koningstein M, van der Bij AK, de Kraker ME, Monen JC, Muilwijk J, de Greeff SC, et al. Recommendations for the empirical treatment of complicated urinary tract infections using surveillance data on antimicrobial resistance in the Netherlands. PLoS One 2014;9(1):e86634. doi: https://doi.org/10.1371/journal.pone.0086634
- Dedeic-Ljubovic A, Hukic M. Catheter-related urinary tract infection in patients suffering from spinal cord injuries. Bosn J Basic Med Sci 2009;9(1):2–9. doi: https://doi.org/10.17305/bjbms.2009.2849
- Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015;13(5):269–84. doi: https://doi.org/10.1038/nrmicro3432
- Garcia Leoni ME, Esclarin De Ruz A. Management of urinary tract infection in patients with spinal cord injuries. Clin Microbiol Infect 2003;9(8):780–5. doi: https://doi.org/10.1046/j.1469-0691.2003.00643.x
- Biardeau X, Corcos J. Intermittent catheterization in neurologic patients: Update on genitourinary tract infection and urethral trauma. Ann Phys Rehabil Med 2016;59(2):125–9. doi: https://doi.org/10.1016/j.rehab.2016.02.006
- Salameh A, Mohajer MA, Daroucihe RO. Prevention of urinary tract infections in patients with spinal cord injury. CMAJ 2015;187(11):807–11. doi: https://doi.org/10.1503/cmaj.141044
- Schaeffer AJ. What do we know about the urinary tract infection-prone individual? J Infect Dis 2001;183(Suppl 1):S66–S69. doi: https://doi.org/10.1086/318837
- Wyndaele JJ. Complications of intermittent catheterization: their prevention and treatment. Spinal Cord 2002;40(10):536–41. doi: https://doi.org/10.1038/sj.sc.3101348
- Garcia-Arguello LY, O’Horo JC, Farrell A, Blakney R, Sohail MR, Evans CT, et al. Infections in the spinal cord-injured population: a systematic review. Spinal Cord 2017;55(6):526–34. doi: https://doi.org/10.1038/sc.2016.173
- Darouiche RO, Al Mohajer M, Siddiq DM, Minard CG. Short versus long course of antibiotics for catheter-associated urinary tract infections in patients with spinal cord injury: a randomized controlled noninferiority trial. Arch Phys Med Rehabil 2014;95(2):290–6. doi: https://doi.org/10.1016/j.apmr.2013.09.003
- Esclarin De Ruz A, Garcia Leoni E, Herruzo Cabrera R. Epidemiology and risk factors for urinary tract infection in patients with spinal cord injury. J Urol 2000;164(4):1285–9. doi: https://doi.org/10.1016/S0022-5347(05)67157-1