Abstract
Context/Objective
Functional electrical stimulation (FES) is commonly used in rehabilitation to generate electrically-induced muscle contractions. FES has been shown to assist in the recovery of voluntary motor functions after stroke or spinal cord injury. However, discomfort associated with FES can motivate patients to withdraw their participation from FES therapy despite its benefits. To address this issue, a functional electrical stimulator, called MyndMove™ (MyndTec Inc., Canada), has been developed to generate more comfortable contractions than conventional stimulators.
Design
Cross-sectional, interventional, with two treatment arms.
Setting
A laboratory within a rehabilitation center.
Participants
Twelve able-bodied participants.
Intervention
FES delivered with two different stimulators, MyndMove™ and Compex Motion (Compex, Switzerland), during muscle contractions of high, moderate and low stimulation intensity.
Outcome Measures
Comfort-related preference to a given stimulator and the discomfort score rated through a Numeric Rating Scale (NRS-101) for both stimulators.
Results
Participants perceived a reduction in discomfort during high-intensity stimulation generated using MyndMove™. In addition, MyndMove™ stimulations were preferred in 60% of all contractions. The reduction in discomfort associated with MyndMove™ might be due the fact that MyndMove™ delivers less charge to generate contractions of equivalent intensity, compared to Compex Motion.
Conclusion
Reducing discomfort during FES may help in generating stronger and more clinically useful contractions, increasing accessibility of FES therapy to include individuals with low tolerance to FES.
Introduction
Functional electrical stimulation (FES) is a technique that uses trains of controlled electrical pulses to artificially excite nerves or muscles.Citation1 Delivering FES to selected muscles in a carefully coordinated manner can generate functional movement, such as grasping,Citation2 walking,Citation3 maintain sittingCitation4 and standing balance,Citation5 among others. As such, the potential of FES to improve motor functions in patients with paralysis due to a neurological injury has been amply documented. Conventionally, FES is used when lower motor neurons are intact and partial innervation remains in the spinal cord, as is the case with stroke, incomplete spinal cord injury,Citation6 or complete spinal cord injury in levels above the cauda equina, approximately L1. Although, FES has also been implemented to maintain denervated muscle mass in patients with lower motor neuron lesions (e.g. cauda equina syndrome).Citation7 In rehabilitation settings, FES has been used to assist patients in movements required for daily activities as a long-term assistive device,Citation8–10 as well as a tool to enhance conventional physical or occupational therapy. In the latter, the patients undergo short-term FES therapy (FEST), in which they relearn to perform the desired movements with the help of FES, and over time, they recover a degree of voluntary control. For example, in upper limb rehabilitation, FEST has resulted in some of the largest improvements in arm and hand function reported to date in individuals with stroke and incomplete spinal cord injury, even in cases when patients had severe impairment.Citation11–14
However, electrical pulses used to artificially contract muscles using FES can produce discomfort in some individuals. This is an important consideration for FEST because such discomfort can lead patients to withdraw from therapy,Citation15 despite concerted effort to optimizing various FES parameters (e.g. electrode size, pulse waveform, pulse duration, frequency of discharge and source regulation) to deliver effective stimulation as well as to minimize the discomfort.Citation16–24 To address the issue of discomfort during FEST, a functional electrical stimulator called MyndMove™Citation12,Citation25,Citation26 has been developed to deliver electrical pulses with a very short rise-time (i.e. 0.03 µs), followed by exponential decay, reaching low steady-state currents. Conversely, Compex Motion,Citation27 a stimulator that has been used extensively in our research group for the past 20 years, delivers rectangular electrical pulses with longer rise times (i.e. 3 µs). In clinical practice, MyndMove™ has been reported to generate less discomfort compared to existing stimulators (e.g. Compex Motion),Citation12 however, objective documentation does not exist.
In this paper, we compared MyndMove™ and Compex Motion in torque-matched conditions during isometric contractions of the biceps brachii muscle in able-bodied individuals. We documented two main aspects of the stimulation related to comfort across three intensities (i.e. low, moderate, and high): preference to a given stimulator, and the discomfort score rated through a Numeric Rating Scale (NRS-101). Reducing discomfort during FES may enable stronger, more clinically useful contractions, making the therapy more accessible for people with low tolerance to FES.
Materials and methods
Participants
Twelve (12) able-bodied participants were recruited for this study. Further details regarding the participants’ demographic information are provided in . The University Health Network Ethics Committee approved the protocols described below. Informed consent was obtained from each participant before undergoing experimentation.
Table 1 Summary of participants’ demographics data.
Experimental setup
Torque
Participants were seated in the chair of a Biodex™ dynamometer (System 3, Biodex Medical System, USA). To measure isometric torque during elbow flexion, the axis of the dynamometer was aligned with the axis of rotation of the participant’s elbow (). The participant’s position in the chair was stabilized with straps over the waist, trunk, and shoulder.
Figure 1 Experimental setup. Isometric contractions of the right biceps brachii muscle during delivery of FES were measured using a dynamometer. The axis of the dynamometer was aligned to the axis of rotation of the elbow joint. A switch was designed to change the stimulator source between MyndMove™ and Compex Motion delivered through the same leads, ensuring consistent electrode placement. Example current pulses for MyndMove™ and Compex Motion are shown in the output stage of the switch, highlighting the difference in shape and amplitude.
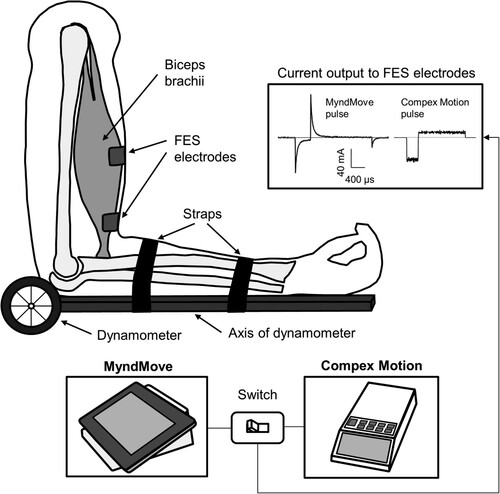
Functional electrical stimulation
Two FES systems were used: Compex Motion (Compex SA, Switzerland) and MyndMove™ (MyndTec Inc, Canada). Electrical pulses were delivered to the right biceps brachii muscle using cutaneous adhesive gel electrodes (5 by 5 cm; Axelgaard Manufacturing, Denmark). A custom-made circuit was designed to switch the stimulator source delivered through the same leads, ensuring electrode placement was consistent between the two stimulators. Both MyndMove™ and Compex Motion stimulators were programed to deliver current pulses with a biphasic asymmetric waveform, pulse duration of 250–400 μs (i.e. values associated with optimal stimulation)Citation22,Citation23 and pulse frequency of 40 Hz. To consider this range of pulse widths (i.e. 250–400 μs), both stimulators were programed to deliver current pulses with a duration of 250–300 μs for 5 participants and 400 μs for 7 participants, to allow for paired statistical comparisons.
Next, the biceps brachii motor points were identified as the location on the skin’s surface where an electrical pulse evoked a visible muscle twitch with the least electrical current. The location of the motor points was used for FES electrode placement. The active cathode was positioned over the proximal motor points of the short and long head of the biceps brachii. The return anode was positioned distally at the end of the muscle belly. The illustration of the experimental setup is shown in .
Data collection
Torque data was recorded at 1 kHz sampling rate using a data acquisition system, amplifier, and software package (LabChart, PowerLab, AD Instruments, USA) and stored on the computer for analysis. A custom-made circuit was designed to record the voltage and current outputs from the stimulators during the experiments, at 100 kHz sample rate. Additionally, to capture the rise time of individual electrical pulses, we used a digital oscilloscope (DSO6012A, 100 MHz, 2 GSA/s, Agilent Technologies, USA), which recorded the voltage and current waveforms at 500 kHz, 2, and 100 MHz.
Experimental procedures
Throughout the study, the participants were blinded to the stimulator type. Experimenters switched the stimulator source using the switch box, which was placed out of the field of view of the participants. The following measurements were recorded during the study.
Maximal voluntary contraction
Before the trials involving electrical stimulation, each participant was asked to perform two maximal voluntary contractions (MVC) in biceps brachii during elbow flexion. Participants were able to monitor their torque production on a computer screen and they were verbally encouraged to apply their maximal effort during each trial. Each trial was two seconds long. The peaks of both recordings were compared, and the greater torque measurement was recorded as the MVC.
Maximally-tolerated contractions and maximally-tolerated torque
After the MVC was recorded, we determined the participants’ maximally-tolerated contractions (MTCs) for each stimulator type. First, the order in which the stimulator type was presented was randomized. Then, for each stimulator, two-second long FES pulse trains were delivered every 10 s with gradually increasing stimulation intensity (i.e. pulse amplitude). The minimum stimulation intensity necessary to produce detectable torque for each stimulator was recorded as the minimally-evoked torque (MET), which differed from the motor threshold (i.e. minimum intensity that causes a visible muscle contraction, even if it does not result in movement). Participants were instructed to inform the researcher should the stimulation intensity become intolerable. The peaks in torque measurements produced during the maximally-tolerated FES trains were recorded as MTCs for the respective stimulators. Finally, the MTCs were compared between the two stimulators and the lower torque was selected as the maximally-tolerated torque (MTT).
Stimulator preference and self-reported discomfort scores
After determining the MTCs for each stimulator and the MTT, we assessed preference and perceived discomfort for each stimulator. Each participant reported their perceived discomfort associated with the stimulation across three intensities using a Numeric Rating Scale (NRS-101). The NRS-101 scale has a range between 0 and 100, where 0 refers to no discomfort and 100 refers to the greatest discomfort (i.e. discomfort associated with the MTC in our study). The three current intensities were matched to torque targets expressed as percentages of the MTT:
Low intensity: 25% of the MTT
Moderate intensity: 50% of the MTT
High intensity: 75% of the MTT
Data analyses were performed in MATLAB (v.2018a, Mathworks, USA). For statistical analysis, two-sided tests were used unless otherwise stated. Non-parametric tests (two-sided Wilcoxon’s signed-rank test) were used to assess the discomfort scores between stimulator type for each stimulation intensity. A Chi-square (χ2) test was used to compare stimulator preference. For each two-second train of pulses, we calculated the charge transfer (i.e. total amount of the electrical charge delivered) as the discrete integral of the leading current pulses (i.e. anodic phase of stimulation) over time, expressed in millicoulombs (mC). The rise time was defined as the time it takes for the leading pulse to go from 10% to 90% of the steady-state amplitude. For the MyndMove™ current pulse, we first used the trailing pulse (i.e. cathodic phase of stimulation; 4 times longer and with ¼ of the amplitude of the leading pulse) to estimate the steady-state amplitude. Finally, this value was multiplied by 4 and used to calculate the rise time of the leading pulse. MyndMove™ displays the steady-state amplitude of the leading pulse as the user-controlled variable of current delivered.
Results
Perceived discomfort
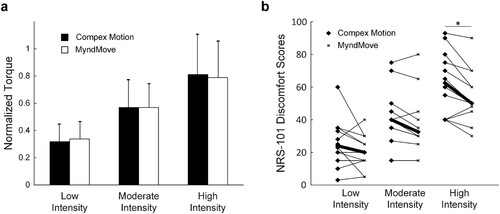
The NRS-101 discomfort scores were recorded for each participant, in three torque-matched conditions (i.e. low, moderate, and high stimulation intensities; a), with two stimulator types (MyndMove™ and Compex Motion), resulting in 72 data points (b). MyndMove™ discomfort scores were significantly lower than Compex Motion scores in the high stimulation intensity condition (Wilcoxon’s signed-rank test, Bonferroni corrected, P = 0.016). However, discomfort scores were not different for moderate (P = 0.0283) or low (P = 0.1328) stimulation intensities.
Figure 2 Torque-matched conditions and discomfort scores. a. Maximal torque values normalized to maximally-tolerated torque for all participants with Compex Motion and MyndMove™ for each stimulation intensity. Normalized torque comparisons between stimulation intensities were not significantly different between stimulator types: low intensity (unpaired Student’s t-test, P = 0.52), moderate intensity (P = 0.99) and high intensity (P = 0.74). b. NRS-101 discomfort scores for Compex Motion and MyndMove™ for each stimulation intensity. Bold lines mark the median discomfort score values for all participants in each stimulation intensity. Discomfort scores between Compex Motion and MyndMove were significantly different in the high intensity stimulation condition.
Stimulator preference
Three preference records were collected per participant for each stimulation intensity, resulting in a total of 108 preference scores. These results are summarized in . Preference was significant for MyndMove™ across all stimulation intensities: χ2 (2, N = 108) = 56.38, P = 5.71e-13. Unlike the discomfort scores, no significant difference in preference was found between stimulation intensities χ2 (2, N = 108) = 1.24, P = 0.54. In other words, preference for MyndMove™ was similar across stimulation intensities.
Table 2 Summary of stimulator preference data.
Pulse rise time, peak current, steady-state current and charge transfer
The estimated pulse rise times for MyndMove™ and Compex Motion stimulators were 0.02 ± 0.01 µs (n = 65) and 0.73 ± 0.54 µs (n = 62), respectively. Also, in 5 participants, Compex Motion current pulses were delivered with a pulse width of 266.3 ± 4.9 µs (range: 260–280 µs), and 305.4 ± 7 µs (range: 280–320 µs) for MyndMove™, while in 7 participants, Compex Motion pulses had a width of 419 ± 3 µs (range: 410–420 µs), and 404.9 ± 5.8 µs (range: 380–410 µs) for MyndMove™.
For individual two-second contractions generated with each stimulator, we plotted the peak vs. steady-state current against torque production (a and b), to assess which current value was a better predictor of torque production, given that MyndMove™ generates inverse exponential current pulses with a large current peak at the beginning of a pulse and then settling at a much lower steady-state current value, which is the user-controlled variable of current delivered using MyndMove™. On the other hand, for Compex Motion’s rectangular pulses, these two current values were expected to be virtually the same. A linear regression model was generated for each stimulator, based on these two values as Torque = Peak Current + Steady-State Current. Peak and steady-state currents were virtually the same in Compex Motion (), as shown in a. The model had an R2 = 0.702. On the other hand, the model of peak and steady-state currents for MyndMove™ (R2 = 0.612; b) highlights the difference between these two values, where peak currents were better able to predict torque production than steady-state currents. A third model was generated from all values, adding stimulator type as a categorical variable (R2 = 0.666; ). This model’s interaction terms confirmed that both peak and steady-state currents from each stimulator had a significantly different effect on torque production, confirming that these two types of current pulses generate torque in distinct ways.
Figure 3 Linear regression models of Torque = Peak Current + Steady-State Current for each stimulator. a. Model for Compex Motion stimulator. Peak and Steady-State Currents were virtually the same because Compex Motion generates rectangular pulses, although the current slightly overshoots before it stabilizes. The bold and dashed lines show the linear fit of the steady-state and peak currents, respectively. b. Model for MyndMove™ stimulator. The difference in Peak and Steady-State Currents is highlighted, where Peak Current was a better predictor of Torque production than Steady-State Currents (). The bold and dashed lines show the linear fit of the steady-state and peak currents, respectively.
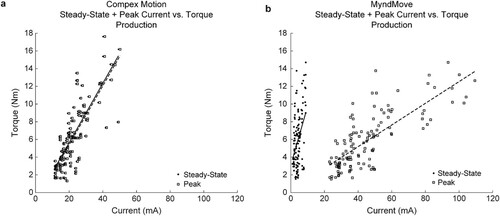
Table 3 Coefficients of linear regression models of Torque = Steady-State Current + Peak Current and Torque = Charge Transfer for each stimulator.
Table 4 Analysis of variance of linear regression models of Torque = Charge * Stimulator Type and Torque = Peak Current * Steady-State Current * Stimulator Type.
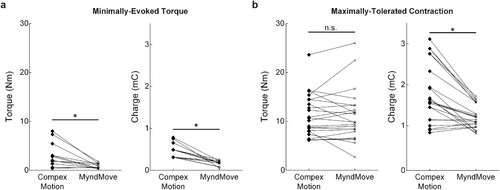
Finally, linear regression models of Torque = Charge Transfer were generated for Compex Motion (R2 = 0.677) and MyndMove™ (R2 = 0.453). In both models (a and b), charge transfer was able to significantly predict torque production (). A third model was generated to compare Torque = Charge Transfer * Stimulator Type (R2 = 0.581; ), in which the interaction term showed that Charge Transfer was significantly different between two stimulators. In other words, MyndMove™ required less charge transfer than Compex Motion to generate a given contraction. Additionally, we compared the charge transfer during METs for each stimulator and found that significantly smaller torques were detected with MyndMove™ compared to Compex Motion (n = 12; Wilcoxon’s signed-rank test, P = 0.0015; a). Naturally, these smaller torques were produced with significantly less charge transfer using MyndMove™ (P = 4.9e-04; a), which suggests that MyndMove™ has greater efficacy than Compex Motion. Similarly, we compared charge transfer during MTCs for each stimulator. One participant reached the maximum stimulation intensity deliverable using MyndMove™ but did not consider it to be their MTC, thus, their data was not considered in this analysis. We found that MTCs were not different between stimulators (n = 22; Wilcoxon’s signed-rank test, P = 0.93; b), however, consistent with our previous observations, charge transfer was significantly lower during MyndMove™ MTCs, compared to Compex Motion (P = 4.3e-04; b).
Figure 4 Linear regression models of Torque = Charge Transfer for each stimulator. a. Model for Compex Motion. The dashed line shows the linear fit. b. Model for MyndMove™, where less charge transfer was generated for a given level of contraction, compared to Compex Motion. The dashed line shows the linear fit.
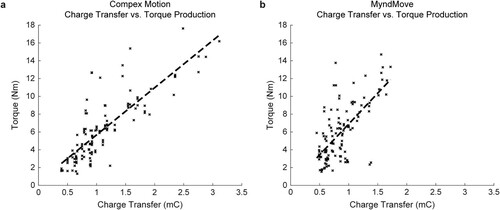
Figure 5 Minimally-evoked torque and maximally-tolerated contraction. a. Torque and charge transfer during the minimally-evoked torque (MET) for each participant (n = 12). b. Torque and charge transfer during the maximally-tolerated contractions (MTC) recorded and the beginning and end of the experiment for 11 participants (n = 22). One participant was not considered because they did not reach a MTC with the highest stimulation intensity delivered with MyndMove™. *P < 0.05.
Discussion
We compared discomfort scores and comfort-based preference between two types of FES pulses. One type of electrical stimulation pulses, produced by MyndMove™, were current- and voltage-regulated with an inverse exponential shape. The other type of pulses, produced by Compex Motion, were current-regulated with a rectangular shape. For both of these types, the pulses were also balanced, biphasic and asymmetric. Preference and discomfort for each stimulation pulse (i.e. stimulator) were compared across high, moderate, and low stimulation intensities in 12 able-bodied participants, in which higher intensities led to more discomfort. We found that discomfort scores for MyndMove™ were significantly lower than scores for Compex Motion in the high stimulation intensity condition. However, scores were not significantly different for moderate or low intensities, even though a trend towards lower discomfort can be observed for the MyndMove™ stimulator. Therefore, MyndMove™ would most likely increase accessibility to FES therapy for people with low tolerance to FES. In other words, one can deliver high intensity stimulation and generate higher muscle torques using MyndMove™ inverse exponential pulses, compared to conventional rectangular stimulation pulses. The generation of stronger contractions is clinically relevant, enabling therapists to increase the therapeutic effect of FEST.
Preference for MyndMove™ across all stimulation intensities (i.e. 60.2%) was significantly greater compared to Compex Motion stimulator, and no preference, suggesting that even when the discomfort was not reduced, participants found an unspecified quality of MyndMove™ stimulation more comfortable. At 60.8%, preference for MyndMove™ over Compex Motion, even though significant, was not beyond doubt, which alludes to the fact that generally most able-bodied participants find electrically-induced muscle contractions uncomfortable even when stimulation parameters have been optimized.
The mechanism by which MyndMove™ is able to generate stronger, more comfortable contractions, might be a result of how the current pulses are shaped. MyndMove™ delivers pulses with very fast rise time (i.e. 0.03 µs), allowing fast electroporation of lipid and cell membranes of the skin, resulting in a drop of resistance.Citation28 The sudden drop of resistance allows more current to be delivered at the beginning of the pulse and lower steady-state currents towards the end of the pulse. This quality of MyndMove™ stimulation might explain why some participants as described it as “sharp at the beginning but becomes comfortable towards the end of the contraction”. However, steady-state currents poorly predict torque production for MyndMove, so the reduction in discomfort was most likely a result of delivering lower charge transfer compared to the Compex Motion system. In a study by Crago et al.Citation16 rectangular current pulses were compared against exponential current pulses during stimulation of equivalent intensity. The authors found that charge transfer was consistently higher in rectangular pulses than exponential pulses. Although the discomfort was not the primary focus of their study, we can deduce that lower charge transfer is most likely the mechanism behind MyndMove™ reducing discomfort during FES interventions. Interestingly, MyndMove™ delivers inverse exponential pulses, as opposed to the positive exponential pulses (i.e. peak current was delivered towards the end of the pulse) studied by Crago et al.Citation16 thus, an exponential-shaped pulse seems optimal during FES, as it delivers lower charge transfer than a rectangular pulse, regardless of whether the peak current is delivered at the beginning or end of the pulse. Similar observations were made by Gracanin and Trnkoczy,Citation19 who concluded that the sensation of pain during stimulation mainly depends on the amount of charge delivered to the tissue.
Another interesting finding from this study is that charge transfer might not be the only predictor of contraction strength, as indicated by the torque values during MTCs (b), in which contractions of equivalent strength were produced with significantly less charge using MyndMove™ compared to Compex Motion. The exact mechanism by which stimulation delivered using exponential current pulses can generate a contraction of equivalent strength with less charge transfer, compared to rectangular pulses, is unknown. During FES, reaching the motor threshold is required to start motor unit recruitment to generate muscle contractions. However, as demonstrated here, inverse exponential vs. rectangular stimulation pulses might result in different patterns of motor unit recruitment. These differences are evident even at low stimulation intensities, as we found in this study during METs (b), where MyndMove™ generated a detectable torque with less charge transfer, compared to Compex Motion. Naturally, when the MET was detected for Compex Motion stimulation, the contraction was significantly stronger compared to MyndMove™.
This difference in the pattern of motor unit recruitment during MyndMove™ stimulation, which might arise from the faster rise time and lower steady-state currents, might also be influencing the discomfort levels perceived by the participants. We speculate that the difference in perceived discomfort arises from the selective activation of nerve fibers, specifically, increasing activation Aα extrafusal muscle fibers and sensory fibers (groups Ia and Ib) innervating muscle spindles and Golgi tendon organs, while decreasing activation of Aδ (group III) and C (group IV) sensory fibers. The Aδ and C sensory fibers innervate free nerve endings and nociceptors, respectively. These fibers have a long time constant (i.e. slow rate of adaptation), which diminishes the probability of their activation during an inverse exponential stimulation pulse because low steady-state stimulation currents are reached before Aδ and C fibers are activated. On the other hand, a rectangular current stimulation pulse would activate Aδ and C sensory fibers. In other words, with the inverse exponential pulse shape, one can drop the steady-state stimulation current below the activation threshold levels just before these fibers are ready to respond to stimulation. This gives an impression of lower intensity stimulation while delivering sufficient charge to activate myelinated and fast responding Aα extrafusal muscle fibers. Further studies will confirm if a faster rise time and lower steady-state currents are contributing factors in reducing discomfort in inverse exponential current pulses, besides a reduction in charge transfer.
Limitations
The primary limitation of this study is that the comparison between the two types of FES pulses were conducted in able-bodied individuals, without the inclusion of participants with sensorimotor impairments due to neurological injuries (e.g. stroke and incomplete spinal cord injury). First, individuals with sensorimotor impairments are more likely to be exposed to FES through rehabilitation interventions for improving their upper or lower limb motor functions. In such applications, FES is delivered at higher intensities (i.e. current amplitudes) in order to generate muscle contractions in weakened and partially paralyzed muscles. Additionally, individuals with stroke or incomplete spinal cord injury also experience impaired somatosensory function, thus, reducing skin discomfort during FES is all the more important to prevent withdrawal from therapy. For these reasons, MTCs would likely be different in the neurologically involved population. Moreover, the stimulation train is usually maintained for much longer than 2 s in therapeutic FES applications. For example, in FEST for the recovery of upper limb function in individuals with stroke, the stimulation may be delivered continuously for 10 s or longer, depending on the target tasks. While the 2-second stimulation train was appropriate for the current experimental design involving able-bodied participants, in which we tried to avoid muscle fatigue, comparing the discomfort of different FES pulse types in individuals with sensorimotor impairments would likely require longer trains of stimulation pulses.
Conclusions
To conclude, future studies where MyndMove™ is tested under different experimental conditions (e.g. other muscles), as well as in patients with neurological conditions, such as incomplete spinal cord injury or stroke, who have various levels of FES tolerance, will help confirm our assertion that MyndMove™ stimulation is preferred over conventional stimulators that deliver rectangular pulses. Finally, the reported reduction in discomfort might lead to an improvement of clinical outcomes in FES rehabilitation therapies due to the generation of stronger muscle contractions.
Abbreviations
| FES | = | functional electrical stimulation |
| FEST | = | functional electrical stimulation therapy |
| MET | = | minimally-evoked torque |
| MTC | = | maximally-tolerated contraction |
| MTT | = | maximally-tolerated torque |
| MVC | = | maximal voluntary contraction |
Disclaimer Statements
Contributors None.
Funding This work was supported by donations from the Toronto Rehabilitation Institute (Internal fund from Toronto Rehab Foundation).
Conflict of interest Milos R. Popovic is the co-founder and director of MyndTec, Inc., a Canadian company that designs, produces, and commercializes functional electrical stimulation therapy systems for upper limb rehabilitation. All other authors have no competing interests.
Ethics approval The University Health Network Ethics Committee approved the protocols described in this manuscript. Informed consent was obtained from each participant before undergoing experimentation. The CAPCR ID is 14-8647.
Acknowledgements
We would like to thank Austin J. Bergquist for his contributions towards designing the study and data collection.
Additional information
Funding
References
- Lynch CL, Popovic MR. Functional electrical stimulation. IEEE Control Syst 2008;28(2):40–50. doi:https://doi.org/10.1109/MCS.2007.914689
- Kapadia N, Popovic M. Functional electrical stimulation therapy for grasping in spinal cord injury: an overview. Top Spinal Cord Inj Rehabil [Internet] 2011;17(1):70–76. Available from http://archive.scijournal.com/doi/abs/. doi:https://doi.org/10.1310/sci1701-70
- Kapadia N, Masani K, Craven CB, Giangregorio LM, Hitzig SL, Richards K, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on walking competency. J Spinal Cord Med 2014;37(5):511–524. doi:https://doi.org/10.1179/2045772314Y.0000000263
- Murphy JO, Audu ML, Lombardo LM, Foglyano KM, Triolo RJ. Feasibility of closed-loop controller for righting seated posture after spinal cord injury. J Rehabil Res Dev 2014;51(5):747–760. doi:https://doi.org/10.1682/JRRD.2013.09.0200
- Mushahwar VK, Jacobs PL, Normann RA, Triolo RJ, Kleitman N. New functional electrical stimulation approaches to standing and walking. J Neural Eng 2007;4(3):S181–S197. doi:https://doi.org/10.1088/1741-2560/4/3/S05
- Martin R, Sadowsky C, Obst K, Meyer B, McDonald J. Functional electrical stimulation in spinal cord injury: from theory to practice. Top Spinal Cord Inj Rehabil 2012;18(1):28–33. doi:https://doi.org/10.1310/sci1801-28
- Kern H, Carraro U, Adami N, Biral D, Hofer C, Forstner C, et al. Home-based functional electrical stimulation rescues permanently denervated muscles in paraplegic patients with complete lower motor neuron lesion. Neurorehabil Neural Repair 2010;24(8):709–721. doi:https://doi.org/10.1177/1545968310366129
- IJzerman MJ, Stoffers TS, in ‘t Groen FACG, Klatte M. AP, Snoek GJ, Vorsteveld JHC, et al. The NESS handmaster orthosis: restoration of hand function in C5 and stroke patients by means of electrical stimulation. J Rehabil Sci 1996;9(3):86–89.
- Sheffler LR, Chae J. Neuromuscular electrical stimulation in neurorehabilitation. Muscle and Nerve 2007;35(5):562–590. doi:https://doi.org/10.1002/mus.20758
- Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med Suppl 2005;37(1):32. doi:https://doi.org/10.1080/16501970410035387
- Popovic MR, Thrasher TA, Zivanovic V, Takaki J, Hajek V. Neuroprosthesis for retraining reaching and grasping functions in severe hemiplegic patients. Neuromodulation 2005;8(1):58–72. doi:https://doi.org/10.1111/j.1094-7159.2005.05221.x
- Hebert DA, Bowen JM, Ho C, Antunes I, O’Reilly DJ, Bayley M. Examining a new functional electrical stimulation therapy with people with severe upper extremity hemiparesis and chronic stroke: a feasibility study. Br J Occup Ther 2017;80(11):651–659. doi:https://doi.org/10.1177/0308022617719807
- Thrasher TA, Zivanovic V, McIlroy W, Popovic MR. Rehabilitation of reaching and grasping function in severe hemiplegic patients using functional electrical stimulation therapy. Neurorehabil Neural Repair 2008;22(6):706–714. doi:https://doi.org/10.1177/1545968308317436
- Popovic MR, Kapadia NM, Zivanovic V, Furlan JC, Craven CB, McGillivray C. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair 2011;25(5):433–442. doi:https://doi.org/10.1177/1545968310392924
- Windholz T, Swanson T, Vanderbyl BL, Jagoe RT. The feasibility and acceptability of neuromuscular electrical stimulation to improve exercise performance in patients with advanced cancer: a pilot study. BMC Palliat Care 2014;13(1):1–8. doi:https://doi.org/10.1186/1472-684X-13-1
- Crago PE, Peckham PH, Mortimer JT, Van Der Meulen JP. The choice of pulse duration for chronic electrical stimulation via surface, nerve, and intramuscular electrodes. Ann Biomed Eng 1974;2:252–264. doi:https://doi.org/10.1007/BF02368496
- Fisher LE, Tyler DJ, Triolo RJ. Optimization of selective stimulation parameters for multi-contact electrodes. J Neuroeng Rehabil 2013;10:25. doi:https://doi.org/10.1186/1743-0003-10-25
- Gorman PH, Mortimer JT. The effect of stimulus parameters on the recruitment characteristics of direct nerve stimulation. IEEE Trans Biomed Eng 1983;BME-30(7):407–414. doi:https://doi.org/10.1109/TBME.1983.325041
- Gracanin F, Trnkoczy A. Optimal stimulus parameters for minimum pain in the chronic stimulation of innervated muscle. Arch Phys Med Rehabil 1975;56(6):243–249.
- Grill WM, Mortimer JT. The effect of stimulus pulse duration on selectivity of neural stimulation. IEEE Trans Biomed Eng 1996;43(2):161–166. doi:https://doi.org/10.1109/10.481985
- Kuhn A, Keller T, Lawrence M, Morari M. The influence of electrode size on selectivity and comfort in transcutaneous electrical stimulation of the forearm. IEEE Trans Neural Syst Rehabil Eng 2010;18(3):255–262. doi:https://doi.org/10.1109/TNSRE.2009.2039807
- Mortimer JT, Shealy CN, Wheeler C. Experimental nondestructive electrical stimulation of the brain and spinal cord. J Neurosurg 1970;32(5):553–559. doi:https://doi.org/10.3171/jns.1970.32.5.0553
- Rouhani H, Rodriguez KE, Bergquist AJ, Masani K, Popovic MR. Minimizing muscle fatigue through optimization of electrical stimulation parameters. J Biomed Eng Informatics 2016;3(1):33. doi:https://doi.org/10.5430/jbei.v3n1p33
- Vodovnik L, Long C, Regenos E, Lippay A. Pain response to different tetanizing currents. Arch Phys Med 1965;46:187–192.
- Huerta SC, Tarulli M, Prodic A, Popovic MR, Lehn PW. A universal functional electrical stimulator based on merged flyback-SC circuit. In: 15th international Power Electronics and Motion Control Conference and Exposition, EPE-PEMC 2012 ECCE Europe; 2012 Sep 4-6 Novi Sad, Serbia: IEEE; 2012. p. LS5a.3-1-LS5a.3-5.
- Tarulli M, Huerta SC, Prodic A, Popovic MR, Lehn PW. Merged flyback-SC-based output stage for versatile portable transcutaneous electrical stimulators. IEEE J Emerg Sel Top Power Electron 2016;4(1):318–331. doi:https://doi.org/10.1109/JESTPE.2015.2509604
- Popovic MR, Keller T. Modular transcutaneous functional electrical stimulation system. Med Eng Phys 2005;27(1):81–92. doi:https://doi.org/10.1016/j.medengphy.2004.08.016
- Chizmadzhev YA, Indenbom AV, Kuzmin PI, Galichenko SV, Weaver JC, Potts RO. Electrical properties of skin at moderate voltages: contribution of Appendageal Macropores. Biophys J 1998;74(2):843–856. doi:https://doi.org/10.1016/S0006-3495(98)74008-1
