Abstract
Purpose
To compare the binding and agonistic activity of Acthar® Gel and synthetic melanocortin receptor (MCR) agonists and examine how the activity of select agonists affects the in vivo production of corticosterone.
Materials and Methods
In vitro binding was determined using concentration-dependent displacement of the ligand [125I]Nle4, D-Phe7-α-melanocyte-stimulating hormone (α-MSH) on cells expressing MC1R, MC3R, MC4R, or MC5R. Functional activity was determined using a time-resolved fluorescence cyclic adenosine monophosphate (cAMP) assay in cells expressing MC1R, MC2R, MC3R, MC4R, or MC5R. In vivo corticosterone analyses were performed by measuring plasma corticosterone levels in Sprague Dawley rats.
Results
Acthar Gel and synthetic MCR agonists exhibited the highest binding at MC1R, lowest binding at MC5R, and moderate binding at MC3R and MC4R. Acthar Gel stimulated the production of cAMP in all 5 MCR-expressing cell lines, with MC2R displaying the lowest level of full agonist activity, 3-, 6.6-, and 10-fold lower than MC1R, MC3R, and MC4R, respectively. Acthar Gel was a partial agonist at MC5R. The synthetic MCR agonists induced full activity at all 5 MCRs, with the exception of α-MSH having no activity at MC2R. Acthar Gel treatment had less of an impact on in vivo production of corticosterone compared with synthetic ACTH1-24 depot.
Conclusions
Acthar Gel bound to and activated each MCR tested in this study, with partial agonist activity at MC5R and the lowest level of full agonist activity at MC2R, which distinguished it from synthetic MCR agonists. The minimal activity of Acthar Gel at MC2R corresponded to lower endogenous corticosteroid production.
Introduction
Melanocortin receptors (MCRs) comprise an evolutionarily conserved system that has many physiological regulatory functions, including energy homeostasis, analgesia, steroidogenesis, and behavioral effects [Citation1,Citation2]. MCRs have been shown to modulate various pathophysiological processes that lead to protection against excitotoxicity, oxidative stress, inflammation, and apoptosis [Citation3–6].
Endogenous MCR agonists include adrenocorticotropic hormone (ACTH) and α-, β-, and γ-melanocyte-stimulating hormones (MSH) [Citation7]. These agonists are derived from post-translational processing of a common protein precursor, proopiomelanocortin, and share a core sequence of 4 amino acids at positions 6–9 (HFRW) that is required for binding to MCRs [Citation8–10]. MCR agonists bind to and activate the 5 known G-protein–coupled transmembrane MCR subtypes (MC1R through MC5R) with varying specificity and affinity [Citation7].
Examples of the physiological processes regulated by MCRs include inflammation and immunoregulation in MC1R-expressing neutrophils, monocytes, macrophages, dendritic cells, and B lymphocytes; corticosteroid production in MC2R-expressing adrenocortical cells; energy homeostasis and control of inflammation in MC3R-expressing monocytes, B lymphocytes, and macrophages; neuroprotection in MC4R-expressing cells within the brain and spinal cord; and immunomodulatory functions in MC5R-expressing B and T lymphocytes, mast cells, and macrophages in peripheral tissues [Citation5,Citation11–13]. MC2R is unique among MCRs in that it binds only to certain ACTH peptides and requires accessory proteins that are essential for binding and receptor trafficking from the endoplasmic reticulum to the cell surface [Citation2,Citation13–17]. As such, α-MSH (ACTH1-13) does not activate MC2R, and animal studies have confirmed that it is not capable of stimulating corticosteroid secretion [Citation18,Citation19]. MCR agonists act as immunomodulators by reducing local and systemic inflammatory responses, rather than by complete immunosuppression [Citation5,Citation20–22].
Acthar® Gel (repository corticotropin injection; Mallinckrodt Pharmaceuticals, Bedminster, NJ, USA) is a naturally sourced complex mixture of ACTH analogs and other pituitary peptides [Citation23]. Acthar Gel engages the MCRs expressed on immune cells and other tissues throughout the body and is thought to produce both indirect anti-inflammatory and direct immunomodulatory effects [Citation24–26]. Acthar Gel is approved for several indications, including treatment of acute exacerbations of multiple sclerosis in adults, as a monotherapy for infantile spasms, and for the induction of diuresis or a remission of proteinuria in nephrotic syndrome without uremia of the idiopathic type or due to lupus erythematosus [Citation23]. Acthar Gel is also US Food and Drug Administration–approved as an adjunctive therapy for short-term administration in psoriatic and rheumatoid arthritis [Citation23]. In support of the considerably broad clinical utility of Acthar Gel [Citation23,Citation27], several studies have revealed anti-inflammatory and immunomodulatory effects in rodent models of multiple sclerosis, systemic lupus erythematosus, nephrotic syndromes, and rheumatoid arthritis [Citation28–31].
The anti-inflammatory effects of Acthar Gel originally were thought to be mediated indirectly through corticosteroid production following the engagement of MC2R-expressing cells in the adrenal cortex [Citation32]; however, recent studies challenge that hypothesis and suggest that Acthar Gel directly modulates the activity of MC1R, MC3R, MC4R, and MC5R on immune cells and various other cell types [Citation24,Citation25].
In the present study, the mechanism of action of Acthar Gel was compared with those of synthetic MCR agonists through the determination of in vitro binding and agonistic activity. The unique physiological effect of Acthar Gel on MC2R functional activity in the adrenal cortex was also assessed through the measurement of rat corticosterone levels in vivo.
Materials and methods
Cell culture and transfections
All cell lines were grown and maintained by Eurofins Cerep (Celle-L'Evescault, France). B16-F1 cells that express endogenous MC1R were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 5% fetal calf serum (FCS) and 1% antibiotics. Cloudman M3 cells were used for MC2R, as they express the required receptor accessory protein, and MC2R in this cell line has demonstrated a response similar to that of endogenous MC2R in adrenal cells [Citation33]. Cloudman M3 cells were cultured in Kaighn's Modification of Ham's F-12 Medium supplemented with 15% horse serum, 2.5% FCS, 1% glutamine, and 1.2% geneticin. Chinese hamster ovary cells were used to express human recombinant MC3R, MC4R, or MC5R and cultured in DMEM supplemented with 5% FCS, 1% nonessential amino acids, and 1.2% geneticin. Appropriate cell lines were stably transfected by Eurofins Cerep using LipofectamineTM (Life Technologies, Rockville, MD) and the pNEO plasmid.
Synthetic MCR agonists
The following synthetic ACTH analogs were used in this study: α-MSH (ACTH1-13; Sigma Aldrich, St. Louis, MO), ACTH1-17 (Bachem Americas, Inc., Torrance, CA), ACTH1-24 (Bachem Americas, Inc., Torrance, CA), human ACTH1-39 (hACTH1-39; Bachem Americas, Inc, Torrance, CA), and porcine ACTH1-39 (pACTH1-39; Bachem Americas, Inc., Torrance, CA). A synthetic N-25 deamidated ACTH1-39 (N25D-ACTH1-39; PolyPeptide Group, Torrance, CA) was also included, as one of the major components of Acthar Gel is N25D-ACTH1-39. The amino acid sequences of the MCR agonists used in this study are listed in .
Table 1. Binding (IC50) Values for Acthar Gel and Synthetic MCR Agonists at MCRs.
Binding assay
The binding assay, performed by Eurofins Cerep, assessed concentration-dependent displacement binding of the ligand [125I]Nle4, D-Phe7-α-MSH (NDP-α-MSH) at MC1R, MC3R, MC4R, and MC5R. Specific binding at MC2R was not observed owing to lack of a commercially available assay. The following conditions were used for this assay: cells expressing MC1R were incubated with 0.05 nM [125I]NDP-α-MSH for 90 min at room temperature (RT); cells expressing MC3R were incubated with 0.75 nM [125I]NDP-α-MSH for 60 min at 37 °C; cells expressing MC4R were incubated with 0.05 nM [125I]NDP-α-MSH for 120 min at 37 °C; and cells expressing MC5R were incubated with 0.05 nM [125I]NDP-α-MSH for 60 min at 37 °C. In combination with [125I]NDP-α-MSH, each cell line was incubated with placebo gel (control), Acthar Gel, or the synthetic MCR agonists in a buffer containing 25 mM N-(2-hydroxyethyl)-piperazine-N′-(2-ethanesulfonic acid) (HEPES)/KOH (pH 7.0), 100 mM NaCl, 1.5 mM CaCl2, 1 mM MgSO4, 0.2 g/L 1–10 phenanthroline, 0.025% NaN3, and 0.1% bovine serum albumin. Prednisolone and 6α-methylprednisolone were included as negative controls.
Following incubation, the samples were rapidly filtered under vacuum through glass fiber filters (GF/B, Packard) presoaked with 0.3% poly(ethyleneimine) and rinsed several times with ice-cold 50 mM Tris-HCl using a 96-sample cell harvester (UniFilter, Packard). The filters were dried and counted for radioactivity in a scintillation counter (TopCount, Packard) using a scintillation cocktail (MicroScintTM 0, Packard). Specific radioligand binding was calculated as the difference between total and nonspecific [125I]NDP-α-MSH binding in the absence and presence of NDP-α-MSH (1 μM), respectively. The results were expressed as percentage inhibition of the control radioligand-specific binding. All estimates of half maximal inhibitory concentration (IC50) were determined with a minimum of 2 replicates using nonlinear regression analysis and a 3-parameter dose-response curve with GraphPad Prism v. 7.03 for Windows (GraphPad Software, San Diego, CA).
Functional activity assay
The functional activity assay was performed by Eurofins Cerep with a homogeneous time-resolved fluorescence cyclic adenosine monophosphate (cAMP) assay (Cisbio Bioassays, Bagnols sur Cèze, France) to measure cellular cAMP levels. Each cell line was incubated with placebo gel (control), Acthar Gel, or the synthetic MCR agonists in buffer containing Hank’s Balanced Salt Solution (Invitrogen, Carlsbad, CA) supplemented with 20 mM HEPES (pH 7.4) and 500 µM 3-isobutyl-1-methylxanthine under the following conditions: MC1R and MC2R cells, 10 min at RT; MC3R and MC4R cells, 30 min at 37 °C; and MC5R cells, 30 min at RT. Prednisolone and 6α-methylprednisolone were included as negative controls.
Following incubation, the cells were lysed, and the fluorescence acceptor (d2-labeled cAMP) and fluorescence donor (anti-cAMP antibody labeled with europium cryptate) were added. After 60 min at RT, the fluorescence transfer was measured at λex = 337 nm and λex = 620 and 665 nm using a microplate reader (Envision®, Perkin Elmer). The cAMP concentration was determined by dividing the signal measured at 665 nm by the signal measured at 620 nm. The results were expressed as a percentage of the control response to 1 µM NDP-α-MSH for MC1R and MC3R, 0.3 to 1 µM ACTH1-39 for MC2R, 30 nm NDP-α-MSH for MC4R, and 10 µM α-MSH for MC5R. All estimates of half maximal effective concentration (EC50) were determined with a minimum of 2 replicates using nonlinear regression analysis and a 3-parameter dose-response curve with GraphPad Prism v. 7.03 for Windows. According to Eurofins Cerep’s experimental parameters, MCR agonists with stimulation values lower than 25% were considered to have no response as the result was attributable to the variability of the signal around the control level.
Rodent corticosterone analyses
A total of 36 female Sprague Dawley rats (n = 6 per treatment group) were acclimated to a Culex cage for 24 h before dosing with Acthar Gel or synthetic ACTH1-24 depot (Mallinckrodt Pharmaceuticals, Bedminster, NJ, US). Following anesthetization with 3% isoflurane, rats were subcutaneously injected with 10, 40, or 400 IU/kg Acthar Gel or 0.6, 1.2, or 2.4 mg/kg synthetic ACTH1-24 depot (doses previously used in several disease models to evaluate corticosteroid levels) in the dorsal area of the back. After prespecified time periods of up to 48 h postdose, blood was collected in lithium heparin tubes lacking separator gel using the Culex automated blood collection system. Plasma was then transferred to a 96-well plate, and corticosterone was quantified with an enzyme-linked immunosorbent assay (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions, as corticosterone is the predominant circulating adrenal corticosteroid in rodents. Area under the curve (AUC) calculations were performed with GraphPad Prism v. 7.03 for Windows. Statistical analyses were performed with a 2-way repeated-measures analysis of variance (ANOVA) or a 1-way ANOVA followed by a Tukey multiple comparisons test.
Ethical considerations
This study was performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals [Citation34]. The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Mallinckrodt Pharmaceuticals.
Results
MCR binding
The binding of placebo control, Acthar Gel, and the synthetic MCR agonists was assessed at MC1R, MC3R, MC4R, and MC5R by measuring concentration-dependent displacement binding of NDP-α-MSH at each receptor (; ). As a negative control, placebo gel did not elicit a response (). Acthar Gel displaced specific binding of [125I]NDP-α-MSH to all 4 MCR subtypes in a concentration-dependent manner and displayed approximately 12- and 16-fold greater binding for MC1R than for the MC3R and MC4R subtypes, respectively (; ). Acthar Gel bound even more weakly to MC5R, with a 46-fold lower binding than for MC1R. On the basis of these values, Acthar Gel binding had the following rank order: MC1R > MC3R≈MC4R > MC5R (). The rank order binding profiles of the synthetic MCR agonists were similar to that of Acthar Gel (). As expected, the corticosteroids prednisolone and 6α-methylprednisolone did not bind to any of the MCRs (data not shown).
Figure 1. Binding of Acthar Gel and synthetic MCR agonists. Data are presented as the mean ± SEM. Placebo gel was used as a control. MC2R was excluded from this analysis owing to lack of a commercially available assay. ACTH: adrenocorticotropic hormone; h: human; MCR: melanocortin receptor; N25D: N-25 deamidated; p: porcine; SEM: standard error of the mean.
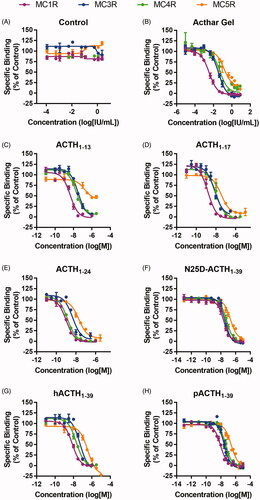
Table 2. Rank order for binding of Acthar Gel and each synthetic MCR agonist.
MCR functional activity
The functional activity of placebo control, Acthar Gel, and the synthetic MCR agonists was assessed at MC1R, MC2R, MC3R, MC4R, and MC5R by measuring the induction of cAMP resulting from activation of each receptor (; ). As a negative control, placebo gel did not elicit a response (). Acthar Gel increased cellular cAMP concentrations in all 5 MCR-expressing cell lines in a concentration-dependent manner (). Acthar Gel had the lowest level of full agonist activity at MC2R, with 3-, 6.6-, and 10-fold lower potency than at MC1R, MC3R, and MC4R, respectively (). Acthar Gel and the synthetic MCR agonists exhibited full agonist activity at MC1R, MC2R, MC3R, and MC4R; however, Acthar Gel behaved as a partial agonist at MC5R (). These data demonstrate that Acthar Gel induced cAMP at each MCR with the following rank order: MC4R > MC3R > MC1R > MC2R > MC5R, whereas the other MCR agonists exhibited functional activity different from that of Acthar Gel (). As expected, the corticosteroids prednisolone and 6α-methylprednisolone had no functional activity at any of the MCRs (data not shown).
Figure 2. Functional activity of Acthar Gel and synthetic MCR agonists. Data are presented as the mean ± SEM. Placebo gel was used as a negative control. ACTH: adrenocorticotropic hormone; cAMP: cyclic adenosine monophosphate; h: human; MCR: melanocortin receptor; N25D: N-25 deamidated; p: porcine; SEM: standard error of the mean.
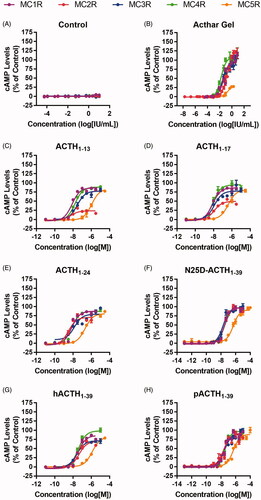
Table 3. Functional activity (EC50) values for Acthar Gel and synthetic MCR agonists at MCRs.
Table 4. Rank order for functional activity of Acthar Gel and each synthetic MCR agonist.
In vivo corticosterone analyses
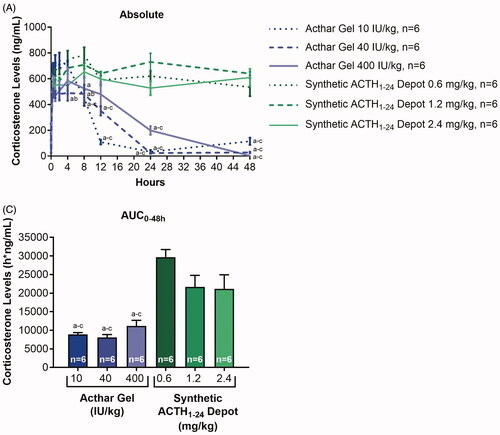
MC2R plays an important role in the regulation of adrenal corticosteroid secretion [Citation35]. Historically, the clinical efficacy of Acthar Gel was thought to be due to its ability to stimulate endogenous cortisol production through the engagement of MC2R [Citation32]. Interestingly, ACTH1-24 demonstrated the greatest MC2R functional activity of any of the synthetic MCR agonists tested, whereas Acthar Gel showed less full agonist activity at MC2R than the other MCRs (). We therefore examined differences between Acthar Gel and synthetic ACTH1-24 depot in eliciting corticosterone response in rats (). There were significant effects on corticosterone levels with treatment (F [5, 275] = 45.03, p < 0.0001) and time (F [10, 55] = 91.07, p < 0.0001). There was also a significant interaction between treatment and time (F [50, 275] = 9.48, p < 0.0001). Corticosterone levels reached their maximum (490–744 ng/mL) at 1, 0.5, and 4 h after administration of 10, 40, and 400 IU/kg Acthar Gel, respectively; whereas maximum corticosterone levels (654–782 ng/mL) following synthetic ACTH1-24 depot administration were reached at 8 h for 0.6 and 2.4 mg/kg and 24 h for 1.2 mg/kg (). Treatment with 10, 40, and 400 IU/kg Acthar Gel resulted in an initial increase followed by a consistent decrease in corticosterone levels, with the 10 and 40 IU/kg Acthar Gel groups returning to baseline within 24 h (). In contrast, treatment with 0.6, 1.2, or 2.4 mg/kg synthetic ACTH1-24 depot resulted in sustained elevations in corticosterone levels throughout 48 h (). Post hoc analysis revealed significant differences in corticosterone levels between the Acthar Gel groups and the synthetic ACTH1-24 depot groups at 24 and 48 h (p < 0.05) ().
Figure 3. In vivo corticosterone induction in response to treatment with Acthar Gel or synthetic ACTH1-24 depot. Data were analyzed with a 2-way repeated-measures ANOVA followed by a Tukey multiple comparisons test in (A) and with a 1-way ANOVA followed by a Tukey multiple comparisons test in (B); a, b, and c correspond to p < 0.05 vs synthetic ACTH1-24 depot 0.6 mg/kg, 1.2 mg/kg, and 2.4 mg/kg, respectively. Data are presented as the mean ± SEM. ACTH: adrenocorticotropic hormone; ANOVA: analysis of variance; AUC: area under the curve; SEM: standard error of the mean.
Discussion
The data presented here confirm the binding of Acthar Gel to MCRs and identify agonistic activity distinct from that of synthetic MCR agonists. Furthermore, in rats, treatment with Acthar Gel led to substantially less corticosterone production than synthetic ACTH1-24 depot. Acthar Gel exhibited the greatest binding at MC1R, moderate binding at MC3R and MC4R, and the lowest binding at MC5R. The other MCR agonists had a similar profile, with the greatest binding at MC1R and lowest at MC5R, but binding at MC3R and MC4R varied from agonist to agonist. Although the binding profile of Acthar Gel was similar to that of the synthetic MCR agonists, its receptor functional activity was remarkably different. Acthar Gel showed the most functional activity at MC4R, a moderate activity level at MC3R and MC1R, the least full agonistic activity at MC2R, and partial agonism at MC5R. Conversely, the MCR agonists ACTH1-24 and pACTH1-39 had the greatest level of functional activity at MC2R. Additionally, to differentiate the activity of Acthar Gel from that of corticosteroids, we confirmed the lack of binding and functional activity of prednisolone and 6α-methylprednisolone at each MCR tested.
These data are supported by prior studies that observed the binding and functional activity of various MCR agonists [Citation36–39]. The rank order binding for ACTH1-13, ACTH1-17, ACTH1-24, pACTH1-39, and hACTH1-39 observed here is similar to that reported by Schiöth et al. [Citation37]. Also, the trend in cAMP generation by ACTH1-13, ACTH1-24, and ACTH1-39 is similar to results from Pritchard et al. [Citation36], who examined MC4R, and Chen et al. [Citation39], who examined MC2R. In addition, Kapas et al. [Citation38] showed that ACTH1-24 induced a greater cAMP response than ACTH1-39, which is consistent with our results. The novelty of the data presented here is the unique engagement and functional activity of Acthar Gel on various MCRs when compared with those of synthetic MCR agonists.
Determining the extent of MCR activation by Acthar Gel and individual MCR agonists has important clinical implications. MCRs downregulate leukocyte activity (e.g. antibody production, inflammatory cytokine release) and potentiate parasympathetic and sympathetic nervous system activity to dampen the immune response [Citation13]. We show that Acthar Gel produced the most robust response at MC4R, which is the predominant MCR in the brain [Citation5,Citation13]. Through the stimulation of MC4R, Acthar Gel may elicit anti-inflammatory effects in the central nervous system, which supports its efficacy in the treatment of acute exacerbations of multiple sclerosis in adults and potentially in infantile spasms [Citation13,Citation40–43]. Acthar Gel had the second largest impact on MC3R, which is expressed in the brain, monocytes, B lymphocytes, and macrophages and controls inflammation [Citation5,Citation13]. In addition, Acthar Gel has activity at MC1R, which is present on neutrophils, monocytes, macrophages, dendritic cells, and B lymphocytes and reduces inflammation [Citation5,Citation13]. MC1R in podocytes has been implicated in providing renoprotective effects in patients with nephrotic syndrome through stabilization of the actin cytoskeleton, and Acthar Gel has been shown to have such renoprotective effects in a rodent model of focal segmental glomerulosclerosis [Citation31,Citation44]. Interestingly, Acthar Gel appeared to be a partial agonist at MC5R, which is expressed on a variety of immune cells, including macrophages and lymphocytes, and may also inhibit the inflammatory response [Citation5,Citation13]. These interactions of Acthar Gel with each MCR likely contribute to its unique mechanism of action.
The ability of Acthar Gel to elicit functional activity at MCRs distinct from that of the synthetic MCR agonists in this study may be attributed to its unique formulation. It has been shown that G-protein−coupled receptors have the ability to heterodimerize, driving a unique pharmacological profile based on ligand binding [Citation45]. The partial agonism of Acthar Gel at MC5R could be attributed to the interactions of its complex mixture, which may ultimately impact net partial agonist activity. This possibility is intriguing, and further research is needed to investigate whether the present data represent the functional profiles of single (i.e. N25D-ACTH1-39) or multiple melanocortin peptides present in Acthar Gel acting alone or in combination with each MCR subtype.
Further confirming the unique mechanism of Acthar Gel is the striking difference between the MC2R-mediated corticosterone response to Acthar Gel and synthetic ACTH1-24 depot. MC2R is critical for ACTH-mediated adrenal glucocorticoid release [Citation46]. Given the in vitro differences between Acthar Gel and ACTH1-24 in functional activity at MC2R, in vivo corticosterone levels were assessed in rats. These in vivo data confirm that Acthar Gel is a weaker agonist of MC2R, leading to significantly less induction of endogenous corticosterone production over time. Conversely, synthetic ACTH1-24 depot led to sustained elevations in corticosterone levels. Although its weaker agonism at MC2R may play an important role in the in vivo response observed here, the distinct clearance mechanisms of Acthar Gel may be another factor to consider.
Overall, reduced corticosterone exposure in Acthar Gel−treated rats suggests a favorable tolerability profile as the adverse effects commonly associated with corticosteroid production may be minimized [Citation47]. In support of this, a recent clinical study in patients with rheumatoid arthritis treated with Acthar Gel reported little to no incidence of adverse events typically associated with corticosteroid use (e.g. hypertension, hyperglycemia, headache, weight gain, edema) [Citation48]. In particular, bone turnover markers were stable, suggesting minimal further impact of Acthar Gel on bone metabolism in patients already receiving corticosteroids [Citation48]. Treatment with Acthar Gel has also been effective in patients with rheumatoid arthritis or glomerular diseases that have been resistant to corticosteroid treatment, further confirming a mechanism of action that is independent of cortisol production [Citation48,Citation49]. Thus, these data, in combination with preclinical and clinical studies [Citation24–26,Citation28,Citation48,Citation49], suggest that Acthar Gel may provide benefit in the treatment of inflammatory disease through mechanisms beyond the induction of steroids.
Taken together, the combined engagement profile of Acthar Gel at MC1R, MC3R, MC4R, and MC5R suggests a distinct direct immune cell effect beyond the indirect anti-inflammatory effect from MC2R-dependent production of corticosteroids. Despite less sustained cortisol induction, Acthar Gel remains efficacious [Citation48,Citation49], suggesting direct interaction with immune cells to treat disease [Citation24–26]. The mechanism of action of Acthar Gel is unique from that of pACTH1-39, ACTH1-24, N25D-ACTH1-39, and other MCR agonists, suggesting that its complex mixture of peptides interacts with MCRs differently than any single MCR agonist does. Although a mixture of peptides may not be the best comparator for single synthetic peptides, these data demonstrate that the various components of Acthar Gel influence its unique MCR agonistic activity. This may result from the proprietary manufacturing process used to produce Acthar Gel, which likely generates peptides that require further characterization, along with its known components (i.e. N25D-ACTH1-39), and contribute to its activity profile. Acthar Gel may therefore have a multimodal and preferential mechanism of regulating the immune response.
Supplemental Material
Download PDF (85.7 KB)Acknowledgments
The authors would like to thank Eurofins Cerep (Celle-L'Evescault, France) for performing the binding and functional activity assays and MedLogix Communications, LLC, (Itasca, IL) for scientific consulting, writing, and editorial support, all of which were funded by Mallinckrodt Pharmaceuticals.
Disclosure statement
ADW, YJH, KG, and BZ, are stock shareholders and employees of Mallinckrodt Pharmaceuticals; LRB is an employee of Mallinckrodt Pharmaceuticals.
Additional information
Funding
References
- Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. 2003;284(3):E468–E474.
- Dores RM, Londraville RL, Prokop J, et al. Molecular evolution of GPCRs: melanocortin/melanocortin receptors. J Mol Endocrinol. 2014;52(3):T29–T42.
- Mykicki N, Herrmann AM, Schwab N, et al. Melanocortin-1 receptor activation is neuroprotective in mouse models of neuroinflammatory disease. Sci Transl Med. 2016;8(362):362ra146
- Zhang L, Dong L, Liu X, et al. α-Melanocyte-stimulating hormone protects retinal vascular endothelial cells from oxidative stress and apoptosis in a rat model of diabetes. PLoS One. 2014;9(4):e93433
- Catania A, Lonati C, Sordi A, et al. The melanocortin system in control of inflammation. ScientificWorldJournal. 2010;10:1840–1853.
- Chai B, Li JY, Zhang W, et al. Melanocortin-4 receptor-mediated inhibition of apoptosis in immortalized hypothalamic neurons via mitogen-activated protein kinase. Peptides. 2006;27(11):2846–2857.
- Lisak RP, Benjamins JA. Melanocortins, melanocortin receptors and multiple sclerosis. Brain Sci. 2017;7(8):104.
- Fridmanis D, Roga A, Klovins J. ACTH receptor (MC2R) specificity: what do we know about underlying molecular mechanisms? Front Endocrinol (Lausanne). 2017;8:13
- Catania A, Gatti S, Colombo G, et al. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56(1):1–29.
- Nakanishi S, Inoue A, Kita T, et al. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979;278(5703):423–427.
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27(7):736–749.
- Li ZX, Liu BW, He ZG, et al. Melanocortin-4 receptor regulation of pain. Biochim Biophys Acta Mol Basis Dis. 2017;1863(10 Pt A):2515–2522.
- Arnason BG, Berkovich R, Catania A, et al. Mechanisms of action of adrenocorticotropic hormone and other melanocortins relevant to the clinical management of patients with multiple sclerosis. Mult Scler. 2013;19(2):130–136.
- Hinkle PM, Sebag JA. Structure and function of the melanocortin2 receptor accessory protein (MRAP). Mol Cell Endocrinol. 2009;300(1–2):25–31.
- Rodrigues AR, Almeida H, Gouveia AM. Intracellular signaling mechanisms of the melanocortin receptors: current state of the art. Cell Mol Life Sci. 2015;72(7):1331–1345.
- Roy S, Rached M, Gallo-Payet N. Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms alpha and beta in isogenic human embryonic kidney 293 cells. Mol Endocrinol. 2007;21(7):1656–1669.
- Sebag JA, Hinkle PM. Regions of melanocortin 2 (MC2) receptor accessory protein necessary for dual topology and MC2 receptor trafficking and signaling. J Biol Chem. 2009;284(1):610–618.
- Nussdorfer GG, Mazzocchi G, Malendowicz LK. Acute effects of alpha-MSH on the rat zona glomerulosa in vivo. Biochem Biophys Res Commun. 1986;141(3):1279–1284.
- Shenker Y, Villareal JZ, Sider RS, et al. Alpha-melanocyte-stimulating hormone stimulation of aldosterone secretion in hypophysectomized rats. Endocrinology. 1985;116(1):138–141.
- Catania A. Neuroprotective actions of melanocortins: a therapeutic opportunity. Trends Neurosci. 2008;31(7):353–360.
- Patel HB, Bombardieri M, Sampaio ALF, et al. Anti-inflammatory and antiosteoclastogenesis properties of endogenous melanocortin receptor type 3 in experimental arthritis. Faseb J. 2010;24(12):4835–4843.
- Patel HB, Leoni G, Melendez TM, et al. Melanocortin control of cell trafficking in vascular inflammation. Adv Exp Med Biol. 2010;681:88–106.
- Acthar Gel [package insert]. Bedminster (NJ): Mallinckrodt ARD LLC; 2019.
- Olsen NJ, Decker DA, Higgins P, et al. Direct effects of HP Acthar Gel on human B lymphocyte activation in vitro. Arthritis Res Ther. 2015;17:300.
- Wright D, Zweifel B, Sharma P, et al. Reduced steroidogenic activity of repository corticotropin injection (RCI) induces a distinct cytokine response following T cell activation in vivo (abstract AB0082). Ann Rheum Dis. 2019;78(suppl 2):1504.
- Benko AL, McAloose CA, Becker PM, et al. Repository corticotrophin injection exerts direct acute effects on human B cell gene expression distinct from the actions of glucocorticoids. Clin Exp Immunol. 2018;192(1):68–81.
- Philbin M, Niewoehner J, Wan GJ. Clinical and economic evaluation of repository corticotropin injection: a narrative literature review of treatment efficacy and healthcare resource utilization for seven key indications. Adv Ther. 2017;34(8):1775–1790.
- Decker DA, Grant C, Oh L, et al. Immunomodulatory effects of H.P. Acthar Gel on B cell development in the NZB/W F1 mouse model of systemic lupus erythematosus. Lupus. 2014;23(8):802–812.
- Brod SA, Bauer V, Hood Z. Oral ACTH (H.P. Acthar(®)Gel) inhibits IL-1 and IL-17 secretion in humans. Biomed Pharmacother. 2012;66(1):36–39.
- Decker D. Repository corticotropin injection attenuates collagen-induced arthritic joint structural damage and has enhanced effects in combination with etanercept. 2020. https://bmcmusculoskeletdisord.biomedcentral.com/.
- Hayes K, Warner E, Bollinger C, et al. Repository corticotropin injection versus corticosteroids for protection against renal damage in a focal segmental glomerulosclerosis rodent model. BMC Nephrol. 2020;21(1):226.
- Ross AP, Ben-Zacharia A, Harris C, et al. Multiple sclerosis, relapses, and the mechanism of action of adrenocorticotropic hormone. Front Neurol. 2013;4:21.
- Penhoat A, Naville D, El Mourabit H, et al. Functional expression of the human ACTH receptor gene. Endocr Res. 2000;26(4):549–557.
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. 8th ed. Washington (DC): National Academies Press (US); 2011.
- Chida D, Nakagawa S, Nagai S, et al. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc Natl Acad Sci USA. 2007;104(46):18205–18210.
- Pritchard LE, Armstrong D, Davies N, et al. Agouti-related protein (83–132) is a competitive antagonist at the human melanocortin-4 receptor: no evidence for differential interactions with pro-opiomelanocortin-derived ligands. J Endocrinol. 2004;180(1):183–191.
- Schiöth HB, Muceniece R, Larsson M, et al. The melanocortin 1, 3, 4 or 5 receptors do not have a binding epitope for ACTH beyond the sequence of alpha-MSH. J Endocrinol. 1997;155(1):73–78.
- Kapas S, Cammas FM, Hinson JP, et al. Agonist and receptor binding properties of adrenocorticotropin peptides using the cloned mouse adrenocorticotropin receptor expressed in a stably transfected HeLa cell line. Endocrinology. 1996;137(8):3291–3294.
- Chen M, Aprahamian CJ, Kesterson RA, et al. Molecular identification of the human melanocortin-2 receptor responsible for ligand binding and signaling. Biochemistry. 2007;46(40):11389–11397.
- Berkovich R, Agius MA. Mechanisms of action of ACTH in the management of relapsing forms of multiple sclerosis. Ther Adv Neurol Disord. 2014;7(2):83–96.
- Nazareth T, Datar M, Yu TC. Treatment effectiveness for resolution of multiple sclerosis relapse in a US health plan population. Neurol Ther. 2019;8(2):383–395.
- Baram TZ, Mitchell WG, Tournay A, et al. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. 1996;97(3):375–379.
- Brunson KL, Khan N, Eghbal-Ahmadi M, et al. Corticotropin (ACTH) acts directly on amygdala neurons to down-regulate corticotropin-releasing hormone gene expression. Ann Neurol. 2001;49(3):304–312.
- Bergwall L, Wallentin H, Elvin J, et al. Amplification of the melanocortin-1 receptor in nephrotic syndrome identifies a target for podocyte cytoskeleton stabilization. Sci Rep. 2018;8(1):15731.
- Gaitonde SA, González-Maeso J. Contribution of heteromerization to G protein-coupled receptor function. Curr Opin Pharmacol. 2017;32:23–31.
- Yang Y, Mishra V, Crasto CJ, et al. Third transmembrane domain of the adrenocorticotropic receptor is critical for ligand selectivity and potency. J Biol Chem. 2015;290(12):7685–7692.
- Yasir M, Sonthalia S. Corticosteroid adverse effects. In StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 [Updated 2019 Oct 1]. https://www.ncbi.nlm.nih.gov/books/NBK531462/.
- Fleischmann R, Furst DE, Connolly-Strong E, et al. Repository corticotropin injection for active rheumatoid arthritis despite aggressive treatment: a randomized controlled withdrawal trial. Rheumatol Ther. 2020;7(2):327–344.
- Bomback AS, Canetta PA, Beck LH, Jr, et al. Treatment of resistant glomerular diseases with adrenocorticotropic hormone gel: a prospective trial. Am J Nephrol. 2012;36(1):58–67.
