ABSTRACT
Background: Compared to adults, children maybe more highly exposed to toxic substances in drinking water because they consume more water per unit of body weight. The U.S. Environmental Protection Agency (USEPA) has developed new guidance for selecting age groups and age-specific exposure factors for assessing children's exposures and risks to environmental contaminants. Research Aim: To demonstrate the application and importance of applying age-specific drinking water intake rates, health reference values, and exposure scenarios when assessing drinking water exposures because these approaches illustrate the potential for greater potential for adverse health effects among children. Methods: manganese, an essential nutrient and neurotoxicant, was selected as a case study and chemical of potential concern for children's health. A screening-level risk assessment was performed using age-specific drinking water intake rates and manganese concentrations from U.S. public drinking water systems. Results: When age-specific drinking water intake rates are used to calculate dose, formula-fed infants receive the highest dose of manganese from drinking water compared to all other age groups. Estimated hazard quotients suggest adverse health effects are possible. Use of USEPA's standardized childhood age groups and childhood exposure factors significantly improves the understanding of childhood exposure and risks.
INTRODUCTION
Children may have increased susceptibility following exposure to environmental hazards, such as drinking water contaminants, because they continue to develop both behaviorally and physiologically throughout childhood (CitationNRC 1993). To account for exposure differences during this period of potential susceptibility, the U.S. Environmental Protection Agency (USEPA) has developed a standard set of age groups based on the behavioral and physiological stages of childhood for use when assessing, modeling or monitoring childhood exposures (CitationUSEPA 2006; CitationFirestone et al. 2007). These age groups have been applied in determining age-specific drinking water intake rates for children ages 0–21 years (CitationUSEPA 2004b, Citation2008).
The USEPA's Child-Specific Exposure Factors Handbook (CS-EFH) includes the standard age groups for children and the resultant revisions of children's drinking water intake rates. The CS-EFH includes an age-group based analysis of children's drinking water intake rates (CitationUSEPA 2008). These intake rates were derived from two 24-hour recalls of food and drinking water intakes from the 1994–1996 and 1998 Continuing Survey of Food Intake by Individuals (CSFII). The drinking water rates have been standardized to USEPA's childhood age groups to estimate children's direct and indirect tap-water ingestion from public drinking water sources ().
Table 1 Recommended community drinking water intake rates.
As shown in , the quantity of water ingested per unit of body weight is at a maximum in the first month of life and decreases with increasing age. When USEPA's adult default drinking water intake rate (2 l/day) is normalized to body weight and compared to that of infants 1 to 3 months, the infants consuming at the 95th percentile of drinking water intake ingest 8 times more water on a ml/kg basis than a 70 kilogram adult; therefore, the youngest children may be more highly exposed to toxic substances in drinking water on a mg/kg basis because they consume more water per unit of body weight (CitationUSEPA 2008).
The use and importance of applying age-specific exposure factors in risk assessment has been discussed in the scientific literature (CitationFirestone et al. 2007; CitationDaston et al. 2004; CitationLandrigan et al. 2004; CitationGinsberg et al. 2004; CitationMiller et al. 2002). However, the development and application of information for implementing children's health risk assessment continues to be an active area of scientific inquiry because many risk assessors continue to use default exposure values, including drinking water default intake values (e.g., an adult intake of 2 l/day per 70 kgr and a child intake of 1 l/day per 10 kgr), for screening level assessments. While the use of such values accounts for differences in drinking water intake rates between children and adults, standard defaults may fail to describe the potentially large variability in exposures between age groups. The goal of this research is to capture the specific differences in children's exposures to drinking water contaminants by: (1) emphasizing the importance of using age-specific drinking water intake rates when assessing children's exposures to drinking water contaminants, and (2) demonstrating how the selection and application different health reference values and drinking water exposure scenarios may result in a greater potential adverse health effects estimate among children.
To achieve the stated goals, this study was completed in two parts. First, in order to assess children's drinking water exposures, existing methodologies were refined to consider infant feeding practices during the first year of life, that is, breast feeding versus formula feeding, and the implications of feeding practice on drinking water exposures. The methodology was then used to define chronic exposure scenarios for children that include feeding practices along with age-specific drinking water intake to estimate exposures. Second, a case study was developed using manganese (Mn) as a chemical of potential concern (COPC) in order to highlight an interesting risk assessment application of age-specific drinking water intake rates and exposures.
Mn was selected as the COPC for the case study because it: (1) is an essential nutrient requiring daily intake; (2) can be a neurotoxicant at higher levels of exposure, with potentially greater toxic effects in children as compared to adults; (3) has various health reference values (HRVs) that account for both essentiality and toxicity; (4) occurs naturally in U.S. drinking water or can be a pollutant that is introduced into the environment through human activity (i.e., the burning of fossil fuels); and (5) has a high frequency of occurrence in U.S. public water systems (PWSs), many of which serve large populations.
This approach has resulted in a nuanced analysis that adds to the existing literature as it is the first to demonstrate the importance of applying new methods for selecting age groups in children's exposure assessment and recently revised age-specific drinking water intake rates for assessing risks due to drinking water contaminants. It is also novel in using Mn exposure as a case study because it underscores how the use of different drinking water exposure scenarios can result in a greater estimated potential for adverse health effects among different age groups.
PART 1: METHODS FOR ASSESSING EARLY LIFE DRINKING WATER EXPOSURES
The USEPA's recommended values for community drinking water intake rates for children from birth to < 21 years of age () were used to assess children's drinking water exposures. Intake rates at the mean and 95th percentiles were selected to illustrate a range of consumer drinking water intakes and resulting exposures. While point estimates were used in this assessment, these estimates are based on datasets from the CSFII, which contain drinking water intake estimates for more than 5000 children (birth to 19 years) (CitationUSEPA 2004b).
To better characterize potential health hazards due to early-life exposures, different analytical approaches were used for breast-fed infants (0 to < 12 months), formula-fed infants (0 to < 12 months), and children (1 to < 21 years). During the first year of life infants are primarily fed either breast milk or formula, and exposures to environmental agents can vary greatly depending on infant feeding practice; it is for this reason a separate risk assessment was conducted for formula-fed infants (0 to < 12 months).
Formula-Fed Infants
The American Academy of Pediatrics (AAP) recommends exclusive breast feeding (no water, juice, nonhuman milk, or foods) for the first 6 months of life (CitationAAP 2005); these recommendations also indicate that infants 6 months to 1 year should continue breastfeeding with the introduction complementary foods. Similarly, both AAP and Institute of Medicine (IOM) Food and Nutrition Board (FNB) recommend infants who are not breast fed should only receive infant feeding formula for the first 6 months of life (CitationIOM FNB 2001; CitationAAP 2005). Based on these recommendations, separate exposure scenarios were developed for exclusively formula-fed and exclusively breast-fed infants. Formula-fed infants represent an important group of children because less than 12 percent of all infants in the United States are exclusively breastfed at 6 months of age and drinking water intake rates are the highest during the first year of life () (CitationCDC 2007; CitationUSEPA 2008).
Assessing formula-fed infant exposures to drinking water COPCs requires additional consideration because some COPCs are both essential nutrients (found in infant formulas) and potential toxicants at high concentrations. When a COPC is found in both infant feeding formula and drinking water, then additional steps must be taken to adequately assess infant exposures, these steps include:
-
Estimate the number of formula servings consumed by age groups < 1 year () based on community drinking water intake rates for children at the mean and 95th percentile standard and formula serving size (Eq. (Equation1))
where FS = the age-specific number of formula servings (ml) per day, IDW = age-specific daily water intake rate (mlwater/day) (see ), and SS = serving size = ml of formula -
Use the number of estimated formula servings consumed by each age group to determine the daily amount of the COPC consumed from formula powder (Eq. (Equation2))
where COPCformula = COPC consumed per day from formula powder (mg/day), Cformula = COPC (mg) in the formula powder used to prepare one serving of formula -
Use age-specific body weights to calculate the potential daily COPC dose from formula powder (Eq. (Equation3)).
where Dformula = potential COPC dose from formula powder (mg/kgBW/day), BW = age-specific body weight (kg) (CitationUSEPA 2008) -
Use COPC drinking water concentrations to calculate the potential COPC dose from the drinking water used to prepare the formula servings (Eq. (Equation4)). For example, the analysis can include the median and 95th percentile concentrations of the COPC to provide a range of concentrations and potential risks.
where Dwater = potential COPC dose from drinking water (mg/kgBW/day), Cwater = concentration of COPC in drinking water (mg/ml)I DW - A age-specific daily water intake rate adjusted for body weight (mlwater intake/ kgBW/day) (see )
-
Sum the doses in formula and drinking water for a total COPC (Eq. (Equation5)).
where Dtotal = total dose from formula powder and water (mg/kgBW/day) -
Calculate age-group specific hazard quotients (HQs) for infants. An HQ is the ratio of the potential exposure to the COPC and corresponding HRVs (a predetermined level at which no adverse effects are expected, e.g., the Reference Dose, RfD, or Adequate Intake, AI); if the HQ is calculated to be less than 1, then adverse health effects are not expected as a result of exposure, and if the HQ is greater than 1, then adverse health effects are possible (CitationUSEPA 1996b). A margin of exposure approach could be used at this step, depending on the interests of the risk assessor.
The age-specific HQs are calculated by applying a HRV and an age-specific relative source contribution (RSC; the percentage of daily exposure allocated to drinking water ingestion) to the calculated dose (Dtotal; Eq. (Equation6 A )). A RSC of one is applied in this analysis for infants less than 6 months based on the assumption that their only exposure to a COPC is from oral ingestion of the water and formula mixture. This assumption is based on the AAP's infant feeding recommendations (CitationAAP 2005). Infants 6–12 months are assigned an RSC of 0.5 because approximately half of older infants daily caloric intake comes from formula while the remaining calories come from weaning foods(CitationAAP 2005), which may contain COPCs.
where HQ A = estimated age-specific hazard quotient, HRV = health reference value, such as the USEPA's Drinking Water RfD or IOM's AI value when adjusted by life-stage body weight, RSC A = age-specific drinking water relative source contribution.
Children 1 to < 21 Years
As discussed earlier, for each age group in Cwater may be used to calculate COPC dose from drinking water (as in Eq. (Equation4)). As the purpose of the analysis is to assess drinking water exposures, additional sources of COPC exposure are not quantified in the assessment of children ages 1 to < 21 years because children at these ages are exposed to COPCs through multiple pathways (including foods and soil ingestion) and limited data are typically lacking to assess these exposures. When there is a lack of age- or COPC-specific data, a default RSC of 0.2 is applied to allow for other sources of exposure in addition to drinking water.
-
Calculate age-group specific HQs for children 1 to < 21 years of age using the age-specific Dwater, a HRV, and a default RSC (Eq. (Equation6 B )).
where HQ A = estimated age-specific hazard quotient, HRV = health reference value, such as the USEPA's Drinking Water RfD or IOM's AI value when adjusted by life-stage body weight, RSC = default relative source contribution of 0.2.
The application of Eqs. (Equation6 A ) and (Equation6 B ) results in an HQ A for each age group (birth to < 1 month, 1 to < 3, 3 to < 6, 6 to < 12 months, 1 to < 2, 2 to < 3, 3 to < 6, 6 to < 11, 11 to < 16, 16 to < 21 years).
Assessment of Chronic Childhood Exposures that Include Formula Feeding
The USEPA defines chronic oral exposures as repeated oral exposure for more than approximately 10% of the life span in humans (CitationUSEPA 2007); based on this definition and the assumption of approximately a 70-year life span, this analysis defines two chronic duration childhood exposure scenarios: (1) early life (the first 7 years of life), and (2) the entire childhood (birth to < 21 years). The chronic HQs are calculated from the aforementioned data as follows:
-
Calculate a weighted HQ for each age group (including infants). The weighted HQ is based on the proportion of a < 7 and < 21 year duration exposure that is represented by the age group (Eq. (Equation7)).
where Weighted HQ A = age-specific hazard quotient that has been weighted for duration of the age group, Weighted Age = duration of age group in months ÷ total number of months for 7 or 21 years. -
Sum the weighted HQs for the age groups to estimate an HQ for the chronic exposure scenario for early life (ages birth to 7 years of age) and for all of childhood (ages birth to 21 years of age) (Eq. (Equation8)).
OR
where HQ(< 7 or < 21 years) = hazard quotient for the < 7 or < 21 years of age chronic exposure scenario.
Assessment of Chronic Childhood Exposures that Include Breast Feeding
It can be assumed that breast-fed infants consume a minimal amount of drinking water during the first year of life based on the AAP recommendation for exclusive breast feeding (no water, juice, nonhuman milk, or foods) for the first 6 months of life and that infants 6 months to 1 year should continue breastfeeding with the introduction complementary foods (CitationAAP 2005). Infant exposures to drinking water agents maybe underestimated for ages 6–12 months using this assumption because complementary foods may be reconstituted using tap water; however, it is difficult to separate the amount of COPC in the infant food as compared to water used to reconstitute food. The breast-fed infant drinking water exposure scenarios can be assessed with a different approach:
-
Calculate age-specific HQs using Eq. (Equation6B ) and weighted HQs using Eq. (Equation7), except the chronic exposure scenarios do not include drinking water exposure for infants < 1 year, such that the early-life scenario is six years (i.e., ages 12 months to 7 years) and childhood scenario is 20 years (i.e., ages 12 months to 21 years) duration (Eq. (Equation9)).
OR
where HQ(6 or 20 years) = hazard quotient for the 6 or 20 year duration breast-fed exposure scenario.
Dose Calculation for Adults
Adult doses are calculated using Eq. (Equation4) and the default exposure factors of 2 l/day drinking water intake and 70 kg body weight. The same exposure factors are used for both short-term and chronic adult exposure scenarios.
PART 2: MANGANESE CASE STUDY
Mn was selected as the COPC for a case study in applying the methodology described in Part 1. Application of this approach allows for comparisons between age groups and between different exposure scenarios (e.g., infant feeding practice, exposure duration). Mn makes an interesting case study because it is an essential nutrient, a potential neurotoxicant with disproportionate effects in children, and a drinking water contaminant.
Daily Mn Intake Requirements and Toxicity
Mn is an essential nutrient for humans; however, excess Mn exposure may lead to impaired neurological function. A recommended daily intake rate (maximum or adequate intake) for Mn must consider both the essentiality and the toxicity of Mn in humans because human disease states have been associated with both Mn deficiencies and excessive intake levels (CitationUSEPA 2004a). The Institute of Medicine (IOM) Food and Nutrition Board, the USEPA, and the California Environmental Protection Agency Office of Environmental Health Hazard Assessment (OEHHA) have all set daily reference values for Mn intake using the different approaches, which account for both essentiality and neurotoxicity, described below.
Because Mn is an essential nutrient (involved in the formation of bone and in amino acid, lipid, and carbohydrate metabolism) the IOM developed a series of adequate intake (AI) and tolerable upper limit intake (UL) dietary references for Mn based on age and sex groupings () (CitationIOM FNB 2001). An AI is developed when the available data are not adequate to establish a more precise Estimated Average Requirement and Recommended Dietary Allowance. The AI, an estimate of daily dietary intakes in a healthy population, was based on median dietary intakes reported from the Food and Drug Administration Total Diet Study for adults and children greater than 1 year of age. For infants ages birth to 6 months, the AI (0.0003 mg/day) reflects the observed mean Mn intake of healthy infants exclusively fed human milk for the first 6 months of life. For children 6 months to 1 year, whose diet includes milk and other foods, the AI (0.6 mg/day) is based on average intake of Mn in a study by CitationGibson and De Wolf (1980) and the adult data extrapolated to children based on reference body weights. The AI for the second 6 months of life is considerably greater then that for the first 6 months because the Mn content of human milk is lower than that for other foods (CitationIOM FNB 2001).
Table 2 IOM recommended daily dietary intakes for Mn (mg/day).
The UL, the highest daily intake of Mn that is not likely to pose a risk of adverse health effects (i.e., neurotoxicity) in almost all individuals, was based on a “no observable adverse effect level” (NOAEL) of 11 mg/day for adults (CitationIOM FNB 2001; CitationGreger 1999) and a LOAEL from a study by CitationDavis and Greger (1992) where there were significant increases in serum Mn after 25 days of total intake of 15 mg/day (of supplemental Mn) and in lymphocyte Mn-dependant superoxide dismustase activity after 90 days. For children ages 1 to 18 years, adult-to-child linear body weight scaling was used to calculate an age-adjusted UL. A UL was not derived for infants < 1 year due to a lack of data and concern about infants' ability to handle excess amounts of Mn (CitationIOM FNB 2001). To prevent excess exposures IOM recommends that infants' only source of Mn intake should be from breast milk, weaning foods, or formula.
The USEPA's Integrated Risk Information System (IRIS) reviewed several observational dietary studies of healthy adults to determine an apparent NOAEL for Mn toxicity and an oral reference dose (RfD) of 0.14 mg/kg/day with neurotoxicity as the critical effect (CitationUSEPA 1996a). IRIS is USEPA's database for health effects information and HRVs, and these values are commonly used in Agency assessments. IRIS defines an RfD as:
an estimate of a daily oral exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime. (CitationUSEPA 2007)
IRIS recommends applying a 3-fold modifying factor to the RfD when assessing Mn exposure from drinking water (yielding an RfD of 0.047 mg/kg/day) because: (1) there is potential for higher Mn absorption from water than from food; (2) an epidemiological study by CitationKondakis et al. (1989) demonstrated impaired neurological function in adults who consumed drinking water containing doses of Mn not far from the range of essentiality; (3) concerns for infants fed formula with higher Mn concentrations than breast milk; and (4) supporting evidence that neonates absorb more and excrete less Mn than adults, combined with data that absorbed Mn in neonates crosses the blood brain barrier more easily than in adults (CitationUSEPA 1996a). The USEPA Office of Water has derived a health-related benchmark for evaluating Mn drinking water occurrence data called the Health Reference Level (HRL), of 0.3 mg Mn/l, based on the IRIS oral RfD with the 3-fold modifying factor and the allocation of a default 20% RSC using the default adult body weight and water ingestion rate (70 kgr and 2 l/d) (CitationUSEPA 2003).
California's CitationOEHHA (2006) has established a child-specific non-dietary reference dose (chRD) for Mn for school-site risk assessment. OEHHA's action was due to the presence of Mn in California school environments and its potential to impact children's neurological function. OEHHA estimated a mid-range dietary intake of 5 mg Mn/day based on the CitationFreeland-Graves et al. (1987) review of available adult human data. The mid-range dietary Mn intake was then subtracted from a NOAEL of 11 mg/day (CitationGreger 1999; same value used by the USEPA and the IOM) to yield a non-dietary NOAEL of 6 mg/day (0.086 mg/kg/day based on 70 kg body weight). Because the NOAEL is based on adult data OEHHA then applied a 3-fold uncertainty factor to account for differences between children and adults in gastrointestinal absorption, biliary excretion, blood-brain barrier, and transferrin receptors, resulting in a chRD of 0.03 mg/kg/day for Mn in school site risk assessments (CitationOEHHA 2006).
In this case study, the USEPA RfD for drinking water and the IOM infant AIs were used as the HRVs (in Eqs. (Equation6 A) and (Equation6 B)). For all age groups older than 1 year, only the USEPA RfD for drinking water exposures (0.047 mg/kg/day) was used to calculate the estimated HQ (note, IOM values were not used because they are similar to the USEPA RfD for ages > 1 year). Older children continue to be exposed to Mn in drinking water, and potential Mn exposures from foods and other sources (i.e., soil) increase. Exposure estimates are not currently available to accurately allocate the total Mn exposure among the various sources of Mn exposure for each of the childhood age groups. The Total Diet Study (CitationUSDA 1998) reported reference dietary intakes for Mn assessed by year, while CitationIOM FNB (2000) estimated Mn intakes via diet in different age groupings (0 to 6 months, 7 to 12 months, 1 to 3 years, 4 to 8 years, 9 to 13 years, and 14 to 18 years). Due this lack of age-group specific data, a default RSC (0.2) was applied to allow for other sources of Mn exposure in addition to drinking water (Eq. (6 C )) (CitationUSEPA 2004a).
Mn Neurotoxic Effects and Children's Health
Mn toxicity is recognized as a serious health hazard, resulting in pathologies of the central nervous system (CNS) characterized by mental disorders and involuntary muscular movements resembling Parkinson's disease (CitationKeen et al. 2000). The association between human exposures to airborne Mn and neurotoxicity has been well documented (CitationMergler et al. 1999; CitationKeen et al. 2000); however, less is known about Mn toxicity resulting from drinking water exposure, especially in children. Recent studies have shown oral exposure to excess Mn can result in measurable signs of neurotoxicity in children; these studies include four epidemiological studies and two case reports (CitationBouchard et al. 2007; CitationWasserman et al. 2006; CitationHe et al. 1994; CitationZhang et al. 1995; CitationWoolf et al. 2002; CitationSahni et al. 2007). These findings are generally supported by the observation of neurotoxicity in early life rodent studies (CitationDorman et al. 2000; CitationBrenneman et al. 1999; CitationTran et al. 2002a,Citationb).
A pilot study of 46 Canadian children (6–15 years of age) assessed differences in children's exposure to well water from two wells with different Mn concentrations: Well 1 (W1): mean 610 μg Mn/l; Well 2 (W2): mean 160 μg Mn/l (CitationBouchard et al. 2007). Researchers found that children whose houses were supplied by W1 had higher hair Mn (MnH) content than those supplied by W2; a significant association was found between elevated MnH content and higher test scores for hyperactive behaviors (oppositional, p = .02; hyperactivity, p = .002) (CitationBouchard et al. 2007).
In another study, a cross-sectional investigation assessed intellectual function among 142 10-year-old children in Bangladesh who had been consuming tube-well water with an average Mn concentration of 793 μg Mn/l; after adjusting for sociodemographic covariates and controlling for lead and arsenic drinking water exposures, a dose–response association was seen between elevated water Mn concentrations and reduced intellectual function (p ≤ .02) (CitationWasserman et al. 2006).
A third epidemiological investigation assessed 92 matched-pair Chinese school pupils, ages 11–13 years, exposed to Mn through drinking water (240–350 μg Mn/l); the contamination originated from sewage irrigation. When compared to matched controls (drinking water concentrations 30–40 μg Mn/l), children in the area with sewage irrigation had a significantly (p < .01) greater MnH content and significantly lower performance scores on a battery of neurobehavioral tests (CitationHe et al. 1994). Using the data from the same study population, a later study by CitationZhang et al. (1995) found blood Mn concentrations were also significantly higher (p < .01) among children exposed to elevated Mn drinking water concentrations; these children also had lower grades in language and mathematics.
A case study report of a 10-year-old boy who had been exposed to high levels of Mn (1.21 mg Mn/l), due to well contamination of his primary drinking water supply, found a marked discrepancy between the child's global cognitive skills and low verbal and visual memory (CitationWoolf et al. 2002). In another case report, a 6-year-old girl was diagnosed with severe Mn neurotoxicity, while Mn drinking water concentrations (1.7 to 2.4 mg/l) at her family's summer cottage were three to five times greater than the World Health Organization (WHO) guideline (0.4 mg/l); the case report authors concluded environmental exposures alone were unlikely to be the cause of her illness (CitationWHO 2006; CitationSahni et al. 2007).
One older study found differing results: CitationKawamura et al. (1941) surveyed the general symptoms of 25 people (including 12 children < 18 years) exposed to elevated well-water Mn concentrations (≥ 29 mg Mn/l) for 2–4 months; upon examination, adults reported more severe neurological symptoms than the children (< 10 years), who appeared to be unaffected by the elevated Mn concentrations; however, clinical signs of Mn toxicity were reported by four adolescents (children > 11 years) (CitationKawamura et al. 1941).
Despite differences in study design, demographic characteristics, diet, elemental drinking water composition, exposure duration, and various confounding factors, all of the available studies on children (except CitationKawamura et al. 1941) have demonstrated a plausible association between elevated Mn concentrations in drinking water and altered neurological function in children (). In the three epidemiological evaluations, intelligence and behavioral test scores were significantly different (p < .05) in the children exposed to excessive Mn concentrations as compared to their demographically matched controls.
Table 3 Summary of children's drinking water exposure epidemiological studies.
The relationship between Mn toxicity resulting from drinking water exposures and infant mortality also remains uncertain. A recent cross-sectional study assessed the association between Mn exposure via drinking water and infant mortality among 3824 Bangladesh infants (CitationHafeman et al. 2007). Results from this study suggest infants exposed to drinking water Mn concentrations in excess of the WHO's standard for drinking water (0.4 mg/l) had increased mortality risk during the first year of life when compared with unexposed infants. However, because of study limitations, the association between drinking water Mn exposure and infant mortality needs to be confirmed through additional research (CitationHafeman et al. 2007).
A number of animal studies have also shown oral exposures to high levels of Mn during early life may result in abnormal neurobehavioral outcomes. For example, a study of infant rhesus monkeys fed commercial infant formulas with varying Mn concentrations found monkeys who consumed formulas with high Mn content, soy formula (300 μg Mn/l), and soy formula with additional Mn (1000 μg Mn/l), were less playful and more clingy than monkeys who received the control formula (50 μg Mn/l) (CitationGolub et al. 2005). Monkeys in the high Mn exposure groups (≥ 300 μg Mn/l) also had shorter wake cycles and periods of daytime inactivity when compared to controls.
While neonatal rats have increased rates of Mn intestinal absorption (70% neonate versus 1–2% adult) and higher Mn absorption into the brain compared to adult rats (CitationMena 1974), the effects findings from rodent studies remain less clear. When given identical oral manganese chloride doses (gavage for adult rats and by mouth using a micropipette for pups), Mn has been shown to accumulate at a higher rate in the brains of young rodents when compared to adult males (0, 25 and 50 mg/kg/d (p < .05); Dorman et al. 2000); moreover, when investigating the relative sensitivity to behavioral effects, these researchers found that pulse-elicited acoustic startle reflex increased in the neonates (p < .05), while neither age exhibited effects in motor activity or passive avoidance tests. These findings suggest that neonate rats are at greater risk of Mn-induced neurotoxicity when compared to adult rats receiving a similar dose (CitationDorman et al. 2000); this conclusion is supported by the observation of increased motor activity in neonatal rats under similar study conditions in a previous study (CitationBrenneman et al. 1999). In a similar study, CitationTran et al. (2002a) observed that dietary exposure to Mn (0, 50, 250, and 500 mg/kg/d) during the neonatal period (Post Natal Day [PND] 1 to 21) resulted in behavioral effects in all measures, including delayed homing ability in the high dose group (PND 10; p < .01), delayed righting in the two high dose groups (PND 5; not significant), and a positive linear relationship across all doses in the passive avoidance test (PND 32; p < .02). However, this research group found that at later time points the measured trends in behavioral outcomes (increased digging latency, PND 58; passive avoidance, PND 60–64) did not attain significance (CitationTran et al. 2002b).
Occurrence of Mn in U.S. Drinking Water
Mn has a high prevalence in ambient U.S. surface and ground waters, 97% and 70%, respectively, based on monitoring data (CitationUSEPA 2003). Mn also has a low aesthetic threshold in tap water because of its visible color impact, and it is for this reason that the USEPA set a secondary drinking water standard (or non-enforceable guideline) of 0.05 mg Mn/l for Mn (40 Code of the Federal Register [CFR] § 143.3). While Mn concentrations in most U.S. public water systems (PWSs) are low, a number of PWSs servicing large populations have reported Mn levels greater than the USEPA HRL of 0.3 mg Mn/l (CitationUSEPA 2003, Citation2004a).
Mn drinking water occurrence data were obtained from USEPA's National Inorganics and Radionuclides Survey (NIRS). This survey was conducted to collect national occurrence data on a select set of contaminants from 989 community PWSs served by ground water in 49 States (CitationUSEPA 2003); national-scale occurrence data for PWSs served by surface water are not currently available. While NIRS data does not provide information on the occurrence of Mn in surface PWSs, NIRS data can be used directly for national Mn ground water occurrence analyses with very few, if any, data quality or use issues (CitationUSEPA 2003).
In this case study NIRS drinking water concentration data were used to calculate the potential Mn dose from the drinking water (Eq. (Equation4)). Three states—Iowa, Minnesota, and Pennsylvania—were selected to illustrate national occurrence of Mn in PWSs throughout the United States. These states were selected based on Mn occurrence frequencies and the number of detects greater than USEPA's Mn HRL (0.3 mg Mn/l). The median and 95th percentile were used to provide a range of Mn concentrations and potential risks ().
Table 4 NIRS drinking water occurrence for Mn.
Mn in Infant Formula
Infant formula contains Mn because Mn is an essential nutrient. However, infant formulas may contain as much as 75 times more Mn, per liter, than breast milk, not including any additional Mn from the water with which it is mixed (CitationCollip et al. 1983; CitationLonnerdal 1994; CitationLjung and Vahter 2007). As a result, Mn concentrations in drinking water may contribute significantly to the total Mn intake of formula-fed infants (CitationSievers 2005).
An informal survey conducted in the local (Washington, DC, USA) marketplace identified 16 powder-based infant feeding formulas from five manufacturers. From this survey, the average formula serving size and the median concentration of Mn in powdered formula were determined. All 16 formulas reported nutrient data for a serving size of 5 ounces (148 ml) diluted formula (). Powdered formula Mn concentrations ranged from 5 μg to 25 μg for each serving of 148 ml; the median concentration of Mn in powdered formula of 15 μg for each 148 ml serving of prepared formula was used in this case study.
Table 5 Mn concentrations in 5 fluid ounces of diluted powder- based infant formulasFootnote*.
CASE STUDY RESULTS
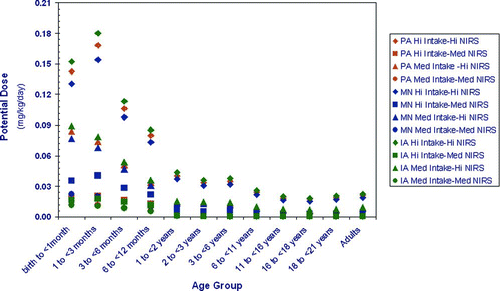
When using age-specific drinking water intakes to calculate dose, formula-fed infants 1 < 3 months receive the highest potential dose of Mn as compared to all other age groups (). In the high exposure scenario (95th percentile for both intake and drinking water concentration), infants 1 < 3 months receive up to an 8-fold greater dose of Mn than adults; estimated doses for neonates are 0.18 mg/kg/day, 0.15 mg/kg/day, and 0.17 mg/kg/day in Iowa, Minnesota and Pennsylvania, respectively, as compared to 0.02 mg/kg/day for adults (the same in all three states; ).
Figure 1 Potential Mn dose from drinking water by age group in Pennsylvania (PA), Iowa (IA), and Minnesota (MN).
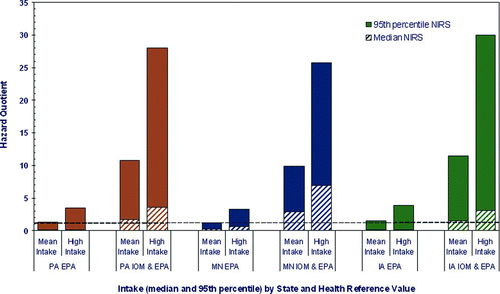
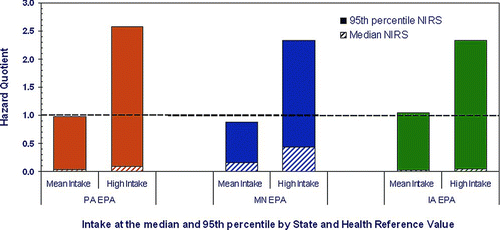
The estimated HQs for the formula-fed early-life chronic scenario (i.e., the first 7 years of life) indicate a greater potential for health risk when IOM AI reference values are applied for children less than 1 year of age as compared to when the USEPA RfD is applied (); the highest estimated HQ is for the high exposure scenario (i.e., 95th percentile for both intake and drinking water concentration) in Iowa, where the early-life chronic scenario yields a HQ of 30.0. HQs for the same high exposure scenario in Pennsylvania and Minnesota were 26 and 28, respectively. The application of IOM AI values to the central tendency exposure (mean intake and median drinking water concentration) scenario yields HQs that range from 1.5 in Iowa to 2.9 in Minnesota. When applying USEPA's drinking water RfD, estimated HQs for the 7-year high exposure scenario range from 3.2 in Minnesota to 3.8 in Iowa; HQs for the 7-year central tendency exposure scenario range from 0.05 in Iowa to 0.25 in Minnesota ().
Figure 2 Potential Mn hazard from 7 years of intake among formula-fed children in Pennsylvania (PA), Minnesota (MN), and Iowa (IA). The Hazard Quotient (HQ) is calculated to be less than 1, then adverse health effects are not expected as a result of exposure, and if the HQ is greater than 1, then adverse health effects are possible (CitationUSEPA 1996b).
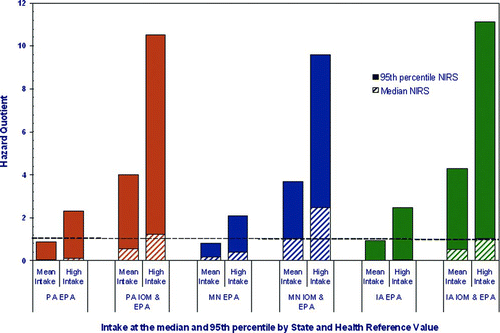
When the entire childhood is considered a chronic exposure (birth to < 21 years) for formula-fed infants, the highest estimated HQ is observed in Iowa when IOM AI reference values are applied to the high exposure scenario (HQ = 11.1; ). The lowest estimated HQ for all of childhood is also observed in Iowa when USEPA's drinking water RfD is applied to the central tendency exposure scenario (HQ = 0.03; ).
Figure 3 Potential Mn hazard from 21 years of intake among formula-fed children in Pennsylvania (PA), Minnesota (MN), and Iowa (IA). The Hazard Quotient (HQ) is calculated to be less than 1, then adverse health effects are not expected as a result of exposure, and if the HQ is greater than 1, then adverse health effects are possible (CitationUSEPA 1996b).
All estimated HQs for breast-fed children exceeded one when drinking water concentrations at the 95th percentile were considered in the chronic exposure scenario; the HQs range from 1.2 in Minnesota to 3.9 in Iowa ( and ). None of the breast- feeding scenarios that included median Mn drinking water concentrations result in HQs greater than one (HQs = 0.03 to 0.60; and ). For breast-fed children, there is smaller variation in the highest observed HQs regardless of the state or the exposure duration period.
Figure 4 Potential Mn Hazard from 6 years of intake among breast-fed children in Pennsylvania (PA), Minnesota (MN), and Iowa (IA). The Hazard Quotient (HQ) is calculated to be less than 1, then adverse health effects are not expected as a result of exposure, and if the HQ is greater than 1, then adverse health effects are possible (CitationUSEPA 1996b).
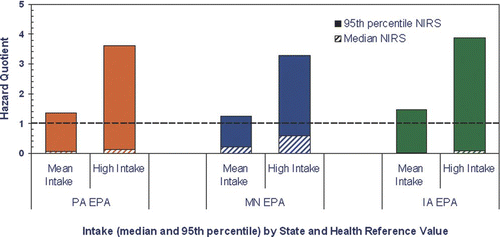
Figure 5 Potential Mn hazard from 20 years intake among breast fed infants in Pennsylvania (PA), Minnesota (MN), and Iowa (IA). The Hazard Quotient (HQ) is calculated to be less than 1, then adverse health effects are not expected as a result of exposure, and if the HQ is greater than 1, then adverse health effects are possible (CitationUSEPA 1996b).
DISCUSSION
Drinking water intakes per kg of body weight are the highest during the first month of life and decrease with increasing age, resulting in higher doses of drinking water contaminants, such as Mn, among the youngest infants. Because of the inverse relationship between dose and age, the smallest infants received up to an 8-fold greater dose of Mn than adults (). In addition to potentially higher doses, it has been suggested that pharmacokinetic processes may contribute to early-life susceptibility to Mn. These processes include: (1) increased absorption from the gastrointestinal tract, (2) decreased excretion, (3) higher tissue retention rates, and (4) different distribution due to the incompletely formed blood-brain barrier (CitationLonnerdal 1994; CitationDorman et al. 2000; CitationStastny et al. 1984). When compared to all other age groups, differences in pharmacokinetic processes and high-dose oral exposures () may place younger infants at the greatest risk for Mn-induced neurotoxicity from drinking water exposures.
While neonates receive the greatest dose of Mn, older children may also be at risk for Mn-induced neurotoxicity. The 95th percentile NIRS drinking water concentrations used in this study ranged from 0.49 to 0.58 mg Mn/l (). At similar concentrations (0.346 and 0.61 mg Mn/l), epidemiological studies have demonstrated an association between exposures to elevated Mn concentrations in drinking water and preclinical signs of neurotoxicity (e.g., lower scores on neurobehavioral tests and oppositional and hyperactive behaviors) in children ages 6–15 (CitationHe et al. 1994; CitationBouchard et al. 2007). Using hair as a biomarker for Mn drinking water exposures, studies by He and Bouchard found association between elevated levels of Mn content in hair and impaired neurobehavioral function in exposed children (CitationBouchard et al. 2007; CitationHe et al. 1994); these findings are consistent with an earlier study that reported an association between elevated levels of Mn in hair and learning disabilities in children (CitationPhil and Parkes 1977). Additionally, Zhang et al. (using the same data as CitationHe et al. 1994) also found an association between Mn drinking water exposures, blood serum neurotransmitters, and school performance (CitationZhang et al. 1995).
The observed differences in HQs resulting from the application of IOM's AI compared to USEPA's RfD are reflective of the methods used to establish these HRVs. The USEPA's RfD is derived from an adult dietary balance study; however, the USEPA recommends applying a 3-fold modifying factor to the oral RfD (0.14 mg/kg/day ÷ 3 = 0.047 mg/kg/day) when assessing exposures to Mn from drinking water. This modifying factor is, in part, to account for childhood concerns, including evidence that infant formulas, which can be prepared with drinking water, contain higher Mn concentrations than human milk, as well as evidence that neonates absorb more Mn, excrete less absorbed Mn, and the absorbed Mn more easily passes the blood-brain barrier (CitationUSEPA 1996a). IOM's and USEPA's HRVs are essentially the same for all ages except birth to < 1 year; to prevent high levels of Mn intake among infants IOM recommends the only source of intake for infants should be from food or formula (CitationIOM FNB 2001). IOM's AI method is based on the observation that there have been no reported case of symptoms of Mn deficiency in newborns exclusively fed breast milk; therefore, IOM's AI is set according to the average breast milk consumption volume (0.78 l/day) multiplied by the average Mn concentration in human milk (3 μg/l) (CitationIOM 2001). IOM did not derive an UL for infants < 1 year due to the lack of data and concern about infants' ability to handle excess amounts of Mn. When the IOM AI is converted to a reference dose (AI (0.003 mg Mn/day) ÷ life-stage body weights (0 < 1 month, 1 < 3 months, and 3 < 6 months)) there is up to a 100-fold difference between USEPA's RfD (0.047 mg/kg/day) and the converted IOM age-specific dose (0.00048 mg/kg/day). Despite method differences in the derivation of HRVs, a smaller difference is seen when USEPA's drinking water RfD is compared to OEHHA's child-specific non-dietary reference dose (chRD) for Mn, 0.047 mg/kg/day and 0.03 mg/kg/day, respectively. The degree of variation between the HRVs highlights the need for additional research to determine an appropriate reference value for Mn, given that most potentially exposed populations include infants and children.
A statistical comparison between formula-fed and breast-fed children is not possible due to exposure scenario differences. The exposure scenarios for the formula-fed infants were 1 year longer in duration based on the assumption that breast-fed infants' exposure to drinking water is negligible during the first year of life (see Methods section for rationale); accordingly, HQs for breast-fed infants were based on six and 20 year exposure scenarios. Acknowledging the difference in duration and the application of different HRVs, estimated hazards for each of the two groups exhibited a wide range when different HRVs were applied; HQs calculated using IOM AI values and the USEPA RfD for the formula-fed children (HQs 7-year duration = 1.5 to 30 and 21-year duration = 0.5 to 11.4) were greater than those calculated using the USEPA RfD for the breast-fed children (HQs 6-year duration = 0.03 to 3.9 and 20-year duration = 0.02 to 2.6) in all high and central tendency exposure scenarios. Observed differences were small between formula-fed and breast-fed children when comparing only the HQs calculated using the USEPA RfD (e.g., HQs for the formula-fed, high exposure scenario infants 7-year duration = 3.2 to 3.8 and 21-year duration = 2.1 to 2.5; HQs for breast-fed, high exposure scenario infants 6-year duration = 3.2 to 3.9 and 20-year duration = 2.3 to 2.6). This observation is related to two characteristics of the methodology used: the USEPA RfD does not account for early life susceptibility in the same way as the IOM AI values, and the first year of life is a relatively short duration in a chronic exposure scenario.
It maybe reasonable to use the IOM infant AI values and the USEPA RfD to evaluate subchronic exposure scenarios, such as for the first year of life or specific age groups, because oral subchronic reference values are not currently available and due to the nature of the data used to derive these values (e.g., IOM AI values for 0–6 months are based on breast feeding studies where Mn breast milk concentrations were observed up to 5 months; CitationIOM 2001). In the calculation of HQs for the chronic scenarios, shorter duration HQs for each age group (birth to < 1 month, 1 to < 3, 3 to < 6, 6 to < 12 months, 1 to < 2, 2 to < 3, 3 to < 6, 6 to < 11, 11 to < 16, 16 to < 21 years) were calculated using Eq. (Equation6A, Equation6B).
When individual age groups were considered a sub-chronic exposure scenario for formula-fed infants and IOM's AI values were used as the benchmark, then observed HQs were as high as 437 (Iowa high exposure scenario for the sub-chronic duration of 1 to < 3 months of age), and subchronic HQs in all states remain greater than one for all age groups in the high exposure scenario. During the same infant time period, the HQs from sub-chronic Mn drinking water exposures in breast-fed infants are assumed to be negligible based on AAP recommendations of exclusive breast feeding (CitationAAP 2005). When Mn concentrations in infant feeding formula are compared to Mn breast milk, differences in the potential dose for the first year of life are also apparent. Numerous studies have shown Mn concentrations in infant formulas at levels 3-fold to more than 100-fold higher than in breast milk (CitationCollip et al. 1983; CitationStastny et al. 1984; Lonnderal 1994); in this study, the infant formula Mn concentration (15 μg Mn/0.148 l without the addition of Mn from drinking water; ) was approximately 34-fold greater than the average breast milk Mn concentration (3 μg Mn/l; CitationIOM 2000). Also of importance, studies have found that formula-fed infants consume, absorb and retain more Mn per day than breast-fed infants (Statsny et al. 1984; CitationDorner et al. 1989). Differences in absorption may be attributed to the transport mechanisms that allow for the uptake of Mn across the gastrointestinal tract since they are likely to differ between formula-fed infants and breast-fed infants (CitationErikson et al. 2007).
Data are not available to indicate whether high drinking water Mn concentrations ingested by a nursing mother may be passed into breast milk (CitationATSDR 2000). This is an important consideration because lactating women have higher drinking water ingestion rates than non-pregnant and non-lactating women (CitationUSEPA 2004).
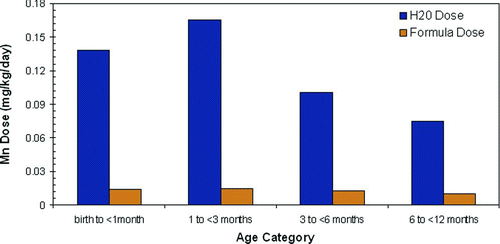
It has been well documented that Mn concentrations are greater in infant formula than in breast milk; however, when the high exposure scenario (95th percentile intake rates and drinking water concentrations) Mn drinking water doses are compared to the Mn powdered formula doses, the drinking water concentrations are the greater source of Mn exposure for formula-fed children less than 1 year of age (). In this scenario, the potential dose from drinking water intake in Iowa is 11-fold greater than the potential dose from the formula. The doses from Mn in drinking water, as shown in , are greater than USEPA's RfD (0.047 mg/kg/day) during the first year of life and IOM's AI age specific values (birth to < 1 month, 4.8 E-04 mg/kg/day; 1 to < 3 months, 4.11 E-04 mg/kg/day; 3 to < 6 months, 3.30 E-04 mg/kg/day) during the first 6 months of life. Where median intake rates and drinking water concentrations are used to assess potential health hazard, the Mn dose from infant feeding formulas was equal to or greater than the dose from drinking water in all three states. The Mn drinking water concentrations used in this assessment were up to 11 times higher than USEPA's current secondary standard for Mn (0.05 mg/l); if the secondary standard was implemented by PWSs, then this would lower children's excess exposures to Mn through drinking water.
Figure 6 Comparison of Mn doses in water and formula in high exposure scenario (95th percentile for both intake and drinking water concentrations) in Iowa).
In addition to the USEPA and IOM HRVs used in the assessment, the World Health Organization's (WHO) has also evaluated the Mn health effects database and established a guideline value for Mn in drinking water (400 μg Mn/l; CitationWHO 2006). Similar to the results reported in the current assessment, a recent review of the WHO guideline presented concern for risk to infants at the current guideline concentration because of the potential for neurotoxicity, and also because reconstituted infant formula generally contains elevated Mn concentrations, even if Mn concentrations are not elevated in drinking water (CitationLjung and Vahter 2007). In their review of the data supporting the WHO value these authors identified a number of unreliable assumptions and concluded a re-evaluation and consideration of a lower guideline value are warranted.
LIMITATIONS AND UNCERTAINTIES
There are a number of limitations and uncertainties associated with this study because several assumptions were made throughout the analysis, including: (1) the use of only published HRVs (i.e., USEPA's RfD and IOM's AIs), which were finalized prior to the publication of recent epidemiological studies that may impact the current RfD and AI values; (2) the use of point estimates (i.e., central tendency and 95th percentile) in the analysis opposed to a probabilistic distributions; (3) the use of median Mn concentrations in powdered infant formulas (100 μg/l) instead of high Mn concentrations (> 170 μg/l, soy-based and milk-based allergy formulas); (4) the evaluation of drinking water monitoring data for Mn from only groundwater sources because similar data for surface water are not available; (5) limited information regarding daily water consumption by children in the available epidemiological studies allowed limited comparison between exposures in the epidemiological studies and this modeling study; (6) few studies have assessed the potential for adverse health effects among children who are exposed to Mn through drinking water; (7) inability to account for additional sources of Mn exposure (i.e., air) or the possible concurrent exposure to other neurotoxicants; (8) scenarios used to assess infant feeding are the most absolute cases, as many infants receive a combination of breast milk and formula.
CONCLUSIONS
The use and importance of children's exposure factors assessment has been discussed in the risk assessment literature (CitationFirestone et al. 2007; CitationDaston et al. 2004; CitationLandrigan et al. 2004; CitationGinsberg et al. 2004: CitationMiller et al. 2002); however, this analysis adds to that literature by demonstrating the importance of applying recently established age groups in children's exposure assessment and recently revised age-specific drinking water intake rates in children's risk assessment (CitationUSEPA 2006a,Citationb). These new age-specific drinking water intake rates (normalized to body weight) indicate that the youngest infants ingest up to eight times more water (on a ml/kg basis) than the default “adult” drinking water intake (2 l/day drinking water intake; 70 kg body weight). These child-specific intake rates can lead to even greater estimated risks when considered various HRVs, including the population-based USEPA RfD (CitationUSEPA 1996a) as well as life stage–based values from the CitationIOM FNB (2001) and California EPA (CitationOEHHA 2006). In this way, this analysis is also novel in using Mn exposure as a case study for illustrating how the use of different drinking water exposure scenarios can result in a greater estimated potential for adverse health effects among different age groups.
Mn is a good case study because it is an essential nutrient that is naturally occurring in food and drinking water, but Mn is also neurotoxic at elevated doses and children may be more sensitive to this effect. While the exposure assessment in this analysis is new, the HRVs may be updated because they were established prior to recent epidemiological studies that have shown an association between elevated Mn concentrations in drinking water and altered neurological function in children. The application of the existing HRVs to early life drinking water exposure scenarios illustrates greater estimated health risk among certain age groups, with infants having the greatest potential for adverse health effects. These results suggest that it is appropriate to use the IOM AI as the HRV for infants less than 1 year, based on the infant approach used to develop this value and on the marked differences in estimated HQs.
In this analysis the estimated HQs for infants were greatest when assessing sub-chronic exposure scenarios (HQ up to 240); however, the estimates were also increased in childhood exposure scenarios of chronic duration (≥ 7 years duration; and ). The estimated HQs were higher for formula-fed infants than for breast-fed infants, with HQs > 1 for formula-fed infants even in the central tendency exposure scenario. Mn is a required nutrient for infant formula (21 Code of the Federal Register [CFR] § 107.10): however, additional intake due to Mn concentrations in the drinking water that is used to reconstitute powdered formulas can lead to excessive Mn intakes for formula-fed infants (e.g., ). While chronic duration childhood exposure scenarios that included formula-feeding yielded the higher estimated doses of Mn when compared to breast-fed children, the analysis indicated that estimated breast-fed child exposures for the chronic scenario can also exceed pre-existing HRVs.
Children's health risk assessment is an issue of on-going interest, which continues to benefit from new scientific developments. Continued research, as well as the potential re-evaluation of reference values, presents the opportunity for further analysis of the application of children's drinking water intake rates and the resulting health risks, and ultimately the potential to protect children's health. In the interim, special concerns for children's health have been expressed in the on-going dialogue about the appropriate Mn drinking water guideline value (CitationLjung and Vahter 2007) and when public health agencies take action to raise awareness of Mn in drinking water (CitationWI DHFS 2007).
ACKNOWLEDGMENT
The authors wish to thank Joyce Donohue, Jacqueline Moya, and Michael Firestone for their expert advice in the preparation of this manuscript.
The views and opinions expressed in this article are those of the authors and are not necessarily representative of an official position of the U.S. Environmental Protection Agency.
Notes
*Case study of a ten year old boy; NA, no available data.
*Mn concentrations only refer to the powder itself (CitationSievers 2005).
REFERENCES
- AAP (American Academy of Pediatrics) . 2005 . Policy statement: Breastfeeding and the use of human milk . Pediatrics , 115 : 496 – 506 .
- ATSDR (Agency for Toxic Substances and Disease Registry) . 2000 . Toxicological Profile for Manganese. Chapters 1, 2, and 5 , Atlanta, GA, , USA : US Department of Health and Human Services . Available at http://www.atsdr.cdc.gov/toxprofiles/tp151.html
- Bouchard , M , Laforest , F Vandelac , L . 2007 . Hair manganese and hyperactive behaviors: Pilot study of school-age children exposed through tap water . Environ Health Perspect , 115 ( 1 ) : 122 – 7 .
- Brenneman , K A , Cattley , R C Ali , S F . 1999 . Manganese-induced developmental neurotoxicity in the CD rat: Is oxidative damage a mechanism of action? . Neurotoxicology , 204 : 77 – 488 .
- CDC (Centers for Disease Control and Prevention) . 2007 . “ Breastfeeding Report Card, United States—2007 ” . In Outcome Indicators United States National Immunization Survey, 2004 Births, CDC Available at http://www.cdc.gov/breastfeeding/data/report_card2.htm
- Collip , P , Chen , S and Matinsky , S . 1983 . Manganese in infant feeding formulas and learning disabilities . Ann Nutr Metab , 27 : 488 – 94 .
- Daston , G , Faustman , E Ginsberg , G . 2004 . A framework for assessing risks to children from exposure to environmental agents . Environ Health Perspect , 112 ( 2 ) : 238 – 56 .
- Davis , C D and Greger , J L . 1992 . Longitudinal changes of manages dependent superoxide dismutase and other indexes of manganese and iron status in women . Am J Clin Nutr , 49 : 170 – 9 .
- Dorman , D , Struve , M Vitarella , D . 2000 . Neurotoxicity of manganese chloride in neonatal and adult cd rats following subchronic (21-day) high dose oral exposure . J App Toxicol , 201 : 79 – 87 .
- Dorner , K , Dziadzka , S Hohn , A . 1989 . Longitudinal manganese and copper balances in young infants and preterm infants fed on breast-milk and adapted cow's milk formula . Br J Nutr , 61 : 559 – 72 .
- Erikson , K , Thompson , K Aschner , J . 2007 . Manganese neurotoxicity: A focus on the neonate . Pharmacol Ther , 113 ( 2 ) : 369 – 77 .
- Firestone , M , Moya , J Choen-Hubal , E . 2007 . Identifying childhood age groups for exposure assessments and monitoring . Risk Anal , 27 : 701 – 14 .
- Freeland-Graves , J H , Bales , C W and Behmardi , F . 1987 . “ Manganese requirements of humans ” . In Nutritional Bioavailability of Manganese , 90 – 104 . Washington, DC, , USA : American Chemical Society .
- Gibson , R S and De Wolf , M S . 1980 . The dietary trace metal intake of some Canadian full-term and low birth weight infants during the first twelve months of infancy . J Canadian Dietary Assoc , 41 : 206 – 15 .
- Ginsberg , G , Slikker , W Bruckner , J . 2004 . Incorporating children's toxicokinetics into a risk framework . Environ Health Perspect , 112 ( 2 ) : 272 – 82 .
- Golub , M , Casey , H Germann , S . 2005 . Neurobehavioral evaluation of rhesus monkey infants fed cow's milk formula, soy formula, or soy formula with added manganese . Neurotoxicol Teratol , 27 : 615 – 27 .
- Greger , J L . 1999 . Nutrition versus toxicology of manganese in humans: Evaluation of potential biomarkers . Neurotoxicology , 20 : 205 – 12 .
- Hafeman , D , Factor-Litvak , P Cheng , Z . 2007 . Association between manganese exposure through drinking water and infant mortality in Bangladesh . Environ Health Perspect , 115 ( 8 ) : 1107 – 12 .
- He , P , Liu , D Zhang , G . 1994 . Effects of high-level manganese sewage irritation on children's neurobehavior . Chinese J Prevent Med , 28 ( 4 ) : 216 – 8 .
- IOMFNB (The National Academies Institute of Medicine Food and Nutrition Board) . 2001 . “ Manganese ” . In Dietary Reference Intakes For Vitamin A, Vitamin K, Arsenic. Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc , 394 – 419 . Washington, DC, , USA : National Academies .
- Kawamura , R , Ikuta , H and Fukuzumi , S . 1941 . Intoxication by manganese in well water . Kitasato Arch Exp Med , 18 : 145 – 69 .
- Keen , C L , Ensuna , J L and Clegg , M S . 2000 . “ Manganese metabolism in animals and humans including the toxicity of manganese ” . In Metal Ions in Biological Systems , Edited by: Siegel , H. and Siegel , A. 89 – 121 . New York, NY, , USA : CRC, Marcel Dekker .
- Kondakis , X G , Markis , N Leostinidis , M . 1989 . Possible health effects of high manganese concentration in drinking water . Arch Environ Health , 44 ( 3 ) : 175 – 8 .
- Landrigan , P , Kimmel , C Correa , A . 2004 . Children's health and the environment: Public health issues and challenges for risk assessment . Environ Health Perspect , 112 ( 2 ) : 257 – 65 .
- Ljung , K and Vahter , M . 2007 . Time to re-evaluate the guideline value for manganese in drinking water? . Environ Health Perspect , 115 ( 11 ) : 1533 – 8 .
- Lonnerdal , B . 1994 . “ Manganese nutrition of infants ” . In Manganese Health and Disease , Edited by: Klimis-Tavantzis , D. 175 – 91 . Boca Raton, FL, , USA : CRC Press .
- Mena , I . 1974 . The role of manganese in human disease . Ann Clin Lab Sci , 4 ( 6 ) : 487 – 91 .
- Mergler , D , Baldwin , M Belanger , S . 1999 . Manganese neurotoxicity, a continuum of dysfunction: Results from a community based study . Neurotoxicology , 20 ( 2–3 ) : 327 – 42 .
- Miller , M , Marty , M Arcus , A . 2002 . Differences between children and adults: Implications for risk assessment at California EPA . Int J Toxicol , 21 : 403 – 18 .
- NRC (National Research Council) . 1989 . Recommended Dietary Allowances, , 10th edit , 230 – 5 . Washington, DC, , USA : Food and Nutrition Board, National Research Council, National Academy Press .
- NRC . 1993 . “ Pesticides in the Diets of Infants and Children ” . In Board on Agriculture and Board on Environmental Studies and Toxicology , Washington, DC, , USA : National Research Council, National Academy Press .
- OEHHA (California Environmental Protection Agency Office of Environmental Health Hazard Assessment) . 2006 . Development of Health Criteria for School Site Risk Assessment Pursuant to Health and Safety Code Section 901 (G): Child Specific Reference Dose (Chrd) for School Site Risk Assessment Edited by: Chan , D . Available at http://www.oehha.ca.gov/public_info/public/kids/pdf/Mn-PCPFinal-070306.pdf
- Phil , R and Parkes , M . 1977 . Hair element content in learning disabled children . Science , 198 : 204 – 206 .
- Sahni , V , Leger , Y Panaro , L . 2007 . Case report: A metabolic disorder presenting as pediatric manganism . Environ Health Perspect , 115 ( 12 ) : 1776 – 9 .
- Sievers , E . 2005 . “ Nutrient minerals in drinking water: implications for the nutrition of infants and young children ” . In Nutrients in Drinking Water (WHO) , 164 – 79 . Geneva, , Switzerland : World Health Organization .
- Stastny , D , Vogel , R S and Picciano , M F . 1984 . Manganese intake and serum manganese concentration of human milk-fed and formula-fed infants . Am J Clin Nutr , : 39872 – 8 .
- Tran , T , Chowanadisa , W Crinella , F . 2002a . Effect of high dietary manganese intake of neonatal rats on tissue mineral accumulation, striatal dopamine levels, and neurodevelopmental status . Neurotoxicology , 23 : 635 – 43 .
- Tran , T , Chowanadisa , W Lonnerdal , B . 2002b . Effect of neonatal dietary manganese exposure on brain dopamine levels and neurocognitive functions . Neurotoxicology , 236 : 45 – 51 .
- USDA (US Department of Agriculture) . 1998 . Continuing of Food Intakes by Individuals: 1994–96, 1998 , Washington, DC, , USA : Agriculture Research Service .
- USEPA (US Environmental Protection Agency) . 1996a . Manganese, CASRN 7439–96-5, IRIS, USEPA Available at http://www.epa.gov/IRIS/subst/0373.htm
- USEPA . 1996b . 1996 National-Scale Air Toxics Assessment Available at http://epa.gov/ttn/atw/nata/gloss1.html
- USEPA . 2003 . Health Effects Support Document For Manganese Available at http://www.epa.gov/safewater/ccl/pdfs/reg_determine1/support_cc1_magnese_healtheffects.pdf
- USEPA . 2004a . Drinking Water Health Advisory for Manganese Available at http://www.epa.gov/safewater/ccl/pdfs/reg_determine1/support_cc1_magnese_dwreport.pdf
- USEPA . 2004b . Estimated Per Capita Water Ingestion and Body Weight in the United States: An Update Available at http://www.epa.gov/waterscience/criteria/drinking/percapita/2004.pdf
- USEPA . 2006 . Guidance on Selecting Age Groups for Monitoring and Assessing Childhood Exposures to Environmental Contaminants (External Review Draft) Available at http://cfpub.epa.gov/ncea/raf/recordisplay.cfm?deid=55887
- USEPA . 2007 . 2007. Glossary of IRIS Terms Available at http://www.epa.gov/iris/gloss8.htm
- USEPA . 2008 . Child-Specific Exposure Factors Handbook Available at http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=199243
- Wasserman , G A , Liu , X Parvez , F . 2006 . Water manganese exposure and children's intellectual function in Araihazar, Bangladesh . Environ Health Perspect , 114 ( 1 ) : 124 – 9 .
- WI DHFS (Wisconsin Department of Health and Family Services) . 2007 . Manganese in Drinking Water Available at http://www.dhfs.state.wi.us/eh/water/fs/manganese.pdf
- WHO (World Health Organization) . 2006 . Guidelines for Drinking—Water Quality, Third Edition, Incorporating First Addendum Available at http://www.who.int/water_sanitation_health/dwq/gdwq3rev/en/index.html
- Woolf , A , Wright , R Amarasiriwardena , C . 2002 . A child with chronic manganese exposure from drinking water . Environ Health Perspect , 110 : 613 – 6 .
- Zhang , G , Liu , D and He , P . 1995 . A preliminary study of the effect of manganese on learning abilities of primary school pupils . Chinese J Preventive Med , 29 ( 3 ) : 156 – 8 .