Abstract
Introduction
Hemiplegic shoulder pain (HSP) is reported in up to 40% of people with stroke and has been associated with spasticity and glenohumeral subluxation. The frequency of HSP has reduced in the last two decades which is most likely due to improved therapy and nursing care. The aim of this systematic literature review was to explore the risk and associated factors for HSP for studies published between 2005 and 2020.
Methods
A systematic online search was conducted of CINAHL, AMED, MEDLINE and the Cochrane library databases using four key terms (risk factors, hemiplegia, shoulder pain and stroke). The search was supplemented by hand searching of relevant journals and citation tracking of the retrieved papers. All primary studies published in English language fulfilling the review’s inclusion criteria were included. Five reviewers extracted the data and independently appraised the methodological quality of the selected studies. Any discrepancies were resolved following discussions.
Results
Of the 50 articles that were identified, 21 studies met the criteria. The common risk factors for HSP were: poor motor function (odds ratio (OR) 0.58–3.19; 95% confidence interval (CI), 1.1–7.7); glenohumeral subluxation (OR 2.48–3.5, 95% CI 1.38–9.37) and reduced range of movement at the shoulder (OR 0.14–4.46, 95% CI 0.99–64).
Conclusion
Despite methodological flaws, complete loss of motor function in the affected arm and glenohumeral subluxation has been recognized as frequently reported risk factors for HSP. Further rigorously designed cohort studies are required to explore the risk factors for HSP.
Introduction
Stroke is one of the largest causes of disability in the western world [Citation1]. Upper limb impairment is a common feature [Citation2] and shoulder problems are the most important component of upper extremity complications [Citation3]. The shoulder is a highly mobile and less stable joint [Citation4] that is vulnerable to a range of post-stroke secondary musculoskeletal complications such as pain, subluxation and restricted joint range of movement [Citation5].
Hemiplegic shoulder pain (HSP) is one of the common medical complications after a stroke [Citation6, Citation7] with the reported incidence of 1.6 to 40% [Citation8]. HSP is difficult to define and is often used to describe a collection of complex problems and diagnoses [Citation9]. Early occurrence of HSP can have adverse effects on rehabilitation [Citation10] and later, on health-related quality of life [Citation11]. Several causes of HSP have been identified and can be broadly classified into neurological (paralysis, spasticity, altered sensation and neuropathic pain) and mechanical factors (glenohumeral subluxation, rotator cuff injury, muscle imbalance and altered scapula position) [Citation10].
Given the implications of HSP on rehabilitation, recent systematic reviews have focused on the effectiveness of varied treatment approaches including physiotherapy, massage therapy, strapping, slings and other supports to minimize glenohumeral subluxation, and local interventions such as nerve blocks and botulinum toxin type A (BTx-A) intramuscular injections for spasticity [Citation12]. Unfortunately, optimal treatment modalities for various types of HSP remain unclear in the literature [Citation10] and, in practice, linking causation with the most effective intervention/s remains problematic [Citation13].
A better understanding of the multifactorial risk and associated factors for HSP will allow improved management and could potentially aid establishment of early preventative measures for hemiplegic shoulder pain [Citation10]. Two recently published systematic reviews have explored the risk factors for hemiplegic shoulder pain [Citation8, Citation14]. One review [Citation8] focused on incidence and prevalence, however, did not include risk factors in the search terms. Holmes et al. [Citation14] included studies if (a) they were prospective cohort studies, (b) they measured any potential risk factor within the first month after stroke, and (c) they measured pain as a key outcome within 1 year after stroke. Also, both reviews included studies from the date of inception.
In the last two decades, the incidence rate of HSP has reduced [Citation11] in comparison to 16 to 72% in a review published in 2001 [Citation15]. A recent systematic review [Citation8] confirms this decline in incidence rate and one of the potential reason could be due to improved nursing and therapy care. According to a recent UK wide survey of therapists, routine screening for HSP was undertaken by 59 (89%) respondents [Citation16]. Education (positioning, appropriate handling of the affected limb) was provided by 51 (77%) respondents. Therefore, the aim of this systematic literature review was to explore the risk and associated factors for HSP in people with stroke for studies published from 2005–2020.
Methods
Search strategy
The structure of the review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [Citation17]. A systematic literature search was performed using the search platforms Medscape, OT Seeker, OVID online, PEDro, and Science Direct. The databases searched included AMED, MEDLINE, CINAHL and Cochrane library and the grey literature was searched using Google Scholar.
The four key terms included: risk factors, hemiplegia, shoulder pain and stroke. A search string was constructed combining the key terms: ‘stroke or cerebrovascular accident or CVA or CVE or hemiplegia’ and ‘shoulder or glenohumeral or upper extremity or upper limb or arm’ AND ‘risk’ or factor or determinant or cause or predictor or pathogenesis or predispose or associate or ‘correlat’ or ‘etiolog’ or incidence or attribute AND range of movement or ROM or spasticity or flexibility or loss of muscle strength or muscle atrophy or severity of stroke or shoulder subluxation’ AND ‘shoulder pain or frozen shoulder or glenohumeral joint pain or rotator cuff pain or GHJpain’. Truncations specific to the databases were also used to widen the search and to ensure that all forms of searched words were hit by the search engine. Finally, reference lists of relevant articles were scanned to identify further relevant studies that had not been identified by the initial search.
Articles were selected based on the following inclusion criteria: (1) Studies published between 2005 and 2020, (2) published in the English language, (3) cohort (prospective, retrospective), case-control and cross-sectional studies, (4) all stroke types, (5) any care settings. Studies which had participants below the age of 18 and non-stroke conditions were excluded. Case reports and case series were also excluded as these types of studies might have a high potential for bias.
Study selection process
Five researchers were involved in the study selection process. The title and abstract of each study were read independently by all the researchers to determine relevance. Relevant full papers were then independently scrutinized to check for the eligibility criteria and to confirm final inclusion of the articles into the review. Any discrepancies were discussed until consensus was reached and where appropriate an independent scrutiniser was involved and only the articles deemed relevant by all the research group members were included for the review purpose.
Quality assessment
To select the quality appraisal tool, each reviewer independently critiqued a randomly selected article (not included in the final review) using widely recognised tools such as Critical Appraisal skills programme (CASP) [Citation18], the Scottish intercollegiate Guidelines Network (SIGN) [Citation19] and the Joanna Briggs Institute Critical Appraisal tool [Citation20]. The Joanna Briggs institute critical appraisal tool was finally selected after group discussion. The methodological quality of each of the selected studies was independently appraised by each reviewer. Any discrepancies were discussed until consensus was reached.
Results
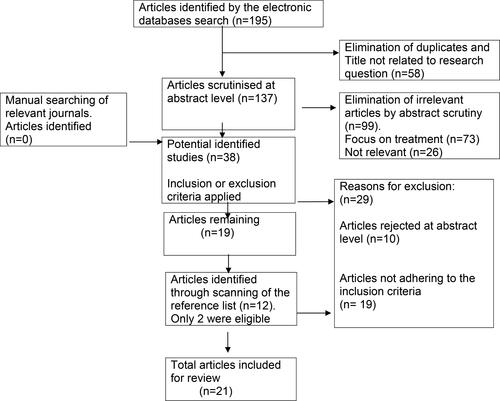
The database search returned 195 studies with a title that related to shoulder pain in people with stroke. There were no articles returned from the Cochrane database and Google Scholar. A further 12 potential articles were identified by searching the reference lists of articles. Of these, 50 potential studies were obtained and scrutinized of which 21 [Citation11, Citation21–40] met the selection criteria. summarizes the results of the search strategy, including the reasons for exclusion of studies from the review.
Description of the studies
The research designs varied considerably across the studies. Seven studies used a cohort design [Citation11, Citation22, Citation23, Citation25, Citation33, Citation34, Citation36], six used a case-control design [Citation21, Citation24, Citation30–32, Citation37], another six used a cross-sectional design [Citation26, Citation27, Citation29, Citation35, Citation38, Citation40], and two others used a retrospective design [Citation28, Citation39].
Participants
Although all 21 studies included patients with stroke, several studies did not specify the type of stroke (infarction or haemorrhage). The time since onset of stroke varied from one week to 82 weeks. The time of follow up across the included cohort studies ranged from four to 70 weeks. The age of the patients ranged from 18 to 102 years across the studies and the mean age varied considerably: between 50–59 years in six studies, 60 and 65 years in 9 studies, 66–70 in three studies and 71–74 years in three studies. All studies had strict inclusion criteria, but the criteria varied considerably across the studies. Sample size varied considerably with the largest sample consisting of 416 patients [Citation22] and smallest consisting of nine [Citation32].
Methodological quality
The methodological quality of the studies varied considerably and some of the studies had serious methodological flaws (). Six out of 21 studies had a sample size ranging from 100–400, but the remaining had a sample size ranging from 9 to 72 and none of the studies provided justification for this. Furthermore, several studies did not state clear participant recruitment criteria, did not justify the rationale for their study design and did not administer appropriate statistical tests; only six studies reported odds ratio [Citation11, Citation23, Citation28, Citation34, Citation36, Citation37] and three [Citation21, Citation22, Citation25] used logistic regression analysis to identify risk factors. Although most studies specified the outcome measures used, only two [Citation31, Citation36] reported using blinding assessment procedures.
Table 1. Quality assessment of included studies in the order of publication.
Outcome measures
A wide range of outcome measures were used in the studies reviewed. Upper limb motor recovery was assessed by Brunnstrom’s motor arm score [Citation23, Citation24, Citation26, Citation27, Citation29, Citation33, Citation36, Citation37, Citation39], upper limb motor function (question 5) from the National Institute of Health Stroke Scale (NIHSS) [Citation11, Citation22, Citation36]. Assessment methods used to assess GHS included radiographic measures [Citation26, Citation30, Citation36], and clinical palpation method [Citation21–23, Citation28, Citation29, Citation33, Citation34, Citation37]. Passive range of movement (PROM) at the shoulder was assessed using goniometery in two studies [Citation25, Citation28, Citation34], while other studies did not specify. Soft tissues in the shoulder were evaluated using sonography [Citation27, Citation29, Citation33, Citation36, Citation39], and magnetic resonance imaging [Citation30]. The Modified Ashworth Scale (MAS) was used to assess muscle tone [Citation23, Citation24, Citation26, Citation29, Citation32–39]. To quantify movement of the shoulder, arm and thorax, an electromagnetic tracking device was used in one study [Citation24] and a computer-based kinematic technique was used in another study [Citation32].
Similarly, a wide range of assessment methods were used to identify HSP. The majority of the studies used vertical visual analogue scale (VAS), however, eight of these used 0–10 scale [Citation25, Citation29, Citation31, Citation37, Citation40], while three studies used 0–100 scale [Citation11, Citation22, Citation26, Citation34]. Other assessment approaches included numerical rating scale (NRS) [Citation32, Citation36], pain on rest and during passive movement recorded as present or absent [Citation21, Citation29, Citation30, Citation37], and three studies did not specify [Citation23, Citation27, Citation39].
Study results
A variety of risk and associated factors were identified in the included studies (). The most frequent risk factors for HSP were poor arm motor function [Citation11, Citation22, Citation28, Citation36, Citation37], glenohumeral subluxation [Citation21, Citation23, Citation36], reduced passive range of movement at the shoulder flexion [Citation28], and shoulder abduction [Citation34].
Table 2. Summary of included studies in the order of publication.
In one of the largest longitudinal studies of 226 patients, absence of arm motor function was strongly associated with the risk of HSP (odds ratio (OR) 3.19; 95% confidence interval (CI), 1.77–6.9) [Citation11]. Similar findings were reported by another longitudinal study of 51 patients that found poor arm motor function as indicated by a poor NIHSS item 5 score (OR = 3.0; 95% CI, 1.1 to 7.7) was a risk factor for HSP [Citation36]. The findings support those of other prospective [Citation22], case-control [Citation37], and retrospective [Citation28] studies that found that poor initial motor function was an independent factor associated with HSP.
Of the studies that identified a link between GHS and HSP, one of the largest prospective studies of 327 patients reported an odds ratio (OR) of 2.48 (95% confidence intervals, 1.38–4.46) suggesting GHS as a potential risk factor for HSP [Citation23]. Similar findings were reported by a longitudinal study that reported an OR = 3.5; 95% CI = 1.4–9.3 at baseline and OR = 2.6; 95%CI = 1.0–6.6 at follow-up [Citation36]. Similarly, a case–controlled study of 107 stroke patients reported that that HSP was significantly higher in the subluxed group and that GHS was independently associated with HSP at follow-up (R2 = 0.458; p < 0.001) [Citation21]. The findings support those of several other longitudinal [Citation22], cross-sectional [Citation38], studies which found an association between GHS and HSP. However, two studies [Citation26, Citation37] reported GHS was not independently associated with HSP.
Reduced ROM was reported as a risk factor for HSP. Decreased passive abduction at 4 months (OR 4.46; 95% CI 0.99–20.10) was reported as a significant risk factor in a prospective study [Citation32]. Similarly, in a retrospective study [Citation28], reduced passive shoulder flexion ROM was found as a risk factor (OR = 0.14, 95% CI 3 to 64) for HSP.
The other risk factors were soft tissue injuries (bicipital tendonitis and rotator cuff tears) [Citation36], pre-morbid shoulder pain [Citation11], left-sided hemiparesis [Citation34], frequency of pain [Citation34], longer duration of disease [Citation37], late initiation of rehabilitation [Citation37], adhesive capsulitis [Citation36], complex regional pain syndrome (CRPS) [Citation26], positive baseline objective assessments (passive external rotation, hand behind neck, the modified Neer test) [Citation11], increased light touch and vibration threshold [Citation25]. In addition, other factors associated with HSP were: spasticity [Citation29, Citation33, Citation38] diabetes mellitus [Citation40], self-perceived ill health [Citation22, Citation25] reduced external rotation ROM at shoulder [Citation29, Citation32], loss of proprioception [Citation29], and higher pain thresholds [Citation35, Citation38].
Discussion
The aim of this systematic literature review was to explore the risk and associated factors for HSP for studies published between 2005 and 2020. The most frequently reported risk factors were poor arm motor function, glenohumeral subluxation, and reduced passive range of movement. The other associated factors were adhesive capsulitis, soft tissue injuries, spasticity, higher pain thresholds and self-perceived ill-health.
Five studies reported that patients with poor or reduced arm motor function had a significantly greater risk of developing HSP on the stroke affected side [Citation11, Citation22, Citation28, Citation36, Citation37]. However, the time points when this was measured varied considerably across the studies. Irrespective of that, the loss of motor function could alter the kinetics and kinematics around the shoulder complex. The suboptimal performance of scapula kinesis and the reduced control of forces around the humeral head on the glenoid has the potential to lead to harmful effects on anatomical structures around the shoulder [Citation41]. Post-stroke loss of motor function can lead to shoulder instability and immobility, which can cause pain directly or place the capsule at risk of trauma, subsequently leading to pain [Citation42].
GHS was a potential risk factor for HSP as reported by three studies [Citation21, Citation23, Citation36]. GHS appears to be caused by a lack of adequate support of the shoulder while the patient is in the upright position [Citation43]. A previous systematic review reported that complete loss of motor function/severity of arm paralysis and apparent absence of supraspinatus contraction are potential risk factors for GHS [Citation44]. The most important function of supraspinatus is to stabilize the humeral head in the glenoid cavity [Citation45]. Tissue damage in the shoulder region may be related to the increase in joint space due to GHS causing passive overstretching and resultant injury and pain [Citation22]. There is some evidence from randomized controlled trials to support the effectiveness of therapeutic interventions including electrical stimulation/functional electrical stimulation of supraspinatus muscle that can reduce/prevent/delay GHS [Citation46]. Two studies had a sound methodology using a prospective design [Citation23, Citation36], a large sample size [Citation23], appropriate statistical tests [Citation21, Citation23, Citation36] and followed patients between 26–64 weeks [Citation23, Citation36]. However, the assessors were not blind, and the outcome measures were not clearly described.
Limited passive range of movement (flexion/abduction) was another reported risk factor for HSP but only two studies reported odds ratio [Citation28, Citation34]. Patients with severe impairment and activity limitations in the upper limb early after stroke are significantly associated with poorer upper limb outcomes [Citation47]. Over time, the central nervous system as well as muscle tissue of the arm adapt to this state of inactivity, often resulting in hypertonia [Citation48, Citation49] and contractures [Citation50] resulting in reduced passive range of movement.
Soft tissue abnormalities (biceps tendon effusion and supraspinatus tendinosis/tear) as assessed using ultrasonography were associated risk factors for HSP [Citation33, Citation36]. The tendon of the supraspinatus runs under the acromion [Citation45] and is susceptible to compression. Degenerative changes are common in rotator cuff muscles and the prevalence of rotator cuff tears increases in people with stroke [Citation51]. A recent study reported that patients with stroke (n = 55) with muscle strength ≤3 on the Medical Research Council grading scale were more likely to have shoulder pain and rotator cuff tears [Citation50]. In addition, Haung et al. [Citation52] found that GHS lateral distance, measured by physical examination, was a predictor for supraspinatus tendonitis. Ultrasound, in addition to diagnosing soft-tissue injuries has the potential to assess GHS by measuring the acromion-greater tuberosity distance, as it may be more sensitive than physical examination [Citation53] and thus can facilitate management of HSP.
Adhesive capsulitis was another risk factor for HSP. The reported incidence of adhesive capsulitis in people with HSP is up to 57% [Citation54, Citation55]. A painful hemiplegic shoulder can develop adhesive capsulitis due to immobilization, disuse atrophy, contracture, or varying degrees of disability [Citation26]. A recent study on patients with stroke (n = 23) reported that rotator cuff tears and adhesive capsulitis might be linked to CRPS [Citation56], while others have found a link between loss of motor control and CRPS [Citation26]. These finding suggests that, to address the multi-factorial nature of HSP, attention should be focused on maintaining shoulder ROM and improving muscle strength.
Poor handling was earlier considered as a contributing factor to HSP in patients who needed help with transfers [Citation15]. In a recent online survey of UK therapists (n = 66), it was reported that positioning (n = 62, 94%), education (n = 51, 77%) regarding appropriate handling to staff, carers/family members was one of the key interventions for HSP [Citation16]. This SLR did not identify poor handling as a potential factor to the HSP suggesting improved awareness among staff and family members regarding appropriate handling.
Overall methodological quality of the studies also needs to be considered, when determining the outcome. A major limitation of the studies reviewed was a lack of description of the methods used to justify sample size. In addition, most of the studies do not report appropriate statistical analysis undertaken to investigate the risk factors. Furthermore, a wide variety of outcome measures were used in the studies reviewed for various clinical outcomes. While most studies assess HSP by visual analogue [Citation25, Citation29, Citation31, Citation37, Citation40] or numerical rating scale [Citation32, Citation36], some do not specify the method used to assess pain [Citation23, Citation27, Citation39]. Measuring pain in people with stroke is a challenge because of its inherently subjective nature. Visual analogue scales are generally reported to have high reliability and validity, however, the validity of their use in stroke patients has been questioned [Citation57, Citation58]. A structured process is therefore required that will facilitate people with HSP to comprehensively describe the nature and impact of their problem. Accurate clinical assessment is vital as this will help improve patient-clinician communication and help establishing targeted management plans [Citation59].
Limitations of this review
The current literature review included all primary data collection studies with all types of study design that were relevant to the aims of the review. The heterogeneity of the studies has made comparability between studies very challenging. The articles published in a language other than English were not included, language bias therefore cannot be excluded.
Implications for practice
People with stroke with persistent motor impairment should be educated regarding positioning and appropriate handling of the affected arm. Patients with little voluntary function may benefit from neuromuscular electrical stimulation. A recent randomized controlled trial (RCT) reported improvement in pain but not in joint range of motion, arm function and activities of daily living after application of electrical stimulation in 36 patients with stroke [Citation60]. Also, given the role of rotator cuff muscles (supraspinatus, infraspinatus, teres minor) in shoulder stability, early rehabilitation programmes should target these muscles to both prevent and reduce secondary complications such as HSP. Evidence from people with shoulder pain in the general population suggests that using concentric and eccentric exercises to rotator cuff muscles are effective in reducing shoulder pain [Citation61, Citation62].
Implications for future research
This systematic literature review has highlighted an apparent paucity in appropriately designed clinical studies on risk factors for HSP. Further rigorously designed research studies using longitudinal cohort design, conducted at multiple rehabilitation centres and over a longer period of time are required. Pain may change over time and therefore their prevalence could be different according to the stage of recovery following stroke. In addition, studies should consider using appropriate statistical tests such as logistic regression analysis/odds ratio to identify potential risk factors for HSP. By doing a robust holistic assessment on symptoms and impact of HSP, other biopsychosocial issues associated with HSP may also be identified that would otherwise be missed.
Conclusion
Despite Hemiplegic shoulder pain being a recognised complication post-stroke, only 21 articles, with heterogeneous designs were identified which investigated the risk factors for HSP, indicating a lack of high-quality research in this area. Despite methodological flaws, complete loss of motor function in the affected arm and glenohumeral subluxation has been recognized as frequently reported risk factors for HSP. Further rigorously designed epidemiology studies (cohort design) are required to explore the risk and associated factors for HSP.
Acknowledgments
This project has been undertaken as part of an undergraduate research study on the BSc (Hons) Physiotherapy programme at the University of the West of England, Bristol. The authors would like to thank Kira Mills and Alexander Pascoe for their help with the systematic review process and Mr Robert Jones, Quality Improvement and Engagement Manager, Stroke Reconfiguration Programme, Bristol North Somerset and South Gloucestershire Clinical Commissioning Group for his critical review of the manuscript.
Disclosure statement
Authors report no conflict of interest.
Additional information
Funding
Notes on contributors
Praveen Kumar
Dr Praveen Kumar is a Senior Lecturer in Physiotherapy at the University of the West of England (UWE), Bristol. Praveen’s passion for improving patient care led him to secure funding from UWE to undertake his PhD on people with stroke. His research interests include upper limb rehabilitation (Lycra Sleeve, electrical stimulation), hemiplegic shoulder pain, glenohumeral subluxation, physical activity/exercises, robotic and virtual rehabilitation in stroke. Praveen has several peer-reviewed publications. He is a peer-reviewer for international journals and also reviews grant application. Praveen’s growing research expertise and portfolio has seen me contribute to the 4th (2012) and 5th (2016) National RCP Stroke Guidelines. Currently, he is a board member for the Association of Chartered Physiotherapists in Neurology (ACPIN) and editor for Synapse-ACPIN journal.
Chiara Fernando
Mrs. Chiara Minoli Fernando is a physiotherapist at Sussex Community NHS Foundation Trust. Chiara graduated from the University of the West of England (UWE) Bristol with a Bachelor of Science honours degree in Physiotherapy. Currently she is a member of Chartered Society of Physiotherapists. Chiara’s Passion for learning and expanding her skills as a physiotherapist has led her to participate in an extensive number of education courses. Similarly, through her profession, she is keen to engage in wide range of research studies to facilitate outstanding patient care through evidence-based practice. Her research interests include physical exercises/activities, upper limb rehabilitation and improving mobility after stroke.
Deanna Mendoza
Deanna Mendoza graduated with a Bachelor of Science (Honours) degree in Physiotherapy in 2020 from the University of the West of England, where she also received a Dean’s List award. She is currently a Musculoskeletal (MSk) Physiotherapist working at the Somerset NHS Foundation Trust and has experience in diagnosing and treating a wide range of MSk conditions. She is also a member of The Chartered Society of Physiotherapy. Deanna has co-presented new research in national and world conferences including the UK Stroke Forum 2021, the Association of Chartered Physiotherapists in Neurology (ACPIN) Online Conference 2021, as well as the World Stroke Congress 2021.
Riya Shah
Riya Shah is a Band 5 rotational physiotherapist at Gloucestershire Hospitals NHS Foundations trust. She recently qualified from the University of the West of England with BSc (Hons) Physiotherapy. Her passion lies in providing people with equal access to healthcare around the world.
References
- Kwakkel G, Wagenaar RC, Twisk JWR, et al. Intensity of leg and arm training after primary Middle-cerebral-artery stroke: a randomised trial. Lancet. 1999;354(9174):191–196.
- Lincoln NB, Parry RH, Vass CD. Randomized, controlled trial to evaluate increased intensity of physiotherapy treatment of arm function after stroke. Stroke. 1999;30(3):573–579.
- Shepherd RB, Carr JH. The shoulder following stroke: preserving musculoskeletal integrity for function. Top Stroke Rehabil. 1998;4(4):35–53.
- Palastanga N, Field D, Soames R. Anatomy and human movement: structure and function. 2nd ed. Oxford: Butterworth-Heinemann; 2013.
- Brandstater EM. Stroke rehabilitation. In: DeLisa JA, Gans BM, editors. Physical medicine and rehabilitation. Principles and practice. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. p. 1655–1676.
- Janus-Laszuk B, Mirowska-Guzel D, Sarzynska-Dlugosz L, et al. Effect of medical complications on the after-stroke rehabilitation outcome. NeuroRehabilitation. 2017;40(2):223–232.
- McLean DE. Medical complications experienced by a cohort of stroke survivors during inpatient, tertiary-level stroke rehabilitation. Arch Phys Med Rehabil. 2004;85(3):466–469.
- Anwer S, Alghadir A. Incidence, prevalence, and risk factors of hemiplegic shoulder pain: a systematic review. IJERPH. 2020;17(14):4962.
- Bender L, McKenna K. Hemiplegic shoulder pain: defining the problem and its management. Disabil Rehabil. 2001;23(16):698–705.
- Vasudevan JM, Browne BJ. Hemiplegic shoulder pain: an approach to diagnosis and management. Phys Med Rehabil Clin N Am. 2014;25(2):411–437.
- Adey-Wakeling Z, Liu E, Crotty M, et al. Hemiplegic shoulder pain reduces quality of life after acute stroke: a prospective population-based study. Am J Phys Med Rehabil. 2016;95(10):758–763.
- Viana R, Pereira S, Mehta S, et al. Evidence for therapeutic interventions for hemiplegic shoulder pain during the chronic stage of stroke: a review. Top Stroke Rehabil. 2012;19(6):514–522.
- Holmes RJ, Connell LA. A survey of the current practice of intramuscular botulinum toxin injections for hemiplegic shoulder pain in the UK. Disab Rehab. 2019;41(6):720–726.
- Holmes RJ, McManus KJ, Koulouglioti C, et al. Risk factors for poststroke shoulder pain: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2020;29(6):104787.
- Walsh K. Management of shoulder pain in patients with stroke. Postgrad Med J. 2001;77(912):645–649.
- Kumar P, Turton A, Cramp M, et al. Management of hemiplegic shoulder pain: a UK-wide online survey of physiotherapy and occupational therapy practice. Physiother Res Int. 2021;26(1):e1874.
- Moher D, Liberati A, Tetzlaff J, The PRISMA Group, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Critical Appraisal Skills Programme (CASP). Appraisal tools [online] [accessed February 2020]. Available from: http://www.phru.nhs.uk/Pages/PHD/resources.htm.
- Scottish Intercollegiate Guidelines Network (SIGN) Appraisal tools, 2019. [online] [accessed February 2020]. Available from: https://www.sign.ac.uk/checklists-and-notes.html.
- Joanna Briggs Institute. Critical appraisal tools. [accessed February 2020]. Available from: https://joannabriggs.org/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Cohort_Studies2017_0.pdf.
- Paci M, Nannetti L, Taiti P, et al. Shoulder subluxation after stroke: relationships with pain and motor recovery. Physiother Res Int. 2007;12(2):95–104.
- Lindgren I, Jönsson AC, Norrving B, et al. Shoulder pain after stroke: a prospective population-based study. Stroke. 2007;38(2):343–348.
- Suethanapornkul S, Kuptniratsaikul PS, Kuptniratsaikul V, et al. Post stroke shoulder subluxation and shoulder pain: a cohort multicentre study. J Med Assoc Thai. 2008;91(12):1885–1893.
- Niessen M, Janssen T, Meskers C, et al. Kinematics of the contralateral and ipsilateral shoulder: a possible relationship with post-stroke shoulder pain. J Rehabil Med. 2008;40(6):482–486.
- Hadianfard H, Hadianfard M. Predictor factors of hemiplegic shoulder pain in a group of stroke patients. Iran Red Crescent Med J. 2008;10:218–222.
- Barlak A, Unsal S, Kaya K, et al. Poststroke shoulder pain in Turkish stroke patients: relationship with clinical factors and functional outcomes. Int J Rehabil Res. 2009;32(4):309–315.
- Lee IS, Shin YB, Moon TY, Jeong YJ, et al. Sonography of patients with hemiplegic shoulder pain after stroke: correlation with motor recovery stage. AJR Am J Roentgenol. 2009;192(2):W40–4.
- Blennerhassett JM, Gyngell K, Crean R. Reduced active control and passive range at the shoulder increase risk of shoulder pain during inpatient rehabilitation post-stroke: an observational study. J Physiother. 2010;56(3):195–199.
- Huang Y, Liang P, Pong Y, et al. Physical findings and sonography of hemiplegic shoulder in patients after acute stroke during rehabilitation. J Rehabil Med. 2010;42(1):21–26.
- Távora DG, Gama RL, Bomfim RC, et al. MRI findings in the painful hemiplegic shoulder. Clin Radiol. 2010;65(10):789–794.
- Pompa A, Clemenzi A, Troisi E, et al. Enhanced-MRI and ultrasound evaluation of painful shoulder in patients after stroke: a pilot study. Eur Neurol. 2011;66(3):175–181.
- Hardwick DD, Lang CE. Scapula and humeral movement patterns and their relationship with pain: a preliminary investigation. Int J Ther Rehabil. 2011;18(4):210–220.
- Pong YP, Wang LY, Huang YC, et al. Sonography and physical findings in stroke patients with hemiplegic shoulders: a longitudinal study. J Rehabil Med. 2012;44(7):553–557.
- Lindgren I, Lexell J, Jönsson AC, et al. Left-sided hemiparesis, pain frequency, and decreased passive shoulder range of abduction are predictors of long-lasting poststroke shoulder pain. PM R. 2012;4(8):561–568.
- Zeilig G, Rivel M, Weingarden H, et al. Hemiplegic shoulder pain: evidence of a neuropathic origin. Pain. 2013;154(2):263–271.
- Kim YH, Jung SJ, Yang EJ, et al. Clinical and sonographic risk factors for hemiplegic shoulder pain: a longitudinal observational study. J Rehabil Med. 2014;46(1):81–87.
- Karaahmet OZ, Eksioglu E, Gurcay E, et al. Hemiplegic shoulder pain: associated factors and rehabilitation outcomes of hemiplegic patients with and without shoulder pain. Top Stroke Rehabil. 2014;21(3):237–245.
- Zeilig G, Rivel M, Doron D, et al. Does hemiplegic shoulder pain share clinical and sensory characteristics with Central neuropathic pain? A comparative study. Eur J Phys Rehabil Med. 2016;52(5):662–671.
- Lin PH. Sonographic findings of painful hemiplegic shoulder after stroke. J Chin Med Assoc. 2018;81(7):657–661.
- Hadianfard M, Taheri P. Association of post-stroke shoulder pain with diabetes mellitus and hyperlipidemia. Phys Med Rehab Electrodiagn. 2018;1:33–36.
- Kibler WB, Sciascia A. The shoulder at risk: scapular dyskinesis and altered glenohumeral rotation. Oper Tech Sports Med. 2016;24(3):162–169.
- Chae J, Mascarenhas D, Yu DT, et al. Poststroke shoulder pain: its relationship to motor impairment, activity limitation, and quality of life. Arch Phys Med Rehabil. 2007;88(3):298–301.
- Ada L, Foongchomcheay A. Efficacy of electrical stimulation in preventing or reducing subluxation of the shoulder after stroke: a meta-analysis. Aust J Physiother. 2002;48(4):257–267.
- Kumar P, Kassam J, Denton C, et al. Risk factors for inferior shoulder subluxation in patients with stroke. Phys Ther Rev. 2010;15(1):3–11.
- Halder AM, Halder CG, Zhao KD, et al. Dynamic inferior stabilisers of the shoulder joint. Clin Biomech. 2001;16(2):138–143.
- Vafadar AK, Côté JN, Archambault PS. Effectiveness of functional electrical stimulation in improving clinical outcomes in the upper arm following stroke: a systematic review and meta-analysis. Biomed Res Int. 2015;2015:729768.
- Coupar F, Pollock A, Rowe P, et al. Predictors of upper limb recovery after stroke: a systematic review and Meta-analysis. Clin Rehabil. 2012;26(4):291–313.
- de Jong LD, Hoonhorst MH, Stuive I, et al. Arm motor control as predictor for hypertonia after stroke: a prospective cohort study. Arch Phys Med Rehabil. 2011;92(9):1411–1417.
- van Kuijk AA, Hendricks HT, Pasman JW, et al. Are clinical characteristics associated with upper-extremity hypertonia in severe ischaemic supratentorial stroke? J Rehabil Med. 2007;39(1):33–37.
- Kwah LK, Harvey LA, Diong JH, et al. Half of the adults who present to hospital with stroke develop at least one contracture within six months: an observational study. J Physiother. 2012;58(1):41–47.
- Yi Y, Shim JS, Kim K, et al. Prevalence of the rotator cuff tear increases with weakness in hemiplegic shoulder. Ann Rehabil Med. 2013;37(4):471–478.
- Huang SW, Liu SY, Tang HW, et al. Relationship between severity of shoulder subluxation and soft-tissue injury in hemiplegic stroke patients. J Rehabil Med. 2012;44(9):733–739.
- Kumar P, Mardon M, Bradley M, et al. Assessment of glenohumeral subluxation in poststroke hemiplegia: comparison between ultrasound and fingerbreadth palpation methods. Phys Ther. 2014;94(11):1622–1631.
- Lo SF, Chen SY, Lin HC, et al. Arthrographic and clinical findings in patients with hemiplegic shoulder pain. Arch Phys Med Rehabil. 2003;84(12):1786–1791.
- Zhu Y, Su B, Li N, et al. Pain management of hemiplegic shoulder pain post stroke in patients from Nanjing, China. Neural Regen Res. 2013;8(25):2389–2398.
- Kim YWM, Kim YM, Kim JMM, et al. Is poststroke complex regional pain syndrome the combination of shoulder pain and soft tissue injury of the wrist? Medicine. 2016;95(31):Pe4388.
- Kahl C, Cleland JA. Visual analogue scale, numeric pain rating scale and the McGill pain questionnaire: an overview of psychometric properties. Phys Ther Rev. 2005;10(2):123–128.
- Pomeroy VM, Tallis R. Neurological rehabilitation: a science struggling to come of age. Physiother Res Int. 2002;7(2):76–89.
- Dromerick AW, Edwards DF, Kumar A. Hemiplegic shoulder pain syndrome: frequency and characteristics during inpatient stroke rehabilitation. Arch Phys Med Rehabil. 2008;89(8):1589–1593.
- Zhou M, Li F, Lu W, et al. Efficiency of neuromuscular electrical stimulation and transcutaneous nerve stimulation on hemiplegic shoulder pain: a randomized controlled trial. Arch Phys Med Rehabil. 2018;99(9):1730–1739.
- Dejaco B, Habets B, van Loon C, et al. Eccentric versus conventional exercise therapy in patients with rotator cuff tendinopathy: a randomized, single blinded, clinical trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(7):2051–2059.
- Cederqvist S, Flinkkilä T, Sormaala M, et al. Non-surgical and surgical treatments for rotator cuff disease: a pragmatic randomised clinical trial with 2-year follow-up after initial rehabilitation. Ann Rheum Dis. 2020;80(6):796–802.