Abstract
One of the widely used approaches for improving the dissolution of poorly water-soluble drugs is particle size reduction. Ball milling is a mechanical, top-down technique used to reduce particle size. The effect of ball number, ball size, and milling speed on the properties of milled Aprepitant is evaluated. A full factorial design was employed to investigate the influence of affecting factors on particle size reduction. The initial suspension was made by suspending the drug in distilled water using excipients followed by milling in a planetary ball mill. Ball size, ball number, and milling speed modulated particle size distribution of Aprepitant. Increasing the number of balls from minimum to maximum for each ball size led to approximately a 28% reduction in mean particle size, a 37% decrease in D90%, and a 25% decrease in the ratio of volume mean particle diameter to numeric mean particle diameter. On average, using 10 mm balls instead of 30 mm balls reduced mean particle size by 1.689 µm. As a result, ball size, ball number, and milling speed are three effective factors in the process of ball milling. By increasing the ball number and decreasing the ball size, efficient micronization of drug particles takes place and the particle size is more uniform.
1. Introduction
The important role of aqueous solubility in drug absorption, and subsequently in bioavailability is well established. This parameter, alongside gastrointestinal permeability, defines whether a drug would deliver the desired effects upon oral administration, or other routes such as parenteral administration should be taken into account (Khadka et al. Citation2014). As roughly 40% of approved drugs and 90% of drug molecules in development are poorly water-soluble, formulating drugs with sufficient bioavailability that would induce anticipated outcomes has become one of the most challenging subjects for pharmaceutical researchers (Khadka et al. Citation2014; Kim et al. Citation2021). Two of the most viable solutions in this regard are lead optimization and formulation redesign; the first option is time-consuming and costly. Therefore, altering the formulation of drugs is preferred in numerous cases (Kumar et al. Citation2018). Improving drug formulation can be related to refining the drug particles or modifying the dosage form (Ganesan et al. Citation2015). Various methods are available to improve the water solubility of drug agents, including, but not limited to; salt forms of the API (Wu et al. Citation2018), lipid-based formulations (Rostamkalaei et al. Citation2019), co-crystal forms (Douroumis et al. Citation2017), and particle size reduction techniques (Shono et al. Citation2010). Particle size reduction is known as one of the most conventional methods used in solubility and/or dissolution rate improvement. The reduction in the size of particles leads to a larger surface-to-volume ratio, increasing the available surface area for water molecules to engulf the drug particles, hence, augmenting the speed by which the drug particles are dissolved in water (Khadka et al. Citation2014). Particle size reduction techniques consist of Top-Down, Bottom-UP, and combination technologies which use both techniques to achieve finer particles (Al-Kassas et al. Citation2017). Media milling is categorized as a Top-Down approach as forces such as crushing and grinding are applied to decrease the particle size (Khadka et al. Citation2014). Wet ball milling is a mechanical particle size reduction technique during which a suspension of drug particles and stabilizers is placed in a container alongside the balls and the rotation of the inner wall of the container causes a collision between drug particles and balls, entailing size reduction. Particles are ground between balls, the container and the balls, or by colliding with the balls (Loh et al. Citation2015; Srivalli and Mishra Citation2016). This method has been applied for manufacturing Aprepitant, an anti-emetic medicine known as Emend® by Merck company (Junyaprasert and Morakul Citation2015). Various parameters in ball milling can significantly impact the resulting particle size and size distribution. The efficacy of the process is influenced by factors such as milling time, the quantity of balls used, the volume of milling medium (i.e. drug suspension), ball size, milling speed, and the quantity of drug material subjected to milling (Peltonen Citation2018).
As a size reduction method that is currently being employed in the industry, it is important to define the parameters that affect the ball milling process and the characteristics of the final particles. Therefore, the purpose of this study is to evaluate the impact of three of the parameters (ball size, ball number, and milling speed) that are likely to have a meaningful effect on the results of ball milling. Assessing these factors, individually and collectively, and defining their influence, is vital for future investigations as applicable data for researchers in upcoming works. The results of this study could be useful in designing oral delivery of Aprepitant.
2. Materials and methods
2.1. Materials
Aprepitant was supplied by Tofigh Daru Research and Engineering Company (Tehran, Iran). Sodium laureth sulfate (SLS) and sucrose were supplied from Merck Chemicals Co. (Darmstadt, Germany). HPC-SL (Viscosity in 20 °C/2% aq. Solution: 3.0–5.9 mPa·s, molecular weight: 100 000) was from Nippon Soda Co. (Tokyo, Japan). Microcrystalline Cellulose PH-101 was provided by NB Entrepreneur (Nagpur, India).
2.2. Experimental design
Parameters were defined as ball size (three levels), ball number (three levels), and milling speed (two levels). Minitab® Statistical Software (version 19, State College, PA) was used to create a general full factorial design that contained 2 three-leveled factors and 1 two-leveled factor. shows each factor and its different states. The number of balls for each size was designated independently according to the fixed volume (approximately) that would be occupied by the balls in each case. The rule was to keep the volume of balls constant for each of the levels of ball number; meaning that for instance, the medium number of 10 mm balls would occupy the same amount of space as the number of 20 mm balls or 30 mm balls. For each of the factors, the Paretro chart was drawn to identify whether they significantly affected the responses (volume mean diameter, D90%, and the ratio of volume mean particle diameter to numeric mean particle diameter) or not. The data with higher R2 were chosen for further assessment. For parameters that were found to be affected by all three factors (ball size, ball number, and rotation speed), the main effect plot was acquired to show the magnitude of the variable’s effect on the responses. Furthermore, interaction plots were used as a means to evaluate the interaction between responses and variables.
Table 1. Factors and factor levels investigated in this study.
To define the ball number for each state, the minimum and maximum values were selected first. Second, the mean of the two numbers was chosen to represent the average number of balls. While selecting, it was not possible to have less than two balls in any of the states as in this case the effect of attrition between two balls would have been eliminated. shows the number of balls used for each ball at minimum, medium, and maximum levels.
Table 2. The number of balls used for each ball size in minimum, medium, and maximum levels.
To define the final suspension volume for each test, the volume of balls was calculated and subtracted from the jar volume (500 ml). The result was divided by two so that the void space volume to suspension volume ratio would be 1:1 and constant for all tests. The order of these tests is shown in .
Table 3. The full factorial design created by Minitab.
In , StdOrder or Standard Order shows the nonrandom order of the tests. RunOrder depicts the order in which the tests were carried out. The quantity of the drug, the weight/weight percentage of the drug in the continuous phase, and the type and amount of excipients were obtained based on the Emend® patent article by Bosch et al. (Citation2012). The drug in the continuous phase of all suspensions was kept between 33% and 36% by weight. illustrates the drug and excipient amounts by weight/weight percent.
Table 4. The percent of Aprepitant and excipients used in the preparation of suspension by weight.
2.3. Suspension preparation
HPC-SL was initially dissolved in distilled water with a magnetic stirrer (Mr Hei-standard, Heidolph, Germany) followed by slow addition of Aprepitant. Next, SLS was added and then, the suspension was left to stir for 20–30 min at 1400 RPM. The last step entailed the addition of sucrose and microcrystalline cellulose along with 5 min of additional stirring. Finally, distilled water was added to the suspension to reach the desired volume.
2.4. Ball milling
Ball milling was conducted using the Planetary Ball Mill (PM 100 Retsch Co., Germany). The 500 ml stainless-steel jar was used in all tests. The milling media consisted of stainless-steel balls with sizes of 10, 20, and 30 mm. The total time of milling was 210 min in all tests. In each run, the planetary ball mill rotated for 3 min and stopped for 15 min to cool down. After each milling run, the direction of rotation was reversed.
2.5. Particle size and size distribution
The particle sizes of the samples were measured by Laser Diffraction Particle Size Analyzer (SALD-2101, SHIMADZU, Japan) and processed by the WingSALD Standard Data Processing program (Wing-1, SHIMADZU). D90% (90% of the total particles are smaller than this size) were acquired as an assessment of particle size. The ratio of volume mean particle diameter to numeric mean particle diameter was calculated and used as a parameter to evaluate particle size distribution.
3. Results
3.1. Size characteristics of drug particles
The mean particle size of volume distribution, D90%, and the ratio of volume mean particle diameter to numeric mean particle diameter for each sample are shown in . According to these results, mean particle size ranged between 2.55 and 6.59 µm. D90% based on volume distribution was in the range of 6.97 − 27.02 µm. The ratio of volume mean particle diameter to numeric mean particle diameter was chosen to represent particle size distribution. This response was ranged from 3.93 to 10.33. These results indicate the effect of the studied parameters (ball size, ball numbers, and milling speed) on the assessed responses.
Table 5. Mean particle size, D90%, and the ratio of volume mean particle diameter to numeric mean particle diameter ratio of samples analyzed by particle size analyzer.
3.2. Effectiveness and the magnitude of effect of process parameters on ball milling
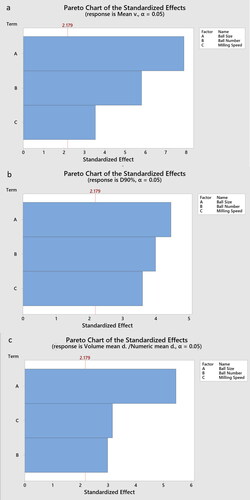
Although all three parameters were effective in influencing the particle size of the drug, the ball size (p-value = 0.000004) was more effective than the ball number (p-value = 0.000083) and milling speed (p-value = 0.004133) (). As illustrated in , D90% is influenced by all the parameters, in which ball size has the greatest effect (p-value = 0.0008) followed by ball number (p-value = 0.0018) and milling speed (p-value = 0.0037). shows that ball size was the most effective parameter on the ratio of volume mean particle diameter to numeric mean particle diameter (p-value = 0.0001). However, the second most effective variable on the ratio of volume mean particle diameter to numeric mean particle diameter was the milling speed (p-value = 0.0082), which was followed closely by ball number (p-value = 0.0114). In a general view, ball size was the most prominent variable for all three responses milling speed had the least or very little effect on all three responses.
3.3. Main effects of parameters on responses
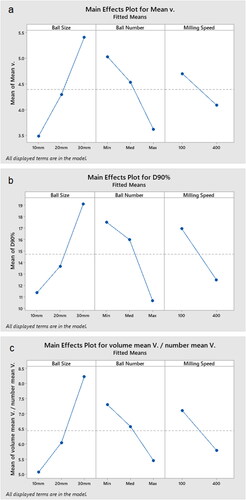
demonstrates the individual effect of each variable. Smaller ball sizes resulted in finer particles. Increased ball number and milling speed both further reduced the particle size (). D90% increased almost two folds when the ball size was increased from 10 to 30 mm. There was a greater decrease in D90% while going from a medium number of balls to the maximum number of balls. Higher milling speed led to about a 50% decrease in D90% (). Smaller ball size, higher ball number, and increased milling speed had a positive effect on particle size distribution as they all resulted in a lower ratio of volume mean particle diameter to numeric mean particle diameter ().
3.4. Interactions between effects of parameters
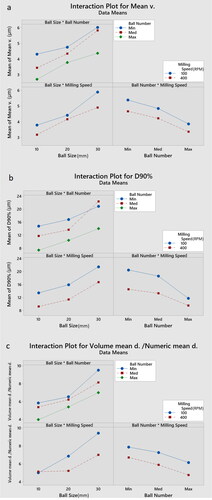
A deviation from the general trend for the ball number’s impact on D90% is seen in . In 30 mm balls, the medium number of balls resulted in a 15% higher D90% compared with the minimum ball number. demonstrates an overlap between high (400 RPM) and low (100 RPM) milling speeds for 10-mm-sized balls, which means that raising the rotation speed failed to produce a lower ratio of volume mean particle diameter to numeric mean particle diameter in 10 mm balls.
4. Discussion
The results of this study demonstrate the significance of ball size, ball number, and milling speed in the ball milling process. Regulating these variables is the key to obtaining adequate particle size reduction by this method.
The reduction in ball size from 30 to 10 mm for the maximum number of balls caused about 40% decrease in particle size in both 100 and 400 RPM milling speed, and the reduction from 20 to 10 mm ball size resulted in approximately 26% decrease in particle size in 400 RPM milling speed and 30% decrease in 100 RPM milling speed. It can be inferred that if all other variables are constant, the smaller balls produce particles with a lower volume mean diameter. This might be because smaller milling balls have a bigger surface-to-volume ratio. Therefore, more surface is present for collision and grinding of drug particles, which are two of the main mechanisms of mechanical milling (Malamatari et al. Citation2018).
The effect of reducing ball size from 30 to 20 mm on D90% is more pronounced (approximately 30%) than reducing ball size from 20 to 10 mm (approximately 15%). This might be justified by the fact that a large proportion of energy input to the system during milling (milling speed and milling time (Peltonen Citation2018)) is utilized to move the balls, thus the larger drug particles are not rotated efficiently in the jar. Therefore, the effective collision between balls and coarse drug particles that leads to grinding and size reduction is decreased, and D90% is increased further. The gap between the weight of 20 and 30 mm balls is larger than that of 20 and 10 mm balls, which justifies the difference between effects created by the reduction of ball size on D90%. This also applies to the ratio of volume mean particle diameter to numeric mean particle diameter which demonstrates the same trend for ball size variations ().
Li et al. demonstrated that very small grinding balls (50 µm), despite producing less force upon impact, were more efficient than larger balls (100 µm). They discussed that in equal loads of grinding balls, the number of balls is higher, and the inter-ball distances are lower for smaller balls. Furthermore, smaller balls break down the drug particles faster, therefore, less milling time is needed with smaller grinding media (Li et al. Citation2015). Although the grinding media used in our study is relatively larger than that of Li’s study, the efficiency of smaller milling balls in this study aligns with Li et al.’s study.
In a study carried out by Ghosh et al. on determining the critical parameters during ball milling, they showed that both RPM and ball size affected the particle size. Their study established that the effect of the milling speed was more significant than the ball size on the micronization process. Similar to our study, the volume of the grinding balls was constant for all the tests. Therefore, Ghosh explained that the same volume of smaller balls contained more grinding media and subsequently more surface for contact and collision. As a result, more effective milling took place with smaller balls. In the case of milling speed, they reported a controversial result compared to our study. Although the effect of RPM was more dominant than the effect of other variables in Ghosh et al.’s research, its increase yielded opposite results for different stabilizers. While higher RPM (400) resulted in smaller particle sizes when polyvinylpyrrolidone (PVP) was used, in the cases of HPMC and HPC-EXF (hydroxypropyl cellulose), lower RPM (250) was able to produce smaller particles. It was also shown that systems containing HPMC and HPC-EXF produced a smaller difference in particle size than the samples containing PVP. Ghosh explained that the viscosity of the drug suspension plays a role in RPM’s effect and a more viscose suspension might result in this reverse effect (Ghosh et al. Citation2013). Despite increasing the milling speed up to four folds in our study, the change in particle size was less than 0.7 µm (). It can be deduced that, although the HPC-SL (hydroxypropyl cellulose) in our study produced a suspension with lower viscosity than Ghosh’s experiment, higher RPM (400) was still able to generate the kinetic energy needed for the breakage of the particles. Yet, the viscosity of the suspension might have been high enough to prevent the increase in milling speed from breaking the particles as much as expected.
The overlap between high (400 RPM) and low (100 RPM) milling speeds for 10-mm-sized balls in further demonstrates that the impact of higher milling speed on the size reduction and particle size uniformity might vary in different milling settings, as increasing the rotation speed has failed to produce a lower volume mean particle diameter to number mean particle diameter ratio in 10 mm balls. It could be inferred that increasing the input energy would have a greater impact on the uniformity of the particles when bigger milling balls are being used. This is because with higher energy, the bigger balls will easily be moved and an efficient collision between particles and milling media would take place. However, while using smaller ball sizes, increasing the energy input of the system is less likely to generate a further positive effect, as low energy input is already enough to efficiently move the smaller balls. In this study, the only parameter that represents the energy input is milling speed.
In another study by Narayan et al., the ball size (5, 10, and 15 mm), stabilizer concentration, amount of drug, and milling time were chosen as variables. The difference between this experiment and this study was that in Narayan’s research, the weight of the balls was kept constant for all tests, whereas in this study and the study carried out by Ghosh et al., the volume of balls was constant. Unlike the results of our study, and that of Li’s and Gosh’s studies, Narayan found that bigger grinding balls were more effective in reducing particle size than small grinding balls (Narayan et al. Citation2017).
The particle size reduction by more grinding media, higher milling speeds, and smaller balls was proven to be more significant which supports our findings (Li et al. Citation2015). It should be noted that the balls utilized in Nayan et al.’s study were 0.3 mm and 0.1 mm in diameter and considerably smaller than our study here, and the milling speeds were 600 and 800 RPM, which were higher than the speeds in this study.
5. Conclusion
In this study, it was demonstrated that in the ball milling process, smaller balls have a better grinding effect on drug particles than larger ones. Moreover, a higher ball number is essential for efficient size reduction and uniformity of particles. The results support that higher RPM contributes to the production of finer particles in most cases. It was observed that in lower ball sizes, the effect of milling speed might not be as significant as it was for bigger ball sizes. In order to achieve a more efficient ball milling process, the impact of ball material and the contamination from ball grinding and the container could be studied in future investigations. Prospective studies may also involve different stabilizer types and concentrations as these variables might also affect the results of the milling process.
| Abbreviations | ||
| API | = | active pharmaceutical ingredient |
| aq. | = | aqueous |
| D90% | = | 90% of the total particles are smaller than this size |
| HPC | = | hydroxypropyl cellulose |
| ml | = | milliliter |
| mm | = | millimeter |
| µm | = | micrometer |
| mPa·s | = | millipascal-second |
| PVP | = | polyvinylpyrrolidone |
| RPM | = | revolutions per minute |
| SLS | = | sodium lauryl sulfate. |
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Al-Kassas R, Bansal M, Shaw J. 2017. Nanosizing techniques for improving bioavailability of drugs. J Control Release. 260:202–212. doi: 10.1016/j.jconrel.2017.06.003.
- Bosch HW, Liversidge E, Shelukar SD, Thompson KC. 2012. Pharmaceutical composition of a tachykinin receptor antagonist. U.S. Patent 8,258,132.
- Douroumis D, Ross SA, Nokhodchi A. 2017. Advanced methodologies for cocrystal synthesis. Adv Drug Deliv Rev. 117:178–195. doi: 10.1016/j.addr.2017.07.008.
- Ganesan P, Soundararajan R, Shanmugam U, Ramu V. 2015. Development, characterization and solubility enhancement of comparative dissolution study of second generation of solid dispersions and microspheres for poorly water soluble drug. Asian J Pharm Sci. 10(5):433–441. doi: 10.1016/j.ajps.2015.05.001.
- Ghosh I, Schenck D, Bose S, Liu F, Motto M. 2013. Identification of critical process parameters and its interplay with nanosuspension formulation prepared by top down media milling technology–a QbD perspective. Pharm Dev Technol. 18(3):719–729. doi: 10.3109/10837450.2012.723720.
- Junyaprasert VB, Morakul B. 2015. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J Pharm Sci. 10(1):13–23. doi: 10.1016/j.ajps.2014.08.005.
- Khadka P, Ro J, Kim H, Kim I, Kim JT, Kim H, Cho JM, Yun G, Lee J. 2014. Pharmaceutical particle technologies: an approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm Sci. 9(6):304–316. doi: 10.1016/j.ajps.2014.05.005.
- Kim DH, Kim YW, Tin YY, Soe MT, Ko BH, Park SJ, Lee JW. 2021. Recent technologies for amorphization of poorly water-soluble drugs. Pharmaceutics. 13(8):1318. doi: 10.3390/pharmaceutics13081318.
- Kumar S, Kaur R, Rajput R, Singh M. 2018. Bio-Pharmaceutics Classification System (BCS) class IV drug nanoparticles: quantum leap to improve their therapeutic index. Adv Pharm Bull. 8(4):617–625. doi: 10.15171/apb.2018.070.
- Li M, Yaragudi N, Afolabi A, Dave R, Bilgili E. 2015. Sub-100 nm drug particle suspensions prepared via wet milling with low bead contamination through novel process intensification. Chem Eng Sci. 130:207–220. doi: 10.1016/j.ces.2015.03.020.
- Loh ZH, Samanta AK, Heng PWS. 2015. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J Pharm Sci. 10(4):255–274. doi: 10.1016/j.ajps.2014.12.006.
- Malamatari M, Taylor KM, Malamataris S, Douroumis D, Kachrimanis K. 2018. Pharmaceutical nanocrystals: production by wet milling and applications. Drug Discov Today. 23(3):534–547. doi: 10.1016/j.drudis.2018.01.016.
- Narayan R, Pednekar A, Bhuyan D, Gowda C, Koteshwara K, Nayak UY. 2017. A top-down technique to improve the solubility and bioavailability of aceclofenac: in vitro and in vivo studies. Int J Nanomed. 12:4921–4935. doi: 10.2147/IJN.S141504.
- Peltonen L. 2018. Design space and QbD approach for production of drug nanocrystals by wet media milling techniques. Pharmaceutics. 10(3):104. doi: 10.3390/pharmaceutics10030104.
- Rostamkalaei SS, Akbari J, Saeedi M, Morteza-Semnani K, Nokhodchi A. 2019. Topical gel of metformin solid lipid nanoparticles: a hopeful promise as a dermal delivery system. Colloids Surf B Biointerfaces. 175:150–157. doi: 10.1016/j.colsurfb.2018.11.072.
- Shono Y, Jantratid E, Kesisoglou F, Reppas C, Dressman JB. 2010. Forecasting in vivo oral absorption and food effect of micronized and nanosized aprepitant formulations in humans. Eur J Pharm Biopharm. 76(1):95–104. doi: 10.1016/j.ejpb.2010.05.009.
- Srivalli KMR, Mishra B. 2016. Drug nanocrystals: a way toward scale-up. Saudi Pharm J. 24(4):386–404. doi: 10.1016/j.jsps.2014.04.007.
- Wu W, Löbmann K, Rades T, Grohganz H. 2018. On the role of salt formation and structural similarity of co-formers in co-amorphous drug delivery systems. Int J Pharm. 535(1–2):86–94. doi: 10.1016/j.ijpharm.2017.10.057.