Abstract
Background
Prehospital care for traumatic brain injury (TBI) is important to prevent secondary brain injury. We aim to compare prehospital care systems within Europe and investigate the association of system characteristics with the stability of patients at hospital arrival.
Methods
We studied TBI patients who were transported to CENTER-TBI centers, a pan-European, prospective TBI cohort study, by emergency medical services between 2014 and 2017. The association of demographic factors, injury severity, situational factors, and interventions associated with on-scene time was assessed using linear regression. We used mixed effects models to investigate the case mix adjusted variation between countries in prehospital times and interventions. The case mix adjusted impact of on-scene time and interventions on hypoxia (oxygen saturation <90%) and hypotension (systolic blood pressure <100mmHg) at hospital arrival was analyzed with logistic regression.
Results
Among 3878 patients, the greatest driver of longer on-scene time was intubation (+8.3 min, 95% CI: 5.6–11.1). Secondary referral was associated with shorter on-scene time (-5.0 min 95% CI: −6.2– −3.8). Between countries, there was a large variation in response (range: 12–25 min), on-scene (range: 16-36 min) and travel time (range: 15–32 min) and in prehospital interventions. These variations were not explained by patient factors such as conscious level or severity of injury (expected OR between countries: 1.8 for intubation, 1.8 for IV fluids, 2.0 for helicopter). On-scene time was not associated with the regional EMS policy (p= 0.58). Hypotension and/or hypoxia were seen in 180 (6%) and 97 (3%) patients in the overall cohort and in 13% and 7% of patients with severe TBI (GCS <8). The largest association with secondary insults at hospital arrival was with major extracranial injury: the OR was 3.6 (95% CI: 2.6–5.0) for hypotension and 4.4 (95% CI: 2.9–6.7) for hypoxia.
Discussion
Hypoxia and hypotension continue to occur in patients who suffer a TBI, and remain relatively common in severe TBI. Substantial variation in prehospital care exists for patients after TBI in Europe, which is only partially explained by patient factors.
Introduction
Traumatic brain injury (TBI) remains an important cause of death and disability globally (Citation1). Although rates vary between countries, TBI is estimated to be responsible for around 300 hospital admissions and 12 deaths per 100,000 persons per year in Europe (Citation2).
After the initial TBI, secondary insults, such as hypotension, hypoxia and intracranial hypertension may worsen the brain damage (Citation3,Citation4). Prehospital care for TBI focuses on preventing secondary brain injury by on-scene stabilization and rapid transportation to an appropriate hospital. There is no universally accepted and implemented international guideline aimed at avoiding secondary injury in the prehospital environment. While national guidelines do exist, these vary substantially. Moreover, the extent to which they are adopted and implemented is unclear, since real-life data on international variations in prehospital care are limited. Provider profiling of study centers in the CENTER TBI study (Citation5–7), a large prospective observational cohort study of TBI across Europe and Israel, highlighted substantial reported variation in advanced life support capability of prehospital staff, degree of preference for stabilizing on scene versus immediate transport, and in preferred destination from scene (specialist center versus nearest hospital) (Citation6). However, these reported preferences were based on clinicians’ reports of local protocols rather than objective patient data.
Objective assessment of such data is important. There is a tradeoff between prehospital stabilization and prompt transportation to hospital. Stabilizing the patient in the prehospital environment with complex interventions can cause an important time delay reaching the hospital and starting appropriate diagnostic and tailored treatments. This delay could worsen outcome (Citation8). Conversely other studies suggest that stabilizing patients on-scene for transportation to more distant specialist centers could improve outcomes (Citation9–12). The decision between prehospital stabilization and immediate transport is made on-scene by prehospital staff based on clinical parameters, injury characteristics, skill levels available and the local policy.
The current study aimed to compare prehospital management of patients with TBI across Europe, and to investigate the association of prehospital care system characteristics with stability of patients at Emergency Department (ED) arrival.
Methods
This study is reported according to the STROBE reporting guidelines (Citation13). Ethical approval was obtained from all local institutional revision boards, according to various national standards (https://www.center-tbi.eu/project/ethical-approval).
Study Design
CENTER-TBI is a multicenter, longitudinal, prospective, observational study in 18 countries across Europe which enrolled patients between December 2014 and December 2017 (5). The core cohort includes patients presenting within 24 hours of injury, with a clinical diagnosis of TBI and an indication for computed tomography (Citation6). Analyses in this manuscript were undertaken on the CENTER-TBI dataset (version 2.0), and accessed using a bespoke data management tool, Neurobot (details available on the SciCrunch Resource Identification Portal, using the Research Resource Identifier RRID/SCR_017004).
Prehospital data were collected by physicians and researchers at participating study centers. Unfortunately, no data was available on prehospital physiology. Response time was defined as time between injury and arrival of first EMS crew. On scene time was defined as time between first EMS crew arrival until the conveying crew left the injury scene. Travel time was the time between patient leaving the scene and arrival at first hospital (Citation14). Major extracranial injury (MEI) was defined as any injury in all areas except head with an Abbreviated Injury Score (AIS) above 3.
Patient Selection
Patients with TBI who were transported by ambulance or helicopter to participating hospitals (n = 56), either directly or by secondary transfer, were included. For the center-level analysis, secondary transfer patients were excluded.
Statistical Analysis
We first compare baseline characteristics between patients that were immediately transported or that were stabilized on scene. This distinction was based on an a-priori defined cutoff of 20 minutes on scene. These two groups (patients who were immediately transported and those who were stabilized on scene) were compared concerning baseline characteristics. Continuous variables were described by the median and interquartile range (IQR). Categorical variables were described by the number of patients and the corresponding percentage.
Second, the drivers of on-scene time, as a continuous variable, were assessed using linear regression. The included predictors were demographic factors (age, sex), severity (GCS, pupil reactivity, major extracranial injury), situational factors (travel time – as proxy to travel distance, physician at scene, road traffic incident, high energy trauma), and interventions (intubation, IV fluids, CPR, ventilation). Within this analysis, we also assessed the adjusted between-country variation in prehospital times and prehospital interventions with mixed effects modeling. A random intercept for centers was applied to correct for between center differences. To assess the effect of between-center differences, the partial R2 for the random intercept was calculated by comparing the R2 of the model with and without random intercept.
Third, the adjusted impact of on-scene times and prehospital interventions (intubation, ventilation, IV fluids, secondary referral) on hypoxia (Saturation <90%) and hypotension (Systolic Blood Pressure <100mmHg) at arrival was assessed with a logistic regression. We adjusted for the following patient characteristics: age, GCS, pupil reactivity, major extracranial injury (Citation15). We also measured the influence of these surrogate prehospital endpoints on functional outcome using ordinal logistic regression, which was adjusted for the aforementioned patient characteristics and utilized the imputed optimized 6-month Extended Glasgow Outcome Scale (GOS-E (6)) as the dependent variable. We allowed for a non-linear effect of systolic blood pressure and saturation with restricted cubic splines (3 degrees of freedom).
Fourth, the unadjusted and adjusted between country variation in prehospital times and rates of prehospital interventions (prehospital intubation, IV fluids, helicopter usage) across Europe were illustrated. Bar charts depict unadjusted variation whilst the aforementioned mixed effects model enabled illustration of adjusted variation. Values of the random intercept for country were visually depicted on a map of Europe. Furthermore, the variation was adjusted for the core variables of the prediction model developed in the International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) study (age, number of reactive pupils, and Glasgow Coma Score at baseline) (Citation15), and the CENTER-TBI stratum (ER/Admission/ICU) in which the patient was enrolled. Also, the median odds ratio (OR) was calculated, which quantifies the expected OR - of interventions performed or times taken - when two randomly picked countries are compared (Citation16).
Additionally, the adjusted on-scene times were compared across centers which had indicated that they have a policy of immediate transportation, or a policy of stabilizing on scene based on the Provider Profiling questionnaires (Citation17). Therefor mixed effects models were applied, with on-scene time as dependent variable, indicating on-scene policy as independent variable and country as random intercept. The on-scene times were adjusted for GCS, travel time to study center, intubation, pupils and sex.
The effects of continuous predictors were presented as the odds ratio for comparing the 75th and the 25th percentile of the specific variable. This was calculated by multiplying the regression coefficient and standard error by the width of the interquartile range of that variable.
We performed the multiple imputation method to impute the covariates for all regression analyses using the mice package in R. The following covariates were included in the imputation model: age, pupil reactivity, GCS, MEI, sex, prehospital intubation, IV fluids, CPR, ventilation, secondary referral and helicopter usage. The percentage of missing data can be found in . These results were compared with complete case analysis as a sensitivity analysis. The results of the complete case analysis of each analysis are shown in the supplemental material.
Table 1. Descriptive analysis of patients who received a short on-scene time (<20 min), or long on-scene time (>20 min)
All analyses were performed using R (R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria). The code applied can be found on https://github.com/bgravesteijn/Code_Core_prehospital.
Results
We included 3878 patients from 56 centers in 17 European Countries from a total of 4509 patients enrolled into the core CENTER TBI study. Patients who had self-presented to hospital without EMS activation (n = 616) or where prehospital details were missing or misreported (one country systematically misreported times, n = 15), were excluded (Figure S1).
Figure 1. A forest plot showing the independent effects on on-scene time of demographic factors, injury severity, situational factors, and interventions given. The estimates can be interpreted as follows: this factor increases or decreases the on-scene time by x minutes, independent of the other factors displayed. This is the result of a multivariable mixed effects linear regression model with a random intercept for center conditional on country. The coefficients (and 95% confidence intervals) of the model are displayed. The partial R2 displayed is the percentage of the full model attributable to between country differences. RTI, Road traffic incident; MEI, major extracranial injury; GCS, Glasgow Coma Scale; IQR, interquartile range; CPR, cardiopulmonary resuscitation; IV, intravenous.
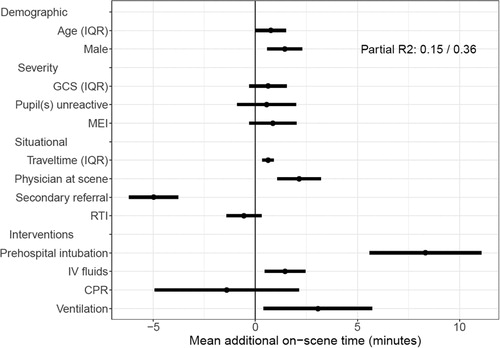
On-Scene Time
The median on-scene time was 22 (IQR: 15-32) minutes, with 1744 (45%) patients having an on-scene time of less than 20 minutes, and 2118 (55%) more than 20 minutes (). Patients with TBI and longer on-scene times were more severely injured (GCS, pupil reactivity, MEI) and had more complex prehospital interventions (CPR, IV fluids, intubation and ventilation). The two characteristics with the largest association with longer on-scene time were prehospital tracheal intubation (+8.3 min, 95% CI: 5.6-11.1), and secondary referral (-5.0 min, 95% CI: −6.2 - −3.8). Other characteristics with smaller (though statistically significant) associations with longer on-scene times were travel time to the hospital (on average +0.6 min, 95% CI: 0.34 − 0.90), having a physician present at scene (+2.1 min, 95% CI: 1.1 − 3.2), administration of IV fluids (+1.5 min, 95% CI: 0.5 − 2.4), initiation of ventilatory support (+3.1 min, 95% CI: 0.4 − 5.7), and male gender (+1.4 min, 95% CI: 0.6-2.3) (; , S1). The full model explained 36% of the variation in on-scene time (R2). Of that variation explained, 42% was due to between center differences.
Predictors of Hypotension and Hypoxia
In total, 159 (5%) of the patients arrived at the ED with hypotension, 76 (2%) with hypoxia, and 21 (1%) with both. The proportions of hypoxia and hypotension were higher in severe TBI patients (defined as a GCS ≤ 8), 90 (11%) arrived with hypotension, 38 (5%) with hypoxia, and 17 (2%) with both (). Moreover, of the patients who were intubated on-scene, 92 (12%) had hypotension, 31 (4%) had hypoxia, and 14 (2%) had both.
Table 2. The number and percentage of patients with hypotension or hypoxia at arrival at the ED
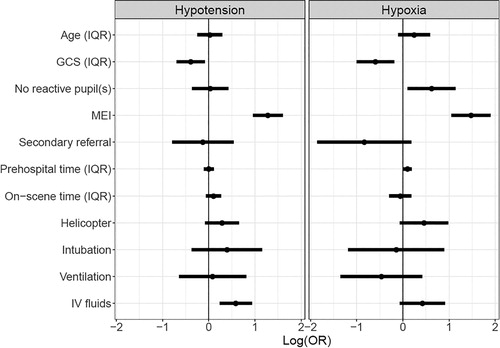
The largest association with secondary insults on arrival was with major extracranial injury: the OR was 3.6 (95% CI: 2.6 − 5.0) for hypotension and 4.4 (95% CI: 2.9 − 6.7) for hypoxia. Other patient factors were also independently associated with arrival secondary insults including a higher GCS at scene, which was associated with less hypotension (OR 0.7, 95% CI: 0.5-0.9) and hypoxia (OR 0.6, 95%CI 0.4-0.8) on arrival; the presence of on scene unilaterally or bilaterally non-reactive pupils(s) predicted arrival hypoxia (OR: 1.9, 95% CI: 1.1 − 3.1). In terms of interventions, the requirement for IV fluids was associated with hypotension at arrival (OR 1.8, 95% CI: 1.3 − 2.5), while prehospital time (average OR 1.1 (1.01-1.20)) predicted hypoxia at arrival (; S1). The complete case analysis showed the same direction and range of effects ( S1). The case mix adjusted variation by country in rates of arrival hypoxia and hypotension was small with a median OR of 1.11 and 1.05 respectively (Figure 6 S1).
Figure 2. The effect of demographic factors, injury severity, situational factors, and interventions given on hypotension (systolic blood pressure < 100 mmHg) or hypoxia (oxygen saturation < 90%) at arrival at the emergency department. The effects are based on a logistic multivariable regression model.
The adjusted association of these surrogate endpoints with functional outcome was significant ( S1): for saturation, lower values were associated with worse GOSE scores, plateauing at a saturation above 95%. For systolic blood pressure, lower (<100 mmHg) as well as higher (>180 mmHg) values were associated with worse functional outcome.
National Variation
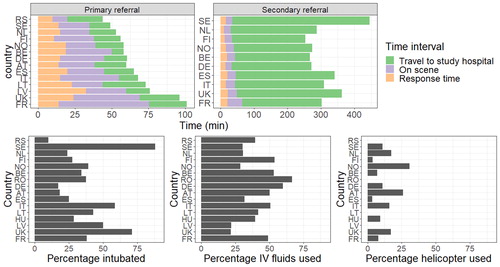
There was large variation between prehospital times across European countries (unadjusted analyses, ). The shortest prehospital times for primary referrals were seen in Sweden (49 [IQR: 39-64] minutes) and Serbia (44 [IQR: 28 − 85] minutes) whereas the longest prehospital times were seen in the United Kingdom (96 [IQR: 72 − 127] minutes) and France (101 [IQR: 74 − 146] minutes). Secondary referral extended the time until arrival at the study hospital to a greater degree (to hours rather than minutes). In Sweden, the time to arrival at the study hospital for secondary referrals was the longest (446 [IQR: 340 − 560] minutes). There was also large between-country variation in therapies the patients were provided with: intubation rates varied from 10% to 88%, iv fluid administration from 22% to 67%, and use of helicopters from 0% to 31%.
Figure 3. Bar charts showing the time spent in different prehospital phases per country (upper row), and the percentage of prehospital interventions (second row) used. In the upper row, only bars based on more than 10 patients are displayed.
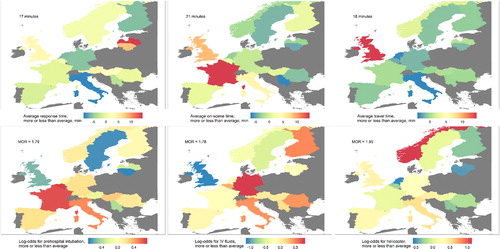
After adjusting for case mix, the variation in prehospital times and interventions within Europe remained substantial (). The range of response times adjusted for injury severity was 12-25 minutes; the range of on-scene times was 16-36 minutes; and the range of travel times was 15-32 minutes. The range of response times adjusted for injury severity and prehospital interventions was 9-31 minutes; the range of on-scene times was 15-34 minutes; and the range of travel times was 14-32 minutes. The median odds ratio, expected when two randomly picked countries are compared, was 1.8 for prehospital intubation, 1.8 for IV fluids and 2.0 for helicopter. If prehospital times were also adjusted for the interventions that individual patients received, the model fit improved significantly (likelihood ratio tests, p < 0.001). However, the values of the random intercepts (which represent the average difference to the European average) did not differ from the models that only adjust for injury severity (Figure S7).
Figure 4. The adjusted variation in prehospital time (upper row), and use of key prehospital interventions (bottom row) across Europe. Every map shows the deviation per country from the overall average. In the upper row, the mean of the median time per country is shown. Moreover, secondarily referred patients are excluded from the analysis of travel times, because the time until arrival in the secondary hospital is unknown. The estimates of the random intercepts for each country are displayed. These are adjusted for the IMPACT core variables (age, pupils, and GCS), the CENTER-TBI stratum in which the patient was included, and the random variation at the center level.
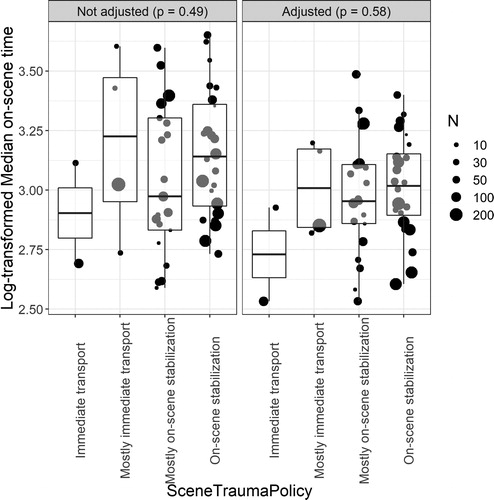
The unadjusted difference between the on-scene times of centers was not significantly different for patients from study hospitals reporting their EMS having a policy of stabilizing on scene versus a policy of immediate transport (p = 0.49) (Citation17). After adjustment, the two centers reporting to have only a policy of immediate transport as part of provider profiling had on average the shortest average on-scene times (). However, the overall difference in on-scene times between hospitals that reported the two different prehospital EMS policies was not significant (p = 0.58).
Figure 5. The unadjusted and adjusted log transformed median on-scene times. The bubbles represent the random intercept value for the model predicting on-scene time with center as random intercept. The right panel shows log transformed median on-scene times adjusted for GCS, traveltime, intubation, pupils, and sex (which were identified drivers of on-scene time).
Discussion
To our knowledge this is the most comprehensive analysis comparing prehospital care for patients after TBI across Europe. Our multicenter, multinational, prospective cohort study suggests large variations across European countries in the prehospital care provided to patients who suffer a TBI, largely unexplained by patient characteristics. Despite the common availability of national guidelines for prehospital care, patients after TBI continue to present at the ER with hypotension and hypoxia, although these are less common than in the past (6% and 3% of cases, respectively). These physiological insults are commonest in severe TBI, where they occur in 13% and 7% of cases, respectively. The main determinant of such physiological instability on arrival at hospital were major extracranial injuries. We found that the main determinants of longer on-scenes time were interventional and situational rather than patient-related, for example on-scene intubation and primary referral to the study center.
However, we also determined that variation across Europe in prehospital times and interventions was only partly concordant with the prehospital policy (immediate transport or stabilize on scene) reported by clinicians in the CENTER TBI provider profiling exercise (Citation6). We discovered that the probability of a patient with TBI being intubated at the injury scene, receiving IV fluids, or being transported by helicopter, was highly dependent on the country where the patient suffered the injury.
Not only did we see variation in prehospital interventions, but also in prehospital times. For on-scene times, this can partially be explained by the variation in provided interventions: for example, we found that prehospital intubation increased the on-scene time by 10 minutes, similar to an American retrospective study (Citation18). Other interventions (IV-fluids, mechanical ventilation) also slightly increased the on-scene times. It is likely that the association of prolonged on-scene time and greater intervention may have been, in part, due to greater injury severity, requiring more on-scene stabilization before transfer. Although this explanation might be true for variations observed concerning the patient-level, the explanation for country-level variation in hospital times requires a different explanation: the diverse geographical landscapes of Europe, and the large between-center variation in the size and type of population of hospital catchment areas are more likely to drive the variation in prehospital times. Unsurprisingly, the use of helicopters was most prevalent in Norway which has large areas with low population density. Interestingly though, the longest total prehospital times (even after adjustment for patient and some situational factors) occurred in France and the United Kingdom. Potential explanations vary: France had the highest case-mix adjusted rates of prehospital intubation concordant with their surveyed response of stabilizing patients on scene; while the United Kingdom had the highest travel times from scene to hospital, perhaps reflecting traffic congestion and/or recent centralization of major trauma care to just 30 out of over 200 hospitals (8 of which participated in CENTER-TBI).
Despite large variation in performed interventions and prehospital times were observed, the rates of hypoxia and hypotension at arrival at the Emergency Department were lower than those in historical TBI studies: for example, even in severe patients, only 11% had hypotension at arrival, compared to 35% in a large historical study (Citation3, Citation19) . In part, these lower rates may be explained by differences in case selection or definitions: While we only report documented hypoxia, the Traumatic Coma Data Bank also inferred hypoxia if there was clinically reported cyanosis or apnea. For example, we included intoxicated GCS < 9 patients in CENTER-TBI, similar to the study by Miller et al (Citation20), who found a similar incidence of hypotension. Historically, TBI patients not in coma were generally not thought to have sustained a significant injury and imaging by CT scan was rarely conducted if intoxication was thought to be the root cause of a low GCS. Therefore, these patients were not included in historical TBI studies. The lower rates of hypoxia and hypotension at arrival can be explained by a higher inclusion rate of mild TBI patients with less severe extracranial injury than in previous studies. Our study reflects modern Emergency Medicine practice, which is to image all severities of TBI. However, there remains the possibility that prehospital care has simply improved over the last decades – in particular the almost universal use of supplemental oxygen, increased use of tracheal intubation, and the common use of prehospital IV fluids, may have markedly reduced the incidence of hypoxia and hypotension. However, there continues to be room for improvement - both physiological insults still occur at significant rates, particularly in patients after severe TBI.
A limitation of this international, multicenter trial is the proportion of missing data. This is unfortunately unavoidable in such a logistically challenging study. Since complete case analysis is both inefficient, and potentially biased, we imputed the data (Citation21): both single imputation for the on-scene time, as well as multiple imputation for the main analyses were used. The single imputation was reliable, but not perfect: 60% of the variation could be explained by the model. The misclassification that could have occurred might have biased our results toward the null hypothesis. For the analysis with multiple imputed datasets, similar results were observed as the complete case analysis. This supports the validity of the selected imputation method.
Another limitation is that some prehospital physiological parameters (oxygen saturations and blood pressure) were not entered into the database. We used hypotension and hypoxia at arrival at the Emergency Department as a proxy for secondary insult. However, interventions such as intubation may have restored normal oxygen levels for some patients who were hypoxic at scene. There were some situational factors such as difficult extrication from the scene due to entrapment or stairs that may be valid factors for prolonging on scene times – and vary by country – that we could not account for using the data.
Finally, we acknowledge the fact that the centers that contributed patients to CENTER-TBI are a selected population of centers: these centers were mostly the equivalent of North American level 1 trauma centers (Citation17). Our conclusions are based on extrapolation of the preferences and policies of these specialized centers toward the entire country.
Nevertheless, the prospective nature of the study, the large number of centers and countries, and the size of the CENTER TBI cohort do provide high external validity. Additionally, the data are acquired as “real-world” data, with lenient exclusion criteria. Therefore, we believe our results are applicable to the majority of settings.
We suggest that the large variation in administered prehospital interventions can be explained by two factors. First, the most relevant guidelines for prehospital management of TBI are national guidelines, which vary substantially across countries (Citation7). However, even within countries, local policies vary according to the Provider Profiling questionnaires (Citation22). Moreover, these local policies might not be concordant with practice, as research suggests that the adherence to guidelines is low (Citation23). However, it is also possible that the prehospital guidelines are not (or not perceived as being) relevant to clinical practice in these contexts, and/or may be difficult to implement (Citation24,Citation25). Understanding and reconciling this discordance is essential if we are to provide a better evidence base for clinical practice in these contexts and ensure its appropriate adoption.
Second, the resources for prehospital care vary substantially across Europe. Even for prehospital intubation, for which the benefit - for severe TBI - has been shown in a randomized controlled trial (Citation26), large variation was observed irrespective of patient factors (Citation27): the practice variation is therefore likely to be also attributable (in part) to variation in resources. In many countries the academic basis for prehospital care is now only becoming a routine part of training for paramedics and other practitioners, whereas it has been established for Hospital based Emergency Medicine for at least 20 years. Some elements of prehospital care – such as helicopters - are costly, so research should also take account of cost-effectiveness. We need to identify prehospital interventions with proven clinical and cost effectiveness, prioritize their integration into guidelines then monitor adherence and impact on outcomes.
Conclusion
Across Europe, there are large variations in prehospital interventions for patients after TBI and in the associated on scene times. This variation is only partially explained by patient factors. Additional drivers of variation are likely to include EMS resource and organizational differences, and a low evidence base. While hypoxia and hypotension are less common than observed in past studies, they continue to occur in a substantial minority of patients after TBI, are particularly frequent following severe TBI or extracranial injury, and are associated with substantially worse outcomes. These data make a strong case for further research to facilitate the development and implementation of guidelines that support best practice in the prehospital care of patients with TBI.
Supplemental Material
Download MS Word (708 KB)Acknowledgments
The CENTER-TBI participants and investigators:
Cecilia Åkerlund1, Krisztina Amrein2, Nada Andelic3, Lasse Andreassen4, Audny Anke5, Anna Antoni6, Gérard Audibert7, Philippe Azouvi8, Maria Luisa Azzolini9, Ronald Bartels10, Pál Barzó11, Romuald Beauvais12, Ronny Beer13, Bo-Michael Bellander14, Antonio Belli15, Habib Benali16, Maurizio Berardino17, Luigi Beretta9, Morten Blaabjerg18, Peter Bragge19, Alexandra Brazinova20, Vibeke Brinck21, Joanne Brooker22, Camilla Brorsson23, Andras Buki24, Monika Bullinger25, Manuel Cabeleira26, Alessio Caccioppola27, Emiliana Calappi 27, Maria Rosa Calvi9, Peter Cameron28, Guillermo Carbayo Lozano29, Marco Carbonara27, Simona Cavallo17, Giorgio Chevallard30, Arturo Chieregato30, Giuseppe Citerio31, 32, Iris Ceyisakar33, Hans Clusmann34, Mark Coburn35, Jonathan Coles36, Jamie D. Cooper37, Marta Correia38, Amra Čović 39, Nicola Curry40, Endre Czeiter24, Marek Czosnyka26, Claire Dahyot-Fizelier41, Paul Dark42, Helen Dawes43, Véronique De Keyser44, Vincent Degos16, Francesco Della Corte45, Hugo den Boogert10, Bart Depreitere46, Đula Đilvesi 47, Abhishek Dixit48, Emma Donoghue22, Jens Dreier49, Guy-Loup Dulière50, Ari Ercole48, Patrick Esser43, Erzsébet Ezer51, Martin Fabricius52, Valery L. Feigin53, Kelly Foks54, Shirin Frisvold55, Alex Furmanov56, Pablo Gagliardo57, Damien Galanaud16, Dashiell Gantner28, Guoyi Gao58, Pradeep George59, Alexandre Ghuysen60, Lelde Giga61, Ben Glocker62, Jagoš Golubovic47, Pedro A. Gomez 63, Johannes Gratz64, Benjamin Gravesteijn33, Francesca Grossi45, Russell L. Gruen65, Deepak Gupta66, Juanita A. Haagsma33, Iain Haitsma67, Raimund Helbok13, Eirik Helseth68, Lindsay Horton 69, Jilske Huijben33, Peter J. Hutchinson70, Bram Jacobs71, Stefan Jankowski72, Mike Jarrett21, Ji-yao Jiang58, Faye Johnson73, Kelly Jones53, Mladen Karan47, Angelos G. Kolias70, Erwin Kompanje74, Daniel Kondziella52, Evgenios Koraropoulos48, Lars-Owe Koskinen75, Noémi Kovács76, Ana Kowark35, Alfonso Lagares63, Linda Lanyon59, Steven Laureys77, Fiona Lecky78, 79, Didier Ledoux77, Rolf Lefering80, Valerie Legrand81, Aurelie Lejeune82, Leon Levi83, Roger Lightfoot84, Hester Lingsma33, Andrew I.R. Maas44, Ana M. Castaño-León63, Marc Maegele85, Marek Majdan20, Alex Manara86, Geoffrey Manley87, Costanza Martino88, Hugues Maréchal50, Julia Mattern89, Catherine McMahon90, Béla Melegh91, David Menon48, Tomas Menovsky44, Ana Mikolic33, Benoit Misset77, Visakh Muraleedharan59, Lynnette Murray28, Ancuta Negru92, David Nelson1, Virginia Newcombe48, Daan Nieboer33, József Nyirádi2, Otesile Olubukola78, Matej Oresic93, Fabrizio Ortolano27, Aarno Palotie94, 95, 96, Paul M. Parizel97, Jean-François Payen98, Natascha Perera12, Vincent Perlbarg16, Paolo Persona99, Wilco Peul100, Anna Piippo-Karjalainen101, Matti Pirinen94, Horia Ples92, Suzanne Polinder33, Inigo Pomposo29, Jussi P. Posti 102, Louis Puybasset103, Andreea Radoi 104, Arminas Ragauskas105, Rahul Raj101, Malinka Rambadagalla106, Jonathan Rhodes107, Sylvia Richardson108, Sophie Richter48, Samuli Ripatti94, Saulius Rocka105, Cecilie Roe109, Olav Roise110,111, Jonathan Rosand112, Jeffrey V. Rosenfeld113, Christina Rosenlund114, Guy Rosenthal56, Rolf Rossaint35, Sandra Rossi99, Daniel Rueckert62, Martin Rusnák115, Juan Sahuquillo104, Oliver Sakowitz89, 116, Renan Sanchez-Porras116, Janos Sandor117, Nadine Schäfer80, Silke Schmidt118, Herbert Schoechl119, Guus Schoonman120, Rico Frederik Schou121, Elisabeth Schwendenwein6, Charlie Sewalt33, Toril Skandsen122, 123, Peter Smielewski26, Abayomi Sorinola124, Emmanuel Stamatakis48, Simon Stanworth40, Robert Stevens125, William Stewart126, Ewout W. Steyerberg33, 127, Nino Stocchetti128, Nina Sundström129, Anneliese Synnot22, 130, Riikka Takala131, Viktória Tamás124, Tomas Tamosuitis132, Mark Steven Taylor20, Braden Te Ao53, Olli Tenovuo102, Alice Theadom53, Matt Thomas86, Dick Tibboel133, Marjolein Timmers74, Christos Tolias134, Tony Trapani28, Cristina Maria Tudora92, Peter Vajkoczy 135, Shirley Vallance28, Egils Valeinis61, Zoltán Vámos51, Mathieu van der Jagt136, Gregory Van der Steen44, Joukje van der Naalt71, Jeroen T.J.M. van Dijck 100, Thomas A. van Essen100, Wim Van Hecke137, Caroline van Heugten138, Dominique Van Praag139, Thijs Vande Vyvere137, Roel P. J. van Wijk100, Alessia Vargiolu32, Emmanuel Vega82, Kimberley Velt33, Jan Verheyden137, Paul M. Vespa140, Anne Vik121, 141, Rimantas Vilcinis132, Victor Volovici67, Nicole von Steinbüchel39, Daphne Voormolen33, Petar Vulekovic47, Kevin K.W. Wang142, Eveline Wiegers33, Guy Williams48, Lindsay Wilson69, Stefan Winzeck48, Stefan Wolf143, Zhihui Yang142, Peter Ylén144, Alexander Younsi89, Frederick A. Zeiler48,145, Veronika Zelinkova20, Agate Ziverte61, Tommaso Zoerle27
1Department of Physiology and Pharmacology, Section of Perioperative Medicine and Intensive Care, Karolinska Institutet, Stockholm, Sweden
2János Szentágothai Research Center, University of Pécs, Pécs, Hungary
3Division of Surgery and Clinical Neuroscience, Department of Physical Medicine and Rehabilitation, Oslo University Hospital and University of Oslo, Oslo, Norway
4Department of Neurosurgery, University Hospital Northern Norway, Tromso, Norway
5Department of Physical Medicine and Rehabilitation, University Hospital Northern Norway, Tromso, Norway
6Trauma Surgery, Medical University Vienna, Vienna, Austria
7Department of Anesthesiology & Intensive Care, University Hospital Nancy, Nancy, France
8Raymond Poincare hospital, Assistance Publique – Hopitaux de Paris, Paris, France
9Department of Anesthesiology & Intensive Care, S Raffaele University Hospital, Milan, Italy
10Department of Neurosurgery, Radboud University Medical Center, Nijmegen, The Netherlands
11Department of Neurosurgery, University of Szeged, Szeged, Hungary
12International Projects Management, ARTTIC, Munchen, Germany
13Department of Neurology, Neurological Intensive Care Unit, Medical University of Innsbruck, Innsbruck, Austria
14Department of Neurosurgery & Anesthesia & intensive care medicine, Karolinska University Hospital, Stockholm, Sweden
15NIHR Surgical Reconstruction and Microbiology Research Center, Birmingham, UK
16Anesthesie-Réanimation, Assistance Publique – Hopitaux de Paris, Paris, France
17Department of Anesthesia & ICU, AOU Città della Salute e della Scienza di Torino - Orthopedic and Trauma Center, Torino, Italy
18Department of Neurology, Odense University Hospital, Odense, Denmark
19BehaviourWorks Australia, Monash Sustainability Institute, Monash University, Victoria, Australia
20Department of Public Health, Faculty of Health Sciences and Social Work, Trnava University, Trnava, Slovakia
21Quesgen Systems Inc., Burlingame, California, USA
22Australian & New Zealand Intensive Care Research Center, Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia
23Department of Surgery and Perioperative Science, Umeå University, Umeå, Sweden
24Department of Neurosurgery, Medical School, University of Pécs, Hungary and Neurotrauma Research Group, János Szentágothai Research Center, University of Pécs, Hungary
25Department of Medical Psychology, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany
26Brain Physics Lab, Division of Neurosurgery, Dept of Clinical Neurosciences, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK
27Neuro ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy
28ANZIC Research Center, Monash University, Department of Epidemiology and Preventive Medicine, Melbourne, Victoria, Australia
29Department of Neurosurgery, Hospital of Cruces, Bilbao, Spain
30NeuroIntensive Care, Niguarda Hospital, Milan, Italy
31School of Medicine and Surgery, Università Milano Bicocca, Milano, Italy
32NeuroIntensive Care, ASST di Monza, Monza, Italy
33Department of Public Health, Erasmus Medical Center-University Medical Center, Rotterdam, The Netherlands
34Department of Neurosurgery, Medical Faculty RWTH Aachen University, Aachen, Germany
35Department of Anaesthesiology, University Hospital of Aachen, Aachen, Germany
36Department of Anesthesia & Neurointensive Care, Cambridge University Hospital NHS Foundation Trust, Cambridge, UK
37School of Public Health & PM, Monash University and The Alfred Hospital, Melbourne, Victoria, Australia
38Radiology/MRI department, MRC Cognition and Brain Sciences Unit, Cambridge, UK
39Institute of Medical Psychology and Medical Sociology, Universitätsmedizin Göttingen, Göttingen, Germany
40Oxford University Hospitals NHS Trust, Oxford, UK
41Intensive Care Unit, CHU Poitiers, Potiers, France
42University of Manchester NIHR Biomedical Research Center, Critical Care Directorate, Salford Royal Hospital NHS Foundation Trust, Salford, UK
43Movement Science Group, Faculty of Health and Life Sciences, Oxford Brookes University, Oxford, UK
44Department of Neurosurgery, Antwerp University Hospital and University of Antwerp, Edegem, Belgium
45Department of Anesthesia & Intensive Care, Maggiore Della Carità Hospital, Novara, Italy
46Department of Neurosurgery, University Hospitals Leuven, Leuven, Belgium
47Department of Neurosurgery, Clinical center of Vojvodina, Faculty of Medicine, University of Novi Sad, Novi Sad, Serbia
48Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK
49Center for Stroke Research Berlin, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany
50Intensive Care Unit, CHR Citadelle, Liège, Belgium
51Department of Anaesthesiology and Intensive Therapy, University of Pécs, Pécs, Hungary
52Departments of Neurology, Clinical Neurophysiology and Neuroanesthesiology, Region Hovedstaden Rigshospitalet, Copenhagen, Denmark
53National Institute for Stroke and Applied Neurosciences, Faculty of Health and Environmental Studies, Auckland University of Technology, Auckland, New Zealand
54Department of Neurology, Erasmus MC, Rotterdam, the Netherlands
55Department of Anesthesiology and Intensive care, University Hospital Northern Norway, Tromso, Norway
56Department of Neurosurgery, Hadassah-hebrew University Medical center, Jerusalem, Israel
57Fundación Instituto Valenciano de Neurorrehabilitación (FIVAN), Valencia, Spain
58Department of Neurosurgery, Shanghai Renji hospital, Shanghai Jiaotong University/school of medicine, Shanghai, China
59Karolinska Institutet, INCF International Neuroinformatics Coordinating Facility, Stockholm, Sweden
60Emergency Department, CHU, Liège, Belgium
61Neurosurgery clinic, Pauls Stradins Clinical University Hospital, Riga, Latvia
62Department of Computing, Imperial College London, London, UK
63Department of Neurosurgery, Hospital Universitario 12 de Octubre, Madrid, Spain
64Department of Anesthesia, Critical Care and Pain Medicine, Medical University of Vienna, Austria
65College of Health and Medicine, Australian National University, Canberra, Australia
66Department of Neurosurgery, Neurosciences Center & JPN Apex trauma center, All India Institute of Medical Sciences, New Delhi-110029, India
67Department of Neurosurgery, Erasmus MC, Rotterdam, the Netherlands
68Department of Neurosurgery, Oslo University Hospital, Oslo, Norway
69Division of Psychology, University of Stirling, Stirling, UK
70Division of Neurosurgery, Department of Clinical Neurosciences, Addenbrooke’s Hospital & University of Cambridge, Cambridge, UK
71Department of Neurology, University of Groningen, University Medical Center Groningen, Groningen, Netherlands
72Neurointensive Care, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK
73Salford Royal Hospital NHS Foundation Trust Acute Research Delivery Team, Salford, UK
74Department of Intensive Care and Department of Ethics and Philosophy of Medicine, Erasmus Medical Center, Rotterdam, The Netherlands
75Department of Clinical Neuroscience, Neurosurgery, Umeå University, Umeå, Sweden
76Hungarian Brain Research Program - Grant No. KTIA_13_NAP-A-II/8, University of Pécs, Pécs, Hungary
77Cyclotron Research Center, University of Liège, Liège, Belgium
78Center for Urgent and Emergency Care Research (CURE), Health Services Research Section, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK
79Emergency Department, Salford Royal Hospital, Salford UK
80Institute of Research in Operative Medicine (IFOM), Witten/Herdecke University, Cologne, Germany
81VP Global Project Management CNS, ICON, Paris, France
82Department of Anesthesiology-Intensive Care, Lille University Hospital, Lille, France
83Department of Neurosurgery, Rambam Medical Center, Haifa, Israel
84Department of Anesthesiology & Intensive Care, University Hospitals Southhampton NHS Trust, Southhampton, UK
85Cologne-Merheim Medical Center (CMMC), Department of Traumatology, Orthopedic Surgery and Sportmedicine, Witten/Herdecke University, Cologne, Germany
86Intensive Care Unit, Southmead Hospital, Bristol, Bristol, UK
87Department of Neurological Surgery, University of California, San Francisco, California, USA
88Department of Anesthesia & Intensive Care, M. Bufalini Hospital, Cesena, Italy
89Department of Neurosurgery, University Hospital Heidelberg, Heidelberg, Germany
90Department of Neurosurgery, The Walton center NHS Foundation Trust, Liverpool, UK
91Department of Medical Genetics, University of Pécs, Pécs, Hungary
92Department of Neurosurgery, Emergency County Hospital Timisoara, Timisoara, Romania
93School of Medical Sciences, Örebro University, Örebro, Sweden
94Institute for Molecular Medicine Finland, University of Helsinki, Helsinki, Finland
95Analytic and Translational Genetics Unit, Department of Medicine; Psychiatric & Neurodevelopmental Genetics Unit, Department of Psychiatry; Department of Neurology, Massachusetts General Hospital, Boston, MA, USA
96Program in Medical and Population Genetics; The Stanley Center for Psychiatric Research, The Broad Institute of MIT and Harvard, Cambridge, MA, USA
97Department of Radiology, University of Antwerp, Edegem, Belgium
98Department of Anesthesiology & Intensive Care, University Hospital of Grenoble, Grenoble, France
99Department of Anesthesia & Intensive Care, Azienda Ospedaliera Università di Padova, Padova, Italy
100Dept. of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands and Dept. of Neurosurgery, Medical Center Haaglanden, The Hague, The Netherlands
101Department of Neurosurgery, Helsinki University Central Hospital
102Division of Clinical Neurosciences, Department of Neurosurgery and Turku Brain Injury Center, Turku University Hospital and University of Turku, Turku, Finland
103Department of Anesthesiology and Critical Care, Pitié -Salpêtrière Teaching Hospital, Assistance Publique, Hôpitaux de Paris and University Pierre et Marie Curie, Paris, France
104Neurotraumatology and Neurosurgery Research Unit (UNINN), Vall d'Hebron Research Institute, Barcelona, Spain
105Department of Neurosurgery, Kaunas University of technology and Vilnius University, Vilnius, Lithuania
106Department of Neurosurgery, Rezekne Hospital, Latvia
107Department of Anaesthesia, Critical Care & Pain Medicine NHS Lothian & University of Edinburg, Edinburgh, UK
108Director, MRC Biostatistics Unit, Cambridge Institute of Public Health, Cambridge, UK
109Department of Physical Medicine and Rehabilitation, Oslo University Hospital/University of Oslo, Oslo, Norway
110Division of Orthopedics, Oslo University Hospital, Oslo, Norway
111Institue of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway
112Broad Institute, Cambridge MA Harvard Medical School, Boston MA, Massachusetts General Hospital, Boston MA, USA
113National Trauma Research Institute, The Alfred Hospital, Monash University, Melbourne, Victoria, Australia
114Department of Neurosurgery, Odense University Hospital, Odense, Denmark
115International Neurotrauma Research Organisation, Vienna, Austria
116Klinik für Neurochirurgie, Klinikum Ludwigsburg, Ludwigsburg, Germany
117Division of Biostatistics and Epidemiology, Department of Preventive Medicine, University of Debrecen, Debrecen, Hungary
118Department Health and Prevention, University Greifswald, Greifswald, Germany
119Department of Anaesthesiology and Intensive Care, AUVA Trauma Hospital, Salzburg, Austria
120Department of Neurology, Elisabeth-TweeSteden Ziekenhuis, Tilburg, the Netherlands
121Department of Neuroanesthesia and Neurointensive Care, Odense University Hospital, Odense, Denmark
122Department of Neuromedicine and Movement Science, Norwegian University of Science and Technology, NTNU, Trondheim, Norway
123Department of Physical Medicine and Rehabilitation, St.Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
124Department of Neurosurgery, University of Pécs, Pécs, Hungary
125Division of Neuroscience Critical Care, John Hopkins University School of Medicine, Baltimore, USA
126Department of Neuropathology, Queen Elizabeth University Hospital and University of Glasgow, Glasgow, UK
127Dept. of Department of Biomedical Data Sciences, Leiden University Medical Center, Leiden, The Netherlands
128Department of Pathophysiology and Transplantation, Milan University, and Neuroscience ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milano, Italy
129Department of Radiation Sciences, Biomedical Engineering, Umeå University, Umeå, Sweden
130Cochrane Consumers and Communication Review Group, Center for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia
131Perioperative Services, Intensive Care Medicine and Pain Management, Turku University Hospital and University of Turku, Turku, Finland
132Department of Neurosurgery, Kaunas University of Health Sciences, Kaunas, Lithuania
133Intensive Care and Department of Pediatric Surgery, Erasmus Medical Center, Sophia Children’s Hospital, Rotterdam, The Netherlands
134Department of Neurosurgery, Kings college London, London, UK
135Neurologie, Neurochirurgie und Psychiatrie, Charité – Universitätsmedizin Berlin, Berlin, Germany
136Department of Intensive Care Adults, Erasmus MC– University Medical Center Rotterdam, Rotterdam, the Netherlands
137icoMetrix NV, Leuven, Belgium
138Movement Science Group, Faculty of Health and Life Sciences, Oxford Brookes University, Oxford, UK
139Psychology Department, Antwerp University Hospital, Edegem, Belgium
140Director of Neurocritical Care, University of California, Los Angeles, USA
141Department of Neurosurgery, St.Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
142Department of Emergency Medicine, University of Florida, Gainesville, Florida, USA
143Department of Neurosurgery, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany
144VTT Technical Research Center, Tampere, Finland
145Section of Neurosurgery, Department of Surgery, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada
Additional information
Funding
References
- Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, Culpepper WJ, Dorsey ER, Elbaz A, Ellenbogen RG, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–80. May 1doi:10.1016/S1474-4422(18)30499-X.
- Majdan M, Plancikova D, Brazinova A, Rusnak M, Nieboer D, Feigin V, et al. Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Heal. 2016;1(2):e76–83. Available from: https://www.thelancet.com/journals/lanpub/article/PIIS2468-2667(16)30017-2/fulltext
- Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma Inj Infect Crit Care. 1993;34(2):216–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8459458
- Davis D, Dunford J, Poste J, Ochs M, Holbrook T, Fortlage D, et al. The Impact of Hypoxia and Hyperventilation on Outcome after Paramedic Rapid Sequence Intubation of Severely Head-injured Patients. J Trauma Inj Infect Crit Care. 2004;57(1):1–10. Available from: https://insights.ovid.com/pubmed?pmid=15284540
- Maas AIR, Menon DK, Steyerberg EW, Citerio G, Lecky F, Manley GT, Hill S, Legrand V, Sorgner A, CENTER-TBI Participants and Investigators Collaborative European neurotrauma effectiveness research in traumatic brain injury (CENTER-TBI): A prospective longitudinal observational study. Neurosurgery. 2015;76(1):67–80. doi:10.1227/NEU.0000000000000575.
- Steyerberg EW, Wiegers E, Sewalt C, Buki A, Citerio G, Keyser VD, et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019;18(10):923–34.
- Cnossen MC, van den Brande R, Lingsma H, Polinder S, Lecky F, Maas A. Prehospital trauma care among 68 European neurotrauma centers: Results of the CENTER-TBI Provider Profiling Questionnaires. J Neurotrauma. 2018;36(1):176–181. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29732946
- Raj R, Siironen J, Kivisaari R, Kuisma M, Brinck T, Lappalainen J, Skrifvars MB. Factors correlating with delayed trauma center admission following traumatic brain injury. Scand J Trauma Resusc Emerg Med. 2013;21(1):67. Available from: https://sjtrem.biomedcentral.com/articles/10.1186/1757-7241-21-67/.
- Lodwick G, Edwards L. Paediatric retrieval services: is it better to 'stay and play' or 'scoop and run'? Br J Hosp Med (Lond). 2017;78(2):118[Internet]. Feb 2 [cited 2019 Aug 13]. Available from: http://www.magonlinelibrary.com/doi/10.12968/hmed.2017.78.2.118.
- Nirula R, Maier R, Moore E, Sperry J, Gentilello L. Scoop and run to the trauma center or stay and play at the local hospital: hospital transfer’s effect on mortality. J Trauma. 2010;69(3):595–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20838131
- Smith RM, Conn AK. Prehospital care − Scoop and run or stay and play? Injury. 2009;40:S23–S6. Available from: https://www.sciencedirect.com/science/article/pii/S0020138309005531?via%3Dihub
- King S. Stay & play vs. scoop & run. JEMS. 2003;28(6):14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12830791
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17947786.
- Ringdal KG, Coats TJ, Lefering R, Di Bartolomeo S, Steen PA, Røise O, Handolin L, Lossius HM, Utstein TCD expert panel The Utstein template for uniform reporting of data following major trauma: a joint revision by SCANTEM, TARN, DGU-TR and RITG. Scand J Trauma Resusc Emerg Med. 2008;16(1):7. Available from: http://sjtrem.biomedcentral.com/articles/10.1186/1757-7241-16-7.
- Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, Murray GD, Marmarou A, Roberts I, Habbema JDF, et al. Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics. Singer M, editor. PLoS Med. 2008;5(8):e165. Available from: http://dx.plos.org/10.1371/journal.pmed.0050165.
- Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, Råstam L, Larsen K. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60(4):290–7. Available from: http://jech.bmj.com/ doi:10.1136/jech.2004.029454.
- Cnossen MC, van der Brande R, Lingsma HF, Polinder S, Lecky F, Maas AIR, the CENTER TBI Investigators and Participants. Prehospital Trauma Care among 68 European Neurotrauma Centers: Results of the CENTER-TBI Provider Profiling Questionnaires. J Neurotrauma. 2019;36(1):176–81. Available from: https://www.liebertpub.com/doi/10.1089/neu.2018.5712.
- Cudnik MT, Newgard CD, Wang H, Bangs C, Herringtion R. Endotracheal Intubation Increases Out-of-Hospital Time in Trauma Patients. Prehospital Emerg Care. Prehosp Emerg Care. 2007;11(2):224–9. Available from: http://www.tandfonline.com/doi/full/10.1080/10903120701205208.
- Chesnut RM, Marshall SB, Piek J, Blunt BA, Klauber MR, Marshall LF. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir Suppl (Wien). 1993;59:121–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8310858.
- Miller JD, Sweet RC, Narayan R, Becker DP. Early Insults to the Injured Brain. JAMA. 1978;240(5):439. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.1978.03290050029011.
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. J Educ Stat. 1991;16(2):150. Available from: https://www.jstor.org/stable/1165119?origin=crossref.
- Cnossen MC, Polinder S, Lingsma HF, Maas AIR, Menon D, Steyerberg EW, CENTER-TBI Investigators and Participants Variation in structure and process of care in traumatic brain injury: Provider profiles of European Neurotrauma Centers participating in the CENTER-TBI study. PLoS One. 2016;11(8):e0161367. doi:10.1371/journal.pone.0161367.
- Ebben RH, Vloet LC, Verhofstad MH, Meijer S, Groot JAM, van Achterberg T. Adherence to guidelines and protocols in the prehospital and emergency care setting: a systematic review. Scand J Trauma Resusc Emerg Med. 2013;21(1):9. Available from: https://sjtrem.biomedcentral.com/articles/10.1186/1757-7241-21-9.
- Cnossen MC, Scholten AC, Lingsma HF, Synnot A, Tavender E, Gantner D, et al. Adherence to Guidelines in Adult Patients with Traumatic Brain Injury: A Living Systematic Review. J Neurotrauma. 2016. Available from: http://www.liebertpub.com/doi/10.1089/neu.2015.4121.
- Neeraj Badjatia A, Carney N, Crocco TJ, Elizabeth Fallat M, Halim A, Hennes FM, Andrew Jagoda FS, et al. Prehospital Guidelines 2 nd Edition Guidelines for Prehospital Management of Traumatic Brain Injury. 2006. Available from: http://www.lifeflightmaine.org/Documents/EMS-and-Hospitals/Research/Brain-Injury/Prehospital-TBI-Management-12-2006.aspx
- Bernard SA, Nguyen V, Cameron P, Masci K, Fitzgerald M, Cooper DJ, et al. Prehospital Rapid Sequence Intubation Improves Functional Outcome for Patients With Severe Traumatic Brain Injury. Ann Surg. 2010;252(6):959–65. doi:10.1097/SLA.0b013e3181efc15f.
- Gravesteijn BY, Sewalt CA, Ercole A, Lecky F, Menon D, Steyerberg EW, et al. Variation in the practice of tracheal intubation in Europe after traumatic brain injury: a prospective cohort study. Anaesthesia. 2019;75:7–10. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/anae.14838.
