ABSTRACT
The herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) has been commercially available since the 1940’s. Despite decades of data on 2,4-D in food, air, soil, and water, as well as in humans, the quality the quality of these data has not been comprehensively evaluated. Using selected elements of the Biomonitoring, Environmental Epidemiology, and Short-lived Chemicals (BEES-C) instrument (temporal variability, avoidance of sample contamination, analyte stability, and urinary methods of matrix adjustment), the quality of 156 publications of environmental- and biomonitoring-based 2,4-D data was examined. Few publications documented steps were taken to avoid sample contamination. Similarly, most studies did not demonstrate the stability of the analyte from sample collection to analysis. Less than half of the biomonitoring publications reported both creatinine-adjusted and unadjusted urine concentrations. The scope and detail of data needed to assess temporal variability and sources of 2,4-D varied widely across the reviewed studies. Exposures to short-lived chemicals such as 2,4-D are impacted by numerous and changing external factors including application practices and formulations. At a minimum, greater transparency in reporting of quality control measures is needed. Perhaps the greatest challenge for the exposure community is the ability to reach consensus on how to address problems specific to short-lived chemical exposures in observational epidemiology investigations. More extensive conversations are needed to advance our understanding of human exposures and enable interpretation of these data to catch up to analytical capabilities. The problems defined in this review remain exquisitely difficult to address for chemicals like 2,4-D, with short and variable environmental and physiological half-lives and with exposures impacted by numerous and changing external factors.
Introduction
The herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) is widely registered globally for use on crops, lawns, rights-of-way, and for aquatic and forestry applications (USEPA Citation2005). This herbicide has been commercially available since the 1940’s. A variety of formulations are available and may contain different forms of 2,4-D, including acid, amine, and ester forms (Peterson et al. Citation2016). Human exposures may occur through consumption of foods with 2,4-D residues, or from exposure to (1) drinking water and recreational water use, (2) residential and/or occupational uses, and (3) post-application exposures associated with use on sites such golf courses, parks and lawns (USEPA Citation2005).
Table 1. Elements of the Biomonitoring, Environmental Epidemiology, and Short-Lived Chemicals (BEES-C) Instrument used in this assessment (from, 2015; LaKind et al. Citation2014, Citation2015)
Table 2. Summary of publications relevant for human exposure assessment by matrix
Understanding the extent, duration, variability, and timing of human exposures is essential for adequately characterizing and classifying exposure in epidemiological research and human health-risk assessment. At the same time, assessing exposures to 2,4-D for specific and general populations is difficult due to a combination of factors. First, as noted above, various forms and formulations 2,4-D are available for use with different physicochemical properties affecting fate and transport, although different forms break down to the acid form (Walters Citation1999). Second, exposures associated with applications of 2,4-D are impacted by meteorological conditions, type of application equipment used, extent of application, personal protective equipment, and other factors (Peterson et al. Citation2016). Third, 2,4-D has a short environmental and physiological half-life, raising complexities related to capturing temporal variability in exposures and determining both peak and long-term exposure levels (USEPA Citation2005).
The research on 2,4-D concentrations in various environmental media and human matrices using biomonitoring approaches is extensive. In addition, there are multiple epidemiology studies examining associations between 2,4-D exposures and human health effects, including those relying on self-reported use and measures of 2,4-D in environmental media and urinary biomarker data (Burns and Swaen Citation2012). With the current shift away from lab animal studies, there will likely be an increased emphasis on the use of human data as the basis for public health decision-making. An assessment of the quality of data for use in human exposure assessments is an integral part of the decision-making process (Barroso et al. Citation2016). Surprisingly, to date, no review has critically examined the quality of the published exposure data to determine whether the 2,4-D data are robust, reliable, and/or generalizable.
The aim of this review was to first examine the quality of the published, peer-reviewed 2,4-D monitoring data from studies of food, air, soil, water, and human matrices by focusing on four quality elements of the Biomonitoring, Environmental Epidemiology, and Short-lived Chemicals (BEES-C) instrument: documentation of avoidance of sample contamination, documentation of analyte stability from time of sample collection to sample analysis, and matrix adjustment methods (specific to interpretation of urinary measurements). Reports were also examined for information on number of samples collected and time between 2,4-D application and sample collection with an eye toward understanding whether the data offered information on peak or long-term exposures as well as the likelihood for exposure misclassification.
Our objective in this review was to examine the strengths and limitations of the international 2,4-D exposure database to inform future research. Our aim was to answer the following questions: (1) Are the published data of sufficient quality for use in assessing general population exposures and/or exposures to specific communities? (2) Are the published data generalizable to broader populations (i.e. can the data be used to generate information on typical peak and/or background exposures in populations other than those specific to an individual study)? (3) What additional information can be gleaned from large-scale population- and media-based studies available from governmental reports (e.g. NHANES, US Department of Agriculture Annual Pesticide Data Program [PDP])? To be clear, this review process results in an overall assessment of various aspects of individual study quality. It is the responsibility of the end user to decide if the data are fit-for-purpose.
Methods
Identification and selection of studies
Recommendations for systematic reviews (Moher and Tricco Citation2008; Shea et al. Citation2007; Sutton et al. Citation1998) and elements of the “assessment tool of multiple systematic reviews” (AMSTAR) guidelines that pertain to exposure data were followed (Shea et al. Citation2007). The search and selection of relevant studies were conducted independently by two study authors (C.J.B and J.S.L.). Electronic data sources including PubMed and Web of Science™ Core Collection were used to conduct the initial literature search. Using keywords “2,4-D,” ‘2,4-dichlorophenoxyacetic acid,” “air,” “blood,” “dust,” “food,” “soil,” “urine,” and “water” as well as various combinations of these keywords, we selected articles that included environmental- and/or biomonitoring-based measurements of 2,4-D. References of retrieved publications were reviewed to identify publications not captured by the electronic search.
The inclusion criteria for this review were as follows:
study includes measurements of 2,4-D in environmental media and/or humans (as opposed to obtaining exposure information through other approaches such as questionnaires or job title),
investigation was published in the peer-reviewed published literature,
publication appeared in English prior to 17 May 2016 (end of literature search),
study measures environmental levels of 2,4-D (as opposed to spiked samples, etc.), and
study provides data that can be linked to human exposure.
The following categories of studies were excluded:
mineralization, sorption, and recovery studies as well as batch and column studies,
methodological studies using pooled urine samples,
groundwater studies with no evidence of potential human exposure to the groundwater,
toxicology studies, including PBPK, lab animal studies, in vitro studies,
studies on exposures to Agent Orange/dioxins,
studies of poisonings via self-administration,
reviews,
regeneration studies and other effects on plants, including plant toxicity,
environmental fate studies that rely upon experimental application, not real world uses (including bioaccumulation),
studies on decontamination/remediation, and
human health studies with no exposure data.
The final body of literature was examined independently by 4 co-authors (C.J.B., J.S.L., J.S.N., A.J.B.). Data from each study were tabulated, and resulting summary tables were again cross-checked (by C.J.B., J.S.L., J.S.N., A.J.B.).
Assessment of individual studies
Principles for characterizing the quality of human studies that rely on biomarkers of chemicals with short physiologic half-lives are described in an evaluative instrument referred to as the BEES-C (LaKind et al. Citation2015, Citation2014). While originally developed for biomonitoring data, the evaluative elements on temporality, selection of analyte, sampling and analysis, and study design are also applicable to evaluating the quality of environmental data. These elements capture core components of the overall quality of measurement data.
To evaluate the scientific quality of each study and strengths and limitations for formulating conclusions (steps 7 and 8 of the AMSTAR guidelines), two co-authors (C.J.B. and J.S.L.) independently reviewed each retrieved study using three exposure-related elements of the BEES-C instrument. The BEES-C instrument uses a three-tier system with Tier 1 indicating the highest quality (LaKind et al. Citation2014). While the instrument includes numerous aspects of study quality, the focus was on elements that are relevant to practices for conducting and reporting exposure: sample contamination, analyte stability, and matrix adjustment (). Temporal variability was explored but was not included in the tiered assessment as many of the investigations were not epidemiology studies and not applicable to the BEES-C tiering approach. The four elements are described here as follows.
Sample contamination
A high-quality study (Tier 1) includes documentation of the procedures used to avoid contamination by the analyte of interest and validates that samples are contamination-free from the time of sample collection to the time of analysis. Approaches include the appropriate use of blanks both in the field and lab. A Tier 2 study has incomplete documentation of the steps taken to avoid contamination, while a Tier 3 study includes no documentation.
Analyte stability
A study is assigned a Tier 1 ranking if it includes details on the history of the sample and has documented analyte stability. If losses of the analyte over time are known, a Tier 2 study has accounted for these losses in the exposure estimates. Finally, a Tier 3 study provides no information on this element.
Matrix adjustment
Measurements of analytes in urine are commonly measured and reported in units of concentration (mass per volume urine). However, urinary output is highly variable and affected by factors including diet, exercise, hydration, and age. A common approach to correcting for variable urine dilution is by adjusting with urinary creatinine concentration or urine specific gravity. However, urinary creatinine levels are also variable and may be affected by age, gender, race/ethnicity, body mass index, fat-free mass, time of day of collection of urine samples, exercise, diet, and health (LaKind and Naiman Citation2015). Currently, there is no consensus on the best method(s) for “correcting” urinary biomarkers measurements for variable urine dilution (LaKind et al. Citation2014). Minimally, both the volume-based and corrected concentrations need to be included to enable appropriate comparison across studies (LaKind et al. Citation2014). A Tier 1 study includes both adjusted and unadjusted urinary concentration data.
Temporal variability
Temporal variability was not formally assessed with a tiering approach. However, study-specific available information on time between 2,4-D application and sample collection, as well as number of samples collected (one-time sampling, multiple samples, and/or longitudinal samples) was tabulated to assess whether the data capture information on short-term or long-term variability/changes in exposure. As noted by Meeker et al. (Citation2013): “Characterizing temporal variability in exposure metrics, especially for biomarkers of nonpersistent compounds… is a critical step in designing and interpreting an epidemiology study related to the potential for exposure measurement error.” If not properly accounted for, temporal variability will impact the ability to interpret study results because of a lack of information on whether peak, chronic, or background concentrations are captured.
Results
Literature search and review
The total number of citations identified from the literature search using PubMed and Web of Science™ was 2628. After removal of publications based on the above-noted exclusion criteria, the total number of publications evaluated was 241. After further review, many of the publications were found not to contain have relevant data (see exclusion criteria in the Methods section), leaving 156 for quality assessment (). The following sections describe the results of the quality review. Full details of the quality assessment of the literature using the BEES-C elements described above are provided in Tables S1-S6.
Exposure data quality assessment
2,4-D and food
Sixteen publications were identified in the peer-reviewed literature that included measurements of 2,4-D in food items. However, nine of these publications did not contain data relevant for human exposure assessment, either because they were primarily methodological papers that used a limited number of samples with no information on source or field handling of food items (Anastassiades and Schwack Citation1998; Lancas, Rissato, and Galhiane Citation1999; Lancas, Rissato, and Mozeto Citation1996; Vdovenko et al. Citation2013), because the items measured were in a form not relevant to direct consumption (e.g. wheat straw) (Cessna and Hunter Citation1993; Gowen, Wiersma, and Tai Citation1976; Lancas, Rissato, and Galhiane Citation1999; Lancas, Rissato, and Mozeto Citation1996), because no primary concentration data were provided (Gartrell et al. Citation1985; Shin et al. Citation2011) or because 2,4-D results were not included (Whitmore et al. Citation1994).
Seven studies (Table S1) contained sufficient information to be evaluated for exposure data quality (Frank, Carpentier, and Mackenzie Citation1987; Melnyk, Berry, and Sheldon Citation1997; Morgan et al. Citation2008; Morgan, Wilson, and Chuang Citation2014; Wilson, Chuang, and Lyu Citation2001; Wilson et al. Citation2003, Citation2010). One study evaluated residue on game fish following treatment for Eurasian water milfoil in two Canada lakes (Frank, Carpentier, and Mackenzie Citation1987). The other studies either collected duplicate diet samples of foods and beverages in household and daycare settings (Morgan et al. Citation2008; Morgan, Wilson, and Chuang Citation2014; Wilson, Chuang, and Lyu Citation2001; Wilson et al. Citation2003, Citation2010) or determined food in households from pesticide applicators (Melnyk et el. 1997).
The 5 studies published after 2000 included both field and lab blanks and were therefore considered of highest quality in terms of addressing avoidance of sample contamination (Table S1). Melnyk, Berry, and Sheldon (Citation1997) documented the use of lab blanks (Tier 2). For assessing analyte stability from time of sampling through time of analysis, none of the studies demonstrated that storage time and conditions did not adversely impact analyte stability.
In addition to the quality elements for internal validity described above, strengths and limitations of the data were also considered for use for more generalized exposure assessments. A strength of the 2,4-D/food studies is that they provide measurements of foods and beverages consumed, as opposed to crops or grains that have not yet been processed or are parts of foods not typically consumed. However, several issues are noted: (1) because the foods were combined into an estimate of 2,4-D in solids and liquids, data on individual foods cannot be extracted and used for people whose diets differ from these cohorts; (2) there is limited information on sources of food and their proximity to use of 2,4-D and so changes in diets throughout a given season or year may place an individual in a different 2,4-D exposure category; (3) studies did not clarify whether these data represent cohort exposures at other times of the year and there is no information on whether the food collected is representative of longer-term food and drink consumption habits; and (4) the data are only from Canada and two states in the USA and the cohorts are relatively small.
Minimal information was provided on timing of the most recent use of 2,4-D related to food and drink-related exposures. The source of 2,4-D was clearly apparent in the study of fish (Frank, Carpentier, and Mackenzie Citation1987) since the lakes were treated with 2,4-D and repeated samples were collected post treatment. Only Melnyk, Berry, and Sheldon (Citation1997) attempted to determine the source of the residue on the household food by evaluating the food from a duplicate meal collected in the field during a 2,4-D application. However, this was limited to a single sample. Only one home owner out of the 135 sampled by Morgan et al. (Citation2008) used a 2,4-D product within 3 days of sample collection. Thus, one cannot determine whether these foods and drinks represent peak or background exposure. These combined issues limit the generalizability of the available food data.
2,4-D and soil/dust
Twenty-four studies were identified that measured 2,4-D in soil/dust. Methodological studies with the objective of evaluating an analytical or data collection approach were excluded (Colt Citation1998; Colt et al. Citation2008; De Amarante et al. Citation2003; Roberts and Camann Citation1989). In addition, omitted from further consideration were studies designed to determine the dissipation of 2,4-D following applications and related evaluations of the microbial characteristics of soil (Grover et al. Citation1985; Taskar et al. Citation1982; Voos and Groffman Citation1997; Wilson, Geronimo, and Armbruster Citation1997).
The remaining 16 publications included sufficient exposure-related information for quality assessment (Table S2). The studies were all conducted in the USA or Canada. Two studies collected soil from agricultural settings or after an application beneath power lines (Gowen, Wiersma, and Tai Citation1976; Meru et al. Citation1990) and one was an occupational setting (Frank, Campbell, and Sirons Citation1985). The other investigations examined indoor soil/dust (i.e. dust on bare surfaces and carpet dust). Several investigations included measurements of soil outside the home and/or daycare settings (Morgan et al. Citation2008; Morgan, Wilson, and Chuang Citation2014; Wilson, Chuang, and Lyu Citation2001; Wilson et al. Citation2010). Regarding documentation of avoidance of sample contamination, four studies included both field and lab blanks and are considered Tier 1 (Morgan et al. Citation2008; Morgan, Wilson, and Chuang Citation2014; Wilson, Chuang, and Lyu Citation2001; Wilson et al. Citation2010). Three studies documented the use of either field blanks or lab blanks and are categorized as Tier 2 (Curwin et al. Citation2005b; Deziel et al. Citation2015; Metayer et al. Citation2013).
Regarding assessment of analyte stability from time of sample collection through time of analysis, only two of the studies provided information on sample holding time or recovery of 2,4-D in field samples (Tier 2) (Curwin et al. Citation2005b; Nishioka et al. Citation1999); the remaining studies are considered Tier 3.
A strength of the studies based in homes and day care centers is that they report exposure data on residues specific to the living environment of adults and children (Curwin et al. Citation2005b; Wilson et al. Citation2010). The generalizability to other geographic regions is limited since variables related to deposition include the structure of the building, cleaning frequency, climate, and number of persons living within the household and might differ from region to region and country to country. The method of collecting samples from household vacuum bags is another limitation. While the approach is simple and noninvasive, the investigator cannot determine the timeframe of soil/dust deposition (i.e. the vacuum bag may have been in use for days, weeks, or longer). Notably, when investigators required that subjects owned their rugs for 5 years or more and used their vacuum within the past year, the resulting sample size was reduced by half (Colt et al. Citation2004). Another limitation of vacuum bags is that the vacuum can be used in another place, such as in a vehicle. Deziel et al. (Citation2015) concluded “While measurements of carpet dust provide information on active ingredients, only interview data can provide information on household behaviors such as rooms occupied by children, timing of pesticide use, and other covariates important to evaluating pesticide exposure.”
An additional issue is raised by the studies by Gowen, Wiersma, and Tai (Citation1976), Frank et al. (Citation1985), Meru et al. (Citation1990) and Whitmore et al. (Citation1994); these may have provided relevant exposure data at the time they were conducted, but various aspects of 2,4-D application practices have changed since the 1970’s–1980’s and before using these data to estimate current exposures, applicability of past practices to current ones must be considered (Peterson et al. Citation2016).
2,4-D and air
Forty publications were identified that included 2,4-D concentration data in air (indoor and outdoor ambient air and samples from personal monitors) from a variety of settings (e.g. urban and rural/farm areas, homes, occupational spraying activities). The studies were conducted in the USA, Canada, Europe, Malaysia and South Africa, and were published over a time span from the 1960’s to the 2000’s.
Eight of these publications did not contain data relevant for human exposure assessment and are not considered further. Reasons for exclusion include: no data or insufficient data (e.g. only minimum concentrations, units in mass per sampling device) were provided (Cessna et al. Citation2000; Grover et al. Citation1985; Messing et al. Citation2014; Raeppel et al. Citation2015; Trevisan et al. Citation1993), 2,4-D was measured in precipitation and data on meteorological conditions or rainfall totals were not included (Asman et al. Citation2005), or measurements focused on 2,4-D butyl ester which was replaced by other 2,4-D formulations (Peterson et al. Citation2016) in the 1980’s (Farwell et al. Citation1976; Hee, Sutherland, and Vetter Citation1975).
Thirty-two studies (Table S3) contained sufficient information to proceed with a quality assessment. These studies included a wide range of study locations, settings and approaches. In terms of documentation of procedures used to prevent and/or quantify the extent of sample contamination, 10 studies included both field and lab blanks and are considered Tier 1 (Aulagnier et al. Citation2008; Hines et al. Citation2001; Messing et al. 2011, 2013; Morgan et al. Citation2008; Morgan, Wilson, and Chuang Citation2014; Thomas et al. Citation2010; Waite et al. Citation2002a; Wilson, Chuang, and Lyu Citation2001; Wilson et al. Citation2010). Five studies included only field blanks (Curwin et al. Citation2005b; Whitmore et al. Citation1994; Yao et al. Citation2006) or lab blanks (Baraud et al. Citation2003; Hawthorne et al. Citation1996) and ranked as Tier 2. The remainder did not document avoidance of sample contamination and are thus Tier 3 for this quality element.
For assessing analyte stability, four studies (Curwin et al. Citation2005b; Messing et al. Citation2013; Nishioka et al. Citation2001; Sandmann, Debeer, and Vandyk Citation1991) provided some information on sample storage time (Tier 2, Table S3) but only two (Abbott et al. Citation1987; Lavy et al. Citation1982) specifically assessed whether any “2,4-D was broken down prior to analysis” (Lavy et al. Citation1982). These two are considered to be Tier 1 studies for this quality element. Most of the air samples in the study by Lavy et al. (Citation1982) had concentrations of 2,4-D below the limit of detection (LOD), thus information on analyte stability could not be extracted. Abbott et al. (Citation1987) reported that “Results of field and laboratory recovery tests showed that all 2,4-D applied to the sampling media was recovered in the analytical process.” The remaining studies were considered Tier 3 for analyte stability.
Several of the studies collected data on the relationship between time of sampling and pesticide application (Baharuddin et al. Citation2011; Curwin et al. Citation2005b, Citation1986a; Grover et al. 1976; Harris, Solomon, and Stephenson Citation1992; Hawthorne et al. Citation1996; Hines et al. Citation2001; Kolmodin-Hedman and Erne Citation1980; Kolmodin-Hedman, Hoglund, and Akerblom Citation1983; Lavy et al. Citation1982; Libich et al. Citation1984; Thomas et al. Citation2010). These studies may likely provide the most useful data for assessing short-term exposure potential associated with either occupational or non-occupational use of 2,4-D. These reports may also be informative regarding whether exposure estimates represent peak or background exposure. In contrast, the timing and/or sources of exposure were not specified for the other investigations.
The generalizability of these data for other populations in other geographic regions and/or other conditions is unclear. Ambient air concentrations are impacted by agricultural practices and meteorological conditions and none of the reviewed publications provided information that would allow for translation of study results to other settings. Even for indoor air sampling (Curwin et al. Citation2005b; Morgan et al. Citation2008; Morgan, Wilson, and Chuang Citation2014; Whitmore et al. Citation1994; Wilson, Chuang, and Lyu Citation2001), monitoring results may be heavily dependent on the type of formulation, extent of application, and time(s) since last application as well as structural factors such as room size and ventilation. Further, longitudinal sampling would be needed to understand whether peak exposures were captured as well as the rate of decrease in air concentrations over time.
2,4-D and water
A total of 78 publications were identified that included 2,4-D water concentration data from a variety of settings such as wetlands, streams, lakes, and surface water. The studies were conducted in the USA, Canada, Mexico, Chili, Korea, Malaysia, Australia, and Europe, and were published over a time span from the 1960’s to the 2000’s.
Thirty-four of these publications did not contain data relevant for human exposure assessment and are not considered further. The excluded studies were experimental, analytical, dissipation, or rainfall studies (Amani et al. Citation2011; Anirudhan and Alexander Citation2014; Degenhardt et al. Citation2011; Farenhorst, Andronak, and McQueen Citation2015; Hill et al. Citation2002, Citation2003; Matthiessen et al. Citation1992; Meru et al. Citation1990; Nam et al. Citation2014; Omidi et al. Citation2014; Osborne et al. Citation2015; Qin, Wei, and Li Citation2002; Quaghebeur et al. Citation2004; Ryals, Genter, and Leidy Citation1998; Sandmann, Debeer, and Vandyk Citation1991; Siemering, Hayworth, and Greenfield Citation2008; Trevisan et al. Citation1993; Waite et al. Citation1995, Citation2002b; Watson Citation1977; Yamini and Saleh Citation2013), provided insufficient data (data only in graphical format) (Botta et al. Citation2012; Cerejeira et al. Citation2003; Davis and Lydy Citation2002; Gilliom Citation2007; Mitchell, Brodie, and White Citation2005; Muir and Grift Citation1987; Nagy and Painter Citation1985; Schultz and Harman Citation1974; Schultz and Whitney Citation1974; Spalding, Junk, and Richard Citation1980; Whitmore et al. Citation1994), were a review (Hallberg Citation1989) or monitored a unique site not applicable to general population exposures (Buczynska and Szadkowska-Stanczyk Citation2005).
Forty-four studies (Table S4) contained sufficient information to be evaluated for exposure data quality. These studies included a wide range of study locations, settings and approaches. In terms of steps taken to avoid and/or quantify the extent of sample contamination, 18 studies are considered Tier 2 as the investigators documented use of either field blanks (Bartlett et al. Citation2016; Loos et al. Citation2009, Citation2010a; Loos, Locoro, and Contini Citation2010b; Phillips and Bode Citation2004) or lab blanks (Birch et al. Citation2015; Buser and Muller Citation1998; DeLorenzo et al. Citation2012, Citation2007; Donald et al. Citation2001; Frank et al. Citation1990; Frank, Logan, and Clegg Citation1991; King and Balogh Citation2010; McManus et al. Citation2014; Park et al. Citation2011; Rawn et al. Citation1999b; Woudneh et al. Citation2006, Citation2007). Nine studies included both and are considered as Tier 1 (Cohen et al. Citation1990; Ensminger et al. Citation2013; Felix-Canedo, Duran-Alvarez, and Jimenez-Cisneros Citation2013; Glozier et al. Citation2012; Messing et al. Citation2011; Palma et al. Citation2004; Struger and Fletcher Citation2007; Waite et al. Citation2002a; Wijnja, Doherty, and Safie Citation2014). All the other studies are Tier 3.
Eight of the studies provided some information on sample storage time (Table S4) (Cohen et al. Citation1990; DeLorenzo et al. Citation2012; Herrero-Hernandez et al. Citation2013; King and Balogh Citation2010; Loos et al. Citation2009, Citation2010a; Loos, Locoro, and Contini Citation2010b; Wiergowski et al. Citation2000) but did not demonstrate that 2,4-D was stable over time from collection through analysis (Tier 2). The remainder of studies were Tier 3 for demonstration of analyte stability.
In terms of capturing data on temporal changes in 2,4-D concentrations in water, many of the water studies collected multiple samples, often weekly or monthly, for example, to track decreases in 2,4-D concentration following an application (Wojtalik, Hall, and Hill Citation1971), to understand differences in land use and agricultural practices (Cessna and Elliott Citation2004; Donald et al. Citation2001) or to follow a presumed high exposure “spill” (Frank et al. Citation1990). Other studies sought to capture the impact of seasonal effects of surface water stratification on 2,4-D water concentrations. The findings of investigations were mixed. Woudneh et al. (Citation2006) observed the highest levels of 2,4-D in surface water during spring sampling (as compared to fall), which was consistent with time of herbicide application. An evaluation of both ground water and surface water in Bulgaria concluded that concentrations were associated with intensity of herbicide use and properties of the pesticide (Balinova and Mondesky Citation1999). Similarly, Hall et al. (Citation1993) noted that 2,4-D in urban creeks mainly had detectable levels of 2,4-D shortly after herbicide applications. In contrast, Waite et al. (Citation1992) found no marked correlation with 2,4-D in surface water and pesticide use in a small agricultural watershed, and similarly found no association between groundwater 2,4-D levels with season or rainfall events. Donald et al. (Citation2001) noted no marked differences in 2,4-D concentrations in wetlands near fields with and without 2,4-D application; in some cases, 2,4-D had not been applied to these lands for many years.
Buser and Muller (Citation1998) detected appreciable variations in 2,4-D levels in Swiss surface water samples collected about a month apart but not timed according to pesticide application. Similarly, seasonal variations in 2,4-D concentrations were observed in some but not all waters collected from urban, agricultural, and mixed sites by Woudneh et al. (Citation2007). However, Glozier et al. (Citation2012) did not report seasonal differences in 2,4-D levels in urban and agriculturally-impacted rivers and streams in a national survey in Canada.
Precipitation might also impact 2,4-D levels in water, either through dilution or run off. In studies of urban surface waters with no agricultural inputs, concentrations of 2,4-D were significantly higher in surface waters during rainstorms (Ensminger et al. Citation2013) and after precipitation events (Glozier et al. Citation2012). In agricultural areas, Donald et al. (Citation1999) found that wetlands had higher levels of pesticides when these chemicals were applied prior to significant rainfall events. Palma et al. (Citation2004) and Phillips and Bode (Citation2004) also observed higher 2,4-D levels in surface waters after rain events.
Difficulties in capturing temporal changes in 2,4-D were highlighted by Rawn et al. (Citation1999a) who noted “that detailed temporal patterns of herbicides and nutrients could only be established if high sampling frequency was maintained during such critical periods such as application periods and during major runoff events. An unexpected finding was that herbicide concentrations were not related to runoff losses, but instead they did correspond to increased levels in precipitation and air measured within the watershed.” These investigators recognized the complicated determinants of movement of herbicides through a watershed, which are influenced by factors such as growing season, soil type, terrain, and meteorological conditions.
Overall, data on 2,4-D levels in water included several types of water systems from various countries and climates, ranging from snowmelt in Canada to rice fields in Malaysia. Most investigations collected multiple samples in a watershed. The most relevant for human exposure were those studies of potable water sources. Higher quality investigations collected frequent samples to determine the range of concentrations during a growing season (Glozier et al. Citation2012; Palma et al. Citation2004; Woudneh et al. Citation2007). However, the factors described above impact the generalizability of 2,4-D water data.
2,4-D and urine
A total of 77 publications were identified that included data on 2,4-D levels in urine from adults and/or children. The studies were conducted in the USA, Canada, Europe, Thailand, Caribbean, Nicaragua, and Costa Rica, and six studies with unspecified locations. The study publication years ranged from 1980 to 2015.
Twenty-five of these publications were not considered further. Many contained data described in other publications reviewed here or were analytical methods papers (Adgate et al. Citation2001; Aprea, Sciarra, and Bozzi Citation1997; Arbuckle et al. Citation2002, Citation2006; Arcury et al. Citation2010; Baker et al. Citation2000; Barr et al. Citation1999; Cessna et al. Citation2000; Coble et al. Citation2005, Citation2011; Davis et al. Citation2013; De Felip et al. Citation1988; Figgs et al. Citation2000; Grover et al. Citation1986b; Harris et al. Citation2001, Citation2002; Hill et al. Citation1995b; Holler et al. Citation1989; Knopp, Schmidt, and Niessner Citation1993; Morgan and Jones Citation2013; Morgan et al. Citation2006; Murphy, Kutz, and Strassman Citation1983; Scher et al. Citation2007; Semchuk et al. Citation2003; Shafik, Sullivan, and Enos Citation1971; Taskar et al. Citation1982).
Fifty-two studies (Table S5) included relevant information on urinary 2,4-D measurements in urine and were evaluated for exposure data quality. Three of these studies included documentation of steps taken to avoid and/or quantify the extent of sample contamination in both the field and lab (Tier 1) (Bradman et al. Citation2015; Morgan Citation2015; Morgan et al. Citation2008). Tier 2 studies included either field blanks (Thomas et al. Citation2010) or lab blanks (or blanks of an unspecified nature) (Arbuckle et al. Citation2005; Arcury et al. Citation2007, Citation2009; Curwin et al. Citation2005a; Hill et al. Citation1989; Hines et al. Citation2003; Kutz et al. Citation1992 ; Nigg and Stamper Citation1983 ; Panuwet et al. Citation2008; Raymer et al. 2014 ; Shealy et al. Citation1996 ; Wilson et al. Citation2010). The remaining studies provided no documentation and are considered Tier 3 studies.
Three studies included information on amount of time between sample collection and analysis (Bhatti et al. Citation2010; Knopp Citation1994; Lavy et al. Citation1982). Lavy et al. (Citation1982) specifically demonstrated an effort to assess whether 2,4-D was stable in the samples until analysis.
Because of the uncertainties with interpreting urinary biomonitoring data introduced by urinary dilution, LaKind et al. (Citation2014) recommended that studies include results for volume-based measures and those adjusted for urinary dilution. Thus, for urinary studies, the additional BEES-C element of matrix adjustment was considered. Reporting both creatinine-adjusted and unadjusted urine concentrations has not been widely adopted in the publications reviewed here, with only 13 of the publications including both (Tier 1) (Alexander et al. Citation2007; Arcury et al. Citation2007; Bradman et al. Citation2015; Curwin et al. Citation2005a; Hill et al. Citation1995a, Citation1989; Jurewicz et al. Citation2012; Knopp and Glass Citation1991; Morgan Citation2015; Morgan et al. Citation2008; Panuwet et al. Citation2009, Citation2008; Rodriguez et al. Citation2012).
A key strength of many of these studies is the collection and reporting of information on the relationship between time of urine sampling and time of pesticide application. In addition, several investigators collected urine samples prior to and after 2,4-D application (Alexander et al. Citation2007; Arbuckle and Ritter Citation2005; Hines et al. Citation2003; Kolmodin-Hedman and Erne Citation1980; Lavy et al. Citation1982; Thomas et al. Citation2010; Zhang et al. Citation2011). This information might be used to distinguish peak exposures from “background” exposures.
A limitation of many of these studies is that only one spot sample per participant was collected; because of the short physiological half-life of 2,4-D (Sauerhoff et al. Citation1977), this one measure does not provide information on temporal variability. Other studies collected 12- or 24-hour samples or conducted repeated sampling. These longitudinal studies offer the best opportunities to draw robust conclusions regarding exposures in the study population and to apply their results to other populations.
2,4-D and other biomonitoring matrices
Five studies evaluated 2,4-D in blood (Knopp Citation1994; Knopp, Schmidt, and Niessner Citation1993; Kolmodin-Hedman and Erne Citation1980; Semchuk et al. Citation2004, Citation2003), but one (Knopp, Schmidt, and Niessner Citation1993) was excluded from further consideration as it was a case study of an unusually highly exposed individual (Table S6). Of the remaining studies, Semchuk et al. (2003, Citation2004) reported the use of different types of lab blanks (Tier 2); none of the studies documented the use of field blanks. Only Knopp (Citation1994) provided information on sample storage time (Tier 2); none of the studies included assessments of analyte stability from time of sample collection through sample analysis.
One study on 2,4-D in semen was identified (Arbuckle et al. Citation1999) (Table S6). Lab blanks were included in the protocol, but use of field blanks was not documented (Tier 2). This is interesting as the likelihood of contamination from handling might be difficult to avoid. No data were provided on analyte stability in semen (Tier 3).
Discussion
In this review, the quality of more than 150 published, peer-reviewed studies that reported concentration data on 2,4-D in various media and matrices was examined using elements of the BEES-C instrument. To our knowledge, this is the first comprehensive quality assessment of 2,4-D exposure data. The focus was on the avoidance of sample contamination and the potential for loss of analyte during collection, shipping and storage of the samples. For urinary measures of 2,4-D, it was noted if results were reported both on a volume and creatinine-adjusted basis. Finally, the extent to which studies provided information from multiples measures and time between 2,4-D application and sample collection was examined. These data may determine the degree to which the investigations captured variability in exposures and whether the data represent peak or background exposures.
In the following sections, three questions are discussed: (1) Are the published data of sufficient quality for use in assessing exposures? (2) Are the published data generalizable to broader populations? And (3) What additional information can be gleaned from large-scale population- and media-based studies available from governmental reports?
Are the published data of sufficient quality for use in assessing exposures?
In terms of the elements of the studies reviewed here, no single study was assigned to the highest quality tier (Tier 1, Tables S1–S6) by documenting the steps taken to avoid sample contamination in the field and lab and by demonstrating analyte stability during sample collection, shipping and storage; both sample contamination and analyte stability may impact the internal validity of individual studies (LaKind et al. Citation2014). Sample contamination was found for many other short-lived, commonly used chemicals, despite great efforts taken to avoid contamination (Barr et al. Citation1999; Calafat and Needham Citation2008, Citation2009; LaKind et al. Citation2014; Needham, Calafat, and Barr Citation2007) and inclusion of field and lab blanks is an integral component of quality control (Bartram and Balance Citation1996; USEPA Citation2008, Citation2009). Because 2,4-D is widely used, contamination at low levels is possible; residues on surfaces and equipment might be the source of exposure and/or transferred to a sample (Zhang et al. Citation2011). The transfer of trace amounts of 2,4-D to condoms may have contributed to detection of 2,4-D in semen samples (Arbuckle et al. Citation1999). Exposure of collection materials or matrix to environmental media might result in falsely elevated concentrations of the target analyte.
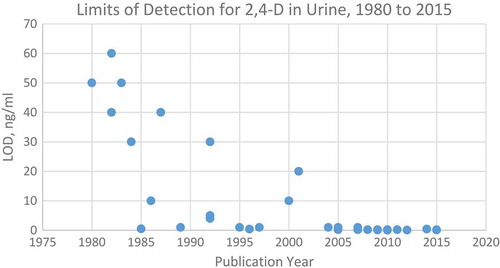
As shown in , analytical LOD for 2,4-D in urine have declined, and therefore the accuracy of measurements of trace levels is increasingly important compared to periods when LOD were comparatively high. For urine biomonitoring, many of the studies reviewed did not include information describing whether participants were instructed on keeping their hands and other body parts free of 2,4-D while collecting samples or how to keep sampling containers free of 2,4-D. Similarly, few studies described field equipment cleaning procedures. This, in combination with lack of blanks, raises concerns regarding the internal validity for these studies, ().
An important factor for internal validity of each study is documentation of 2,4-D stability from the time of sample collection to the time of analysis. Considering the short environmental half-life of 2,4-D and potentially long sample holding times under variable conditions, substantial degradation may occur. Walters (Citation1999) noted that environmental half-lives-ranging 1 to 393 days- depended upon medium, pH, presence of sunlight, oxygen, and microbial activity. 2,4-D in water samples stored at low temperatures (4° C) declined substantially over a period of less than a month (McManus et al. Citation2014). Data on sample stability for 2,4-D in air were available for only 1 week (25°C) (NIOSH Citation1994). While short-term concentrations of 2,4-D-spiked urine in gauze pads kept at 37° C in a water bath did not change markedly over 24 hr (Hu et al. Citation2000), Davis et al. (Citation2013) indicated that additional work is needed to determine the long-term storage stability of 2,4-D conjugates in urine. Harris et al. (Citation2002) who stored urine samples in the freezer for up to 9 months prior to analyses reported no significant differences in mean recoveries among five different storage methods. Most publications reviewed here did not report sample storage times and conditions (Tables S2–S6). Researchers may store samples before and/or after shipping for months or years. A lack of information on storage times or conditions and information on stability of analyte raises questions regarding robustness of the generated data.
Uncontrolled sample contamination may lead to falsely elevated concentrations while analyte degradation during storage may result in falsely lower concentrations. Both erode the accuracy of the exposure information, the validity of using the data in exposure assessments, and ultimately the ability to employ the data to make informed public health decisions. Documentation of quality control is essential. The increasing availability of online publications without page limits provides an opportunity for researchers to make more quality information available, either from primary investigators or by contract labs. Tier 2 and Tier 3 study data may be informative depending upon the purpose of the data. Not all uses require that all elements be Tier 1.
Are the published data generalizable to other populations?
The published studies reviewed here provide an opportunity to develop an understanding of relative sources and ranges of exposures to 2,4-D in a variety of populations. The data provide a starting point for comparing concentrations in the context of use patterns from one location to another. However, the external validity of the body of literature is also important.
Geography and setting
Most of the reports reviewed focused on relatively small, specific geographic regions. To some extent, this is a strength for exposure assessment as this approach enables coordination with local applications and collection of data on conditions relevant to exposures in food, soil, air, and water including meteorological conditions, food consumed, and contact with environmental media since time of application. Equally, exposure models are often population- and geography-specific. At the same time, assumptions regarding generalizability of these data needs to be made with care. With respect to geography, diet studies reviewed were all from North America, which are not necessarily representative of foods consumed elsewhere. Similarly, 2,4-D data for soil and dust were limited to the USA and Canada. Transfer of contaminated soil into the home depends upon the structural characteristics of the dwelling, which vary by geographic region, culture, climate, and economic status. Further, actual exposure to the residents may also be impacted by personal hygiene practices such as showering after work and frequency of cleaning the home.
In contrast, the studies of water and air exhibited a more global scope, spanning North America, Europe, and other continents. However, the generalizability of a given air sample to another population/geographic region is similarly complex as factors that impact air and water concentrations such as meteorological conditions also vary across regions and countries.
Many of the water studies were conducted to assess changes in concentration in a water body over various time periods post-application. While this provides useful surveillance information, other information needs to be known before applying these data to more general models of human exposures. This includes details of the 2,4-D application and meteorological conditions, as well as other factors that affect surface water concentrations such as thermal gradients in the water body. Perhaps the most useful water data are those derived from investigations of drinking water, including dugouts used for potable water which represent a worst-case exposure scenario due to their proximity to tilled farmland (Cessna and Elliott Citation2004; Grover et al. Citation1997; Waite et al. Citation2002a). However, even these data are difficult to interpret; as observed by Donald et al. (Citation2001), levels of 2,4-D in wetlands were similar regardless of whether 2,4-D was applied a few days prior or not for years. This suggests that 2,4-D may be transported from the site of application and be regionally and evenly distributed (Donald et al. Citation2001).
In terms of the biomonitoring studies, there are numerous issues that limit the generalizability of urinary 2,4-D data. These include variations in (1) direct contact with the 2,4-D application process, (2) number of acres treated, (3) gloves and other personal protective equipment use, (4) time between exposure and sample collection, (5) formulation, and (6) environmental factors such as temperature, humidity, wind speed, and direction, and application method (Alexander et al. Citation2007; Arbuckle et al. Citation2005; Garry et al. Citation2001). Collection of these types of data enhance the ability to use biomonitoring results to inform exposure modeling of other global settings, where, as noted by Panuwet et al. (Citation2008), farmers may handle pesticides inappropriately.
Temporal changes
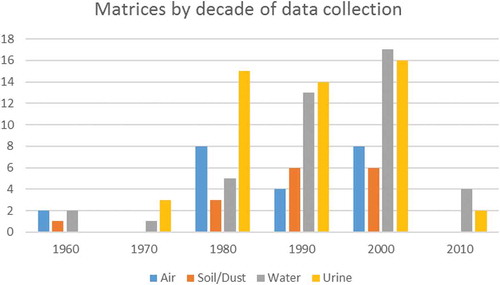
The studies reviewed here span 5 decades (Figure 2) and data were generated using a wide variety of sampling and analytical approaches. The year/decade the experiment was conducted might be highly relevant in weighing the value of data for conducting current exposure assessments as 2,4-D formulations, applications techniques, farming practices, and analytical methods have advanced since the time of the earliest studies. An assessment of temporal changes related to these issues is necessary to understand whether older study results are relevant to today’s exposures in North America and elsewhere.
Various changes occurred since the 1960’s that likely resulted in decreased human exposure to 2,4-D. Arbuckle et al. (Citation2005) found lower urinary 2,4-D concentrations in farmer applicators after 1995 and attributed improvements in education, equipment, and labeling to declining occupational exposure. Early investigators acknowledged that differences in volatility by formulation type impact amounts of drift and volatilization (Farwell et al. Citation1976). The form of the 2,4-D in the formulation also determines its behavior in the environment (Waite et al. Citation2002a). More volatile 2,4-D esters such as propylene glycol butyl ester, methyl ester, isopropyl ester and butyl ester were replaced in the 1980’s by less volatile 2,4-D esters, thereby reducing volatilization after application (Peterson et al. Citation2016). These changes in moiety and agricultural practices are positive steps toward reducing both occupational and non-occupational exposures. In addition, agricultural practices changed to reduce soil erosion and water runoff. In turn, these temporal changes led to reductions in herbicide residue loss. Frank, Logan, and Clegg (Citation1991) attributed decrease in herbicides in water levels from the mid-1970’s to the time of their study in the late 1980’s to improved educational programs and conservation practices. As a result, care needs to be taken in reconstructing exposure in the distant past if using data from the present (and vice versa), particularly for use in epidemiology investigations (Alexander et al. Citation2007). While exposure opportunities today may be higher due to increased uses for agricultural practices and urban encroachment, it may also be lower due to other temporal variations.
Analytical methods have also changed over time resulting in lowered LOD for 2,4-D in urine (). The LOD for 2,4-D in water samples ranged from 50 ng/L for samples collected in 1989 (Waite et al. Citation2002a) to 0.2 ng/L for samples collected in 2013 (Birch et al. Citation2015). This presents a challenge when comparing results across studies. Between 1988–1994, initial general population surveys in the US demonstrated minimal to no detectable 2,4-D exposures in urine sampled when LOD was 1 µg/L (Centers for Disease Control and Prevention, Citation2015; Hill et al. Citation1995a). By 2009–2010, the LOD was almost an order of magnitude lower (0.15 µg/L), and levels of urinary 2,4-D were detectable for half of the sampled population (Centers for Disease Control and Prevention, Citation2015).
Peak versus background exposures
A challenge to estimating human exposures is understanding whether exposure data represent peak or background. For 2,4-D concentration data in environmental media, the half-life varies by environmental conditions such as amount of sunlight, presence of oxygen and microbes, as well as weather conditions. In assessing environmental data for external validity, these factors need to be measured and considered.
For interpreting biomonitoring data, the short physiological half-life in humans (17.7 hr) (Sauerhoff et al. Citation1977; Walters Citation1999) also complicates the ability to infer exposure to the past or future months or years. In general, one cannot state with confidence that a single measurement represents a reliable longer-term exposure estimate. Studies that collected repeated samples during and after work/application demonstrated temporal variability in urinary 2,4-D levels (Alexander et al. Citation2007; Arbuckle et al. 2005; Kolmodin-Hedman and Ene 1980). To illustrate, among corn farmers, Bakke et al. (Citation2009) detected higher urinary 2,4-D levels compared to controls, even during periods when pesticides were not recently applied, suggesting that farmers may be exposed to 2,4-D through sources that are not related to the actual application of 2,4-D. More detailed information is needed when attempting to use study-specific data to generalize to other populations.
More to this point, many of the investigations reviewed here collected only one sample. The validity of a single sample improves with repeatability of the activity or exposure source (Aylward et al. Citation2014). Arcury et al. (Citation2009) recommended that research should “include multiple measures of dose or exposure collected at frequent intervals (daily or weekly) over an extended period with close evaluation of risk factors for exposure. Repeated exposure measures will allow us to discern the trajectory of exposure and the factors that are associated with exposure.” In addition, timing of urine collection is important when assessing exposure. In conclusion, improper use of the available biomonitoring data for estimating exposures within or beyond the examined population may result in exposure misclassification (LaKind et al. Citation2014).
What additional information can be gleaned from large-scale population- and media-based studies available from governmental reports?
Large-scale governmental surveys are undertaken to shed light on sources, routes, and levels of exposure to the general population. Surveys such as the US Pesticide Data Program and US Geological Survey National Water Quality Assessment Program evaluate up to thousands of samples annually in food and drinking water, respectively (Gilliom Citation2007; USDA Citation2004). The European Food Safety Authority also reviews residues on plants and foods as part of its risk assessment procedures (EFSA Citation2016). These large studies report the presence of 2,4-D in various fractions of environmental samples and humans. In the USA, 2,4-D was detected in 16% of 24 drinking water samples in 2002 but in no food samples in 2014 (USDA Citation2004, Citation2015). In Europe, 2,4-D was found in fewer than 5% of the food samples (PDP 2014 ; EFSA Citation2014). A compilation of ambient air data from the USA (14 locations excluding remote locations and areas with direct source impacts; N > 1163) reported a range of ambient air concentrations for 2,4-D (from < LOD to 4 ng/m3) (Kelly et al. Citation1994), although these data may not be relevant to today’s air concentrations due to changes in practices and extent of applications since 1994.
As previously noted, nationally representative biomonitoring studies of urinary 2,4-D from the US and Canada from 1999 to 2010 reported detectable concentrations for up to half of the sampled population (Canada Citation2010; Centers for Disease Control and Prevention Citation2015) (LOD ≤ 0.2 µg/l). This population percentage with detectable levels suggests routes of exposure other than actual applications of 2,4-D and drift from proximity to application. However, these surveys did not include specific information on sources of exposure. Associated data reported by the Add, Centers for Disease Control and Prevention (CDC) as part of the US National Health and Nutrition Examination Survey (NHANES) may be used to test certain hypotheses related to 2,4-D exposure. The publicly available NHANES data were utilized on fasting time as a proxy for contribution of dietary exposure to urinary 2,4-D levels. In adults 21 years and older (N = 1,925), the associations between fasting hours and unadjusted and creatinine-adjusted log urinary 2,4-D levels were not statistically significant. This agrees with results of the study of pregnant women (Lewis et al. Citation2014), in which no significant associations were noted between urinary 2,4-D and consumption of nearly all fruits, vegetables, and legumes (except collards or spinach). In contrast, for children participating in NHANES (<21 years of age, N = 822), significant inverse associations between fasting hours and log urinary 2,4-D were observed (unadjusted: slope = −0.001, p-value = 0.00083; creatinine-adjusted: slope = −0.015, p-value = 0.00033). In other words, longer fasting times were correlated with lower concentrations of 2,4-D in urine, suggesting food as a possible source of exposure in US children. This is in agreement with results of an exposure study of children (Wilson et al. Citation2010) which found that dietary exposures accounted for almost 90% of the aggregate dose of 2,4-D.
The NHANES questionnaire information on application of lawn herbicides was utilized as a proxy for contact with 2,4-D recognizing that not all herbicides contain 2,4-D. The relationship was examined between urinary 2,4-D concentrations and response to the NHANES question “In the past 7 days, were any chemical products used in the lawn or garden to kill weeds?”. The association was positive and significant for adults (N = 1,715) for both unadjusted and creatinine-adjusted urinary levels (p = 0.00021 and 0.0011, respectively); the correlation was not significant for children (N = 807). In adults, a positive response to this question had the effect of increasing urinary 2,4-D concentration by an estimated 75%. Thus, recent use of these products appeared to exert an observable effect on the general US population.
In summary, for governmental reports described here, large studies up to thousands of samples offer information regarding the distribution of exposure within a region or population. However, in the absence of data on timing and uses of 2,4-D, generalizability of this information regarding specific target groups or peak versus background exposure is poor. Nonetheless, data from large-scale governmental investigations may generate hypotheses on which to base future research.
Conclusions
The quality of human health studies is directly reliant upon quality of exposure data. With growing interest in using human exposure and epidemiology data for public health decision-making, the importance of high-quality exposure data has increased. A published quality assessment instrument was utilized to systematically review the large body of literature on 2,4-D exposure data and it was found that few publications (1) documented steps taken to avoid sample contamination in the field and/or lab or (2) demonstrated the stability of the analyte over the time from sample collection through analysis. The possibility that this may reflect incomplete documentation in the final publication(s) is recognized. Only a small number of studies included duration of time between 2,4-D application and sample collection, limiting our ability to evaluate whether results reflected peak or background exposures. Other investigations focused on general surveillance and did not seek to examine the relationship between reported concentrations of 2,4-D and temporal or geographic proximity to 2,4-D applications.
To use the 2,4-D concentration data effectively to support epidemiological investigations, risk assessments and regulatory decisions, improved demonstration of quality control measures in existing and future published studies is needed. Studies with high quality elements were identified in this review, demonstrating the feasibility of using and reporting on quality control elements, either in the body of a publication or as supplemental material. Difficulties in conducting exposure studies of short-lived chemicals with variable formulations, uses, and application processes are recognized. Additional complexities are introduced by factors such as meteorological conditions and human behavior. It is necessary to emphasize that categorization of a quality element as Tier 2 or 3 does not necessarily preclude its use; this decision would depend on professional judgment.
Perhaps the greatest challenge for the exposure community is not the inclusion and documentation of quality control measures, but rather the ability to reach consensus addressing problems specific to short-lived chemical exposures in observational studies. A more comprehensive conversation is needed to address critical issues such as (1) feasibility of timed data collection to minimize exposure misclassification, (2) minimum data requirements for distinguishing peak from background concentrations, and (3) study design improvements to enhance generalizability of study data for advancing our understanding of human exposures to short-lived chemicals, as well as to allow interpretation of these data to catch up to analytical capabilities. Exposure science problems described in this review remain exquisitely difficult to address for chemicals like 2,4-D, with short half-lives and with exposures impacted by numerous and changing external factors including those related to application practices and formulations.
JTEH_MS___R-402_SUPPLEMENTAL_INFORMATION.docx
Download MS Word (133.8 KB)Acknowledgments
The Task Force was not involved in the design, collection, management, analysis, or interpretation of the data; or in the preparation or approval of the manuscript.
Funding
This work was supported by the Industry Task Force II on 2,4-D Research Data. The 2,4-D Research Task Force is made up of those companies owning the technical registrations on the active ingredient in 2,4-D herbicides. They are Dow AgroSciences (U.S.), Nufarm, Ltd. (Australia) and Agro-Gor Corp., a U.S. corporation jointly owned by Alb augh, LLC. (U.S.) and PBI-Gordon Corp. (U.S.).
Supplemental data
Supplemental data for this article can be accessed at the publisher’s website.
Additional information
Funding
References
- Abbott, I. M., J. L. Bonsall, G. Chester, T. B. Hart, and G. J. Turnbull. 1987. Worker exposure to a herbicide applied with ground sprayers in the United Kingdom. AIHA J. 48:167–175. doi:10.1080/15298668791384571.
- Adgate, J. L., D. B. Barr, C. A. Clayton, L. E. Eberly, N. C. Freeman, P. J. Lioy, L. L. Needham, E. D. Pellizzari, J. J. Quackenboss, A. Roy, and K. Sexton. 2001. Measurement of children’s exposure to pesticides: Analysis of urinary metabolite levels in a probability-based sample. Environ. Health Perspect. 109:583–590.
- Alexander, B. H., J. S. Mandel, B. A. Baker, C. J. Bums, M. J. Bartels, J. F. Acquavella, and C. Gustin. 2007. Biomonitoring of 2,4-dichlorophenoxyacetic acid exposure and dose in farm families. Environ. Health Perspect. 115:370–376. doi:10.1289/ehp.8869.
- Amani, V., S. Roshan, A. A. Asgharinezhad, E. Najafi, H. Abedi, N. Tavassoli, and H. Zhad. 2011. Determination of 2,4-D in environmental samples by three phases directly suspended LPME combined with HPLC-UV. Anal. Methods 3:2261–2267. doi:10.1039/c1ay05150d.
- Anastassiades, M., and W. Schwack. 1998. Analysis of carbendazim, benomyl, thiophanate methyl and 2,4-dichlorophenoxyacetic acid in fruits and vegetables after supercritical fluid extraction. J. Chromatogr. A. 825:45–54. doi:10.1016/s0021-9673(98)00691-8.
- Anirudhan, T. S., and S. Alexander. 2014. Multiwalled carbon nanotube based molecular imprinted polymer for trace determination of 2,4-dichlorophenoxyaceticacid in natural water samples using a potentiometric method. Appl. Surface Sci. 303:180–186. doi:10.1016/j.apsusc.2014.02.139.
- Aprea, C., G. Sciarra, and N. Bozzi. 1997. Analytical methods for the determination of urinary 2,4-dichlorophenoxyacetic acid and 2-methyl-4-chlorophenoxyacetic acid in occupationally exposed subjects and in the general population. J. Anal. Toxicol. 21:262–267.
- Arbuckle, T. E., D. Bruce, L. Ritter, and J. C. Hall. 2006. Indirect sources of herbicide exposure for families on Ontario farms. J. Expo Sci. Environ. Epidemiol. 16:98– 104. doi:10.1038/sj.jea.7500441.
- Arbuckle, T. E., R. Burnett, D. Cole, K. Teschke, M. Dosemeci, C. Bancej, and J. Zhang. 2002. Predictors of herbicide exposure in farm applicators. Int. Arch. Occup. Environ. Health. 75:406–414. doi:10.1007/s00420-002-0323-7.
- Arbuckle, T. E., D. C. Cole, L. Ritter, and B. D. Ripley. 2005. Biomonitoring of herbicides in Ontario farm applicators. Scand J. Work Environ. Health. 31 (Suppl 1):90–97. discussion 63-95.
- Arbuckle, T. E., and L. Ritter. 2005. Phenoxyacetic acid herbicide exposure for women on Ontario farms. J. Toxicol. Environ. Health Part A 68:1359–1370. doi:10.1080/15287390590953635.
- Arbuckle, T. E., S. M. Schrader, D. Cole, J. C. Hall, C. M. Bancej, L. A. Turner, and P. Claman. 1999. 2,4-Dichlorophenoxyacetic acid residues in semen of Ontario farmers. Reprod. Toxicol. 13:421–429.
- Arcury, T. A., J. G. Grzywacz, D. B. Barr, J. Tapia, H. Y. Chen, and S. A. Quandt. 2007. Pesticide urinary metabolite levels of children in eastern North Carolina farmworker households. Environ. Health Perspect. 115:1254–1260. doi:10.1289/ehp.9975.
- Arcury, T. A., J. G. Grzywacz, S. Isom, L. E. Whalley, Q. M. Vallejos, H. Chen, L. Galvan, D. B. Barr, and S. A. Quandt. 2009. Seasonal variation in the measurement of urinary pesticide metabolites among Latino farmworkers in eastern North Carolina. Int. J. Occup. Environ. Health 15:339–350. doi:10.1179/oeh.2009.15.4.339.
- Arcury, T. A., J. G. Grzywacz, J. W. Talton, H. Y. Chen, Q. M. Vallejos, L. Galvan, D. B. Barr, and S. A. Quandt. 2010. Repeated pesticide exposure among North Carolina migrant and seasonal farmworkers. Am. J. Ind. Med. 53:802–813. doi:10.1002/ajim.20856.
- Asman, W. A. H., A. Jørgensen, R. Bossi, K. V. Vejrup, B. Bügel Mogensen, and M. Glasius. 2005. Wet deposition of pesticides and nitrophenols at two sites in Denmark: Measurements and contributions from regional sources. Chemosphere 59:1023–1031. doi:http://dx.doi.org/10.1016/j.chemosphere.2004.11.048.
- Aulagnier, F., L. Poissant, D. Brunet, C. Beauvais, M. Pilote, C. Deblois, and N. Dassylva. 2008. Pesticides measured in air and precipitation in the Yamaska Basin (Québec): Occurrence and concentrations in 2004. Sci. Total Environ. 394:338–348. doi:http://dx.doi.org/10.1016/j.scitotenv.2008.01.042.
- Aylward, L. L., S. M. Hays, R. Smolders, H. M. Koch, J. Cocker, K. Jones, N. Warren, L. Levy, and R. Bevan. 2014. Sources of variability in biomarker concentrations. J. Toxicol. Environ. Health B 17:45–61. doi:10.1080/10937404.2013.864250.
- Baharuddin, M. R., I. B. Sahid, M. A. Noor, N. Sulaiman, and F. Othman. 2011. Pesticide risk assessment: A study on inhalation and dermal exposure to 2,4-D and paraquat among Malaysian paddy farmers. J. Environ. Sci. Health. Part. B 46:600–607. doi:10.1080/03601234.2011.589309.
- Baker, S. E., D. B. Barr, W. J. Driskell, M. D. Beeson, and L. L. Needham. 2000. Quantification of selected pesticide metabolites in human urine using isotope dilution high-performance liquid chromatography/tandem mass spectrometry. J. Expo Anal. Environ. Epidemiol. 10:789–798.
- Bakke, B., A. J. De Roos, D. B. Barr, P. A. Stewart, A. Blair, L. B. Freeman, C. F. Lynch, R. H. Allen, M. C. Alavanja, and R. Vermeulen. 2009. Exposure to atrazine and selected non-persistent pesticides among corn farmers during a growing season. J Expo Sci Environ Epidemiol 19(6):544–554. doi: 10.1038/jes.2008.53.
- Balinova, A. M., and M. Mondesky. 1999. Pesticide contamination of ground and surface water in Bulgarian Danube plain. J. Environ. Sci. Health. Part. B 34:33–46. doi:10.1080/03601239909373182.
- Baraud, L., D. Tessier, J. J. Aaron, J. P. Quisefit, and J. Pinart. 2003. A multi-residue method for characterization and determination of atmospheric pesticides measured at two French urban and rural sampling sites. Anal. Bioanal. Chem. 377:1148–1152. doi:10.1007/s00216-003-2196-3.
- Barr, D. B., J. R. Barr, W. J. Driskell, R. H. Hill, D. L. Ashley, L. L. Needham, S. L. Head, and E. J. Sampson. 1999. Strategies for biological monitoring of exposure for contemporary-use pesticides. Toxicol. Ind. Health 15:168–179. doi:10.1191/074823399678846556.
- Barroso, J., I. Y. Ahn, C. Caldeira, P. L. Carmichael, W. Casey, S. Coecke, R. Curren, B. Desprez, C. Eskes, C. Griesinger, J. Guo, E. Hill, A. J. Roi, H. Kojima, J. Li, C. H. Lim, W. Moura, A. Nishikawa, H. Park, S. Peng, O. Presgrave, T. Singer, S. J. Sohn, C. Westmoreland, M. Whelan, X. Yang, Y. Yang, and V. Zuang. 2016. International Harmonization and Cooperation in the Validation of Alternative Methods. Adv. Exp. Med. Biol. 856:343–386. doi:10.1007/978-3-319-33826-2_14.
- Bartlett, A. J., J. Struger, L. C. Grapentine, and V. P. Palace. 2016. Examining impacts of current-use pesticides in Southern Ontario using in situ exposures of the amphipod Hyalella azteca. Environ. Toxicol. Chem. 35:1224–1238. doi:10.1002/etc.3265.
- Bartram, J., and R. Balance. 1996. Water quality monitoring : A practical guide to the design and implementation of freshwater quality studies and monitoring programs. London: United Nations Environment Programme and the World Health Organization.
- Bhatti, P., A. Blair, E. M. Bell, N. Rothman, Q. Lan, D. B. Barr, L. L. Needham, L. Portengen, L. W. Figgs, and R. Vermeulen. 2010. Predictors of 2,4-dichlorophenoxyacetic acid exposure among herbicide applicators. J. Expo Sci. Environ. Epidemiol. 20:160–168. doi:10.1038/jes.2009.14.
- Birch, G. F., D. S. Drage, K. Thompson, G. Eaglesham, and J. F. Mueller. 2015. Emerging contaminants (pharmaceuticals, personal care products, a food additive and pesticides) in waters of Sydney estuary, Australia. Mar. Pollut. Bull. 97:56–66. doi:10.1016/j.marpolbul.2015.06.038.
- Botta, F., N. Fauchon, H. Blanchoud, M. Chevreuil, and B. Guery. 2012. Phyt’Eaux Cites: Application and validation of a programme to reduce surface water contamination with urban pesticides. Chemosphere 86:166–176. doi:10.1016/j.chemosphere.2011.10.005.
- Bradman, A., L. Quiros-Alcala, R. Castorina, R. A. Schall, J. Camacho, N. T. Holland, D. B. Barr, and B. Eskenazi. 2015. Effect of organic diet intervention on pesticide exposures in young children living in low-income urban and agricultural communities. Environ. Health Perspect. 123:1086–1093. doi:10.1289/ehp.1408660.
- Buczynska, A., and I. Szadkowska-Stanczyk. 2005. Identification of health hazards to rural population living near pesticide dump sites in Poland. Int. J. Occup Med. Environ. Health 18:331–339.
- Burns, C. J., and G. M. Swaen. 2012. Review of 2,4-dichlorophenoxyacetic acid (2,4-D) biomonitoring and epidemiology. Crit. Rev. Toxicol. 42:768–786. doi:10.3109/10408444.2012.710576.
- Buser, H. R., and M. D. Muller. 1998. Occurrence and transformation reactions of chiral and achiral phenoxyalkanoic acid herbicides in lakes and rivers in Switzerland. Environ. Sci. Technol. 32:626–633. doi:10.1021/es970652w.
- Calafat, A. M., and L. L. Needham. 2008. Factors affecting the evaluation of biomonitoring data for human exposure assessment. Int. J. Androl. 31:139–143. doi:10.1111/j.1365-2605.2007.00826.x.
- Calafat, A. M., and L. L. Needham. 2009. What additional factors beyond state-of-the-art analytical methods are needed for optimal generation and interpretation of biomonitoring data? Environ. Health Perspect. 117:1481–1485. doi:10.1289/ehp.0901108.
- Canada, H. 2010. Report on human biomonitoring of environmental chemicals in Canada. Results of the Canadian Health Measures Survey Cycle 1 (2007–2009).
- Centers for Disease Control and Prevention. 2015. Fourth report on human exposure to environmental chemicals, Updated Tables, (February, 2015). U.S. Department of Health and Human Services, Atlanta, GA.
- Cerejeira, M. J., P. Viana, S. Batista, T. Pereira, E. Silva, M. J. Valerio, A. Silva, M. Ferreira, and A. M. Silva-Fernandes. 2003. Pesticides in Portuguese surface and ground waters. Water Res. 37:1055–1063. doi:10.1016/s0043-1354(01)00462-6.
- Cessna, A. J., and J. A. Elliott. 2004. Seasonal variation of herbicide concentrations in prairie farm dugouts. J. Environ. Qual. 33:302–315.
- Cessna, A. J., and J. H. Hunter. 1993. Residues of 2,4-D and dicamba in wheat following postemergence field application as a tank mixture. Can. J. Plant Sci. 73:345–349.
- Cessna, A. J., D. T. Waite, L. A. Kerr, and R. Grover. 2000. Duplicate sampling reproducibility of atmospheric residues of herbicides for paired pan and high-volume air samplers. Chemosphere 40:795–802.
- Coble, J., T. Arbuckle, W. J. Lee, M. Alavanja, and M. Doserneci. 2005. The validation of a pesticide exposure algorithm using biological monitoring results. J. Occup. Environ. Hyg. 2:194–201. doi:10.1080/15459620590923343.
- Coble, J., K. W. Thomas, C. J. Hines, J. A. Hoppin, M. Dosemeci, B. Curwin, J. H. Lubin, L. E. B. Freeman, A. Blair, D. P. Sandler, and M. C. R. Alavanja. 2011. An updated algorithm for estimation of pesticide exposure intensity in the Agricultural Health Study. Int. J. Environ. Res. Public Health 8:4608–4622. doi:10.3390/ijerph8124608.
- Cohen, S. Z., S. Nickerson, R. Maxey, A. Dupuy, and J. A. Senita. 1990. A ground-water monitoring study for pesticides and nitrates associated with golf-courses on Cape-Cod. Groundwater Monit. Rem. 10:160–173. doi:10.1111/j.1745-6592.1990.tb00333.x.
- Colt, J. S. 1998. Comparison of pesticides and other compounds in carpet dust samples collected from used vacuum cleaner bags and from a high-volume surface sampler. Environ. Health Perspect. 106:721–724.
- Colt, J. S., R. B. Gunier, C. Metayer, M. G. Nishioka, E. M. Bell, P. Reynolds, P. A. Buffler, and M. H. Ward. 2008. Household vacuum cleaners vs. the high-volume surface sampler for collection of carpet dust samples in epidemiologic studies of children. Environ. Health 7:6. doi:10.1186/1476-069X-7-6.
- Colt, J. S., J. Lubin, D. Camann, S. Davis, J. Cerhan, R. K. Severson, W. Cozen, and P. Hartge. 2004. Comparison of pesticide levels in carpet dust and self-reported pest treatment practices in four US sites. J. Expo. Anal. Environ. Epidemiol. 14:74–83. doi:10.1038/sj.jea.7500307.
- Curwin, B. D., M. J. Hein, W. T. Sanderson, D. B. Barr, D. Heederik, S. J. Reynolds, E. M. Ward, and M. C. Alavanja. 2005a. Urinary and hand wipe pesticide levels among farmers and nonfarmers in Iowa. J. Expo. Anal. Environ. Epidemiol. 15:500–508. doi:10.1038/sj.jea.7500428.
- Curwin, B. D., M. J. Hein, W. T. Sanderson, M. G. Nishioka, S. J. Reynolds, E. M. Ward, and M. C. Alavanja. 2005b. Pesticide contamination inside farm and nonfarm homes. J. Occup. Environ. Hyg. 2:357–367. doi:10.1080/15459620591001606.
- Davis, M. D., E. L. Wade, P. R. Restrepo, W. Roman-Esteva, R. Bravo, P. Kuklenyik, and A. M. Calafat. 2013. Semi-automated solid phase extraction method for the mass spectrometric quantification of 12 specific metabolites of organophosphorus pesticides, synthetic pyrethroids, and select herbicides in human urine. J. Chromatogr. B-Anal Technol. Biomed. Life Sci. 929:18–26. doi:10.1016/j.jchromb.2013.04.005.
- Davis, N. M., and M. J. Lydy. 2002. Evaluating best management practices at an urban golf course. Environ. Toxicol. Chem. 21:1076–1084.
- De Amarante, O. P.Jr., N. M. Brito, T. C. Dos Santos, G. S. Nunes, and M. L. Ribeiro. 2003. Determination of 2,4-dichlorophenoxyacetic acid and its major transformation product in soil samples by liquid chromatographic analysis. Talanta 60:115–121. doi:10.1016/s0039-9140(03)00113-9.
- De Felip, E., A. Di Domenico, F. Tancredi, F. Volpi, and G. Bagnasco. 1988. Levels of 4-chloro-2-methylphenoxyacetic acid (MCPA) in the urine of northern Italy occupationally exposed agricultural workers. Ecotoxicol. Environ. Saf. 16:170–175.
- Degenhardt, D., A. J. Cessna, R. Raina, A. Farenhorst, and D. J. Pennock. 2011. Dissipation of six acid herbicides in water and sediment of two Canadian prairie wetlands. Environ. Toxicol. Chem. 30:1982–1989. doi:10.1002/etc.598.
- DeLorenzo, M. E., B. Thompson, E. Cooper, J. Moore, and M. H. Fulton. 2012. A long-term monitoring study of chlorophyll, microbial contaminants, and pesticides in a coastal residential stormwater pond and its adjacent tidal creek. Environ. Monit. Assess. 184:343–359. doi:10.1007/s10661-011-1972-3.
- Deziel, N. C., J. S. Colt, E. E. Kent, R. B. Gunier, P. Reynolds, B. Booth, C. Metayer, and M. H. Ward. 2015. Associations between self-reported pest treatments and pesticide concentrations in carpet dust. Environ. Health 14:27. doi:10.1186/s12940-015-0015-x.
- Donald, D. B., A. J. Cessna, E. Sverko, and N. E. Glozier. 2007. Pesticides in surface drinking-water supplies of the northern Great Plains. Environ. Health Perspect. 115:1183–1191. doi:10.1289/ehp.9435.
- Donald, D. B., N. P. Gurprasad, L. Quinnett-Abbott, and K. Cash. 2001. Diffuse geographic distribution of herbicides in northern prairie wetlands. Environ. Toxicol. Chem. 20:273–279. doi:10.1897/1551-5028(2001)020<0273:dgdohi>2.0.co;2.
- Donald, D. B., J. Syrgiannis, F. Hunter, and G. Weiss. 1999. Agricultural pesticides threaten the ecological integrity of northern prairie wetlands. Sci. Total Environ. 231:173–181. doi:10.1016/s0048-9697(99)00091-1.
- EFSA. 2016. 2014 European Union Report on Pesticide residues in Food. EFSA Journal. doi:10.2903/j.efsa.2016.4611.
- Ensminger, M. P., R. Budd, K. C. Kelley, and K. S. Goh. 2013. Pesticide occurrence and aquatic benchmark exceedances in urban surface waters and sediments in three urban areas of California, USA, 2008-2011. Environ. Monit. Assess 185:3697–3710. doi:10.1007/s10661-012-2821-8.
- Farenhorst, A., L. A. Andronak, and R. D. A. McQueen. 2015. Bulk deposition of pesticides in a Canadian City: Part 1. Glyphosate and other agricultural pesticides. Water Air Soil Pollut. 226. doi:10.1007/s11270-015-2343-4.
- Farwell, S. O., E. Robinson, W. J. Powell, and D. F. Adams. 1976. Survey of airborne 2,4,-D in South-Central Washington. J. Air Pollut. Control Assoc. 26:224–230.
- Felix-Canedo, T. E., J. C. Duran-Alvarez, and B. Jimenez-Cisneros. 2013. The occurrence and distribution of a group of organic micropollutants in Mexico City’s water sources. Sci. Total Environ. 454-455:109–118. doi:10.1016/j.scitotenv.2013.02.088.
- Figgs, L. W., N. T. Holland, N. Rothman, S. H. Zahm, R. E. Tarone, R. Hill, R. F. Vogt, M. T. Smith, C. D. Boysen, F. F. Holmes, K. VanDyck, and A. Blair. 2000. Increased lymphocyte replicative index following 2,4-dichlorophenoxyacetic acid herbicide exposure. Cancer Causes Control 11:373–380. doi:10.1023/a:1008925824242.
- Frank, R., H. E. Braun, B. D. Ripley, and B. S. Clegg. 1990. Contamination of rural ponds with pesticide, 1971-85, Ontario, Canada. Bull. Environ. ContamToxicol. 44:401–409.
- Frank, R., R. A. Campbell, and G. J. Sirons. 1985. Forestry workers involved in aerial application of 2,4-dichlorophenoxyacetic acid (2,4-D): Exposure and urinary excretion. Arch Environ Contam Toxicol 14(4):427–435.
- Frank, R., A. G. Carpentier, and D. L. Mackenzie. 1987. Monitoring for 2,4-residues in fish species resident in treated lakes in east-central Ontario 1977-80. Environ. Monit. Assess. 9:71–82. doi:10.1007/bf00394217.
- Frank, R., L. Logan, and B. S. Clegg. 1991. Pesticide and polychlorinated biphenyl residues in waters at the mouth of the Grand, Saugeen, and Thames Rivers, Ontario, Canada, 1986-1990. Arch. Environ. Contam. Toxicol. 21:585–595.
- Garry, V. F., R. E. Tarone, I. R. Kirsch, J. M. Abdallah, D. P. Lombardi, L. K. Long, B. L. Burroughs, D. B. Barr, and J. S. Kesner. 2001. Biomarker correlations of urinary 2,4-D levels in foresters: Genomic instability and endocrine disruption. Environ. Health Perspect. 109:495–500.
- Gartrell, M. J., J. C. Craun, D. S. Podrebarac, and E. L. Gunderson. 1985. Pesticides, selected elements, and other chemicals in infant and toddler total diet samples, October 1978-September 1979. J. Assoc. Off Anal. Chem. 68:842–861.
- Gilliom, R. J. 2007. Pesticides in U.S. streams and groundwater. Environ. Sci. Technol. 41:3407–3413.
- Glozier, N. E., J. Struger, A. J. Cessna, M. Gledhill, M. Rondeau, W. R. Ernst, M. A. Sekela, S. J. Cagampan, E. Sverko, C. Murphy, J. L. Murray, and D. B. Donald. 2012. Occurrence of glyphosate and acidic herbicides in select urban rivers and streams in Canada, 2007. Environ. Sci. Pollut. Res. Int. 19:821–834. doi:10.1007/s11356-011-0600-7.
- Gowen, J. A., G. B. Wiersma, and H. Tai. 1976. Mercury and 2,4-D levels in wheat and soils from sixteen states, 1969. Pest Monit. J. 10:111–113.
- Grover, R., A. J. Cessna, N. I. Muir, D. Riedel, C. A. Franklin, and K. Yoshida. 1986a. Factors affecting the exposure of ground-rig applicators to 2,4-D dimethylamine salt. Arch. Environ. Contam. Toxicol. 15:677–686.
- Grover, R., C. A. Franklin, N. I. Muir, A. J. Cessna, and D. Riedel. 1986b. Dermal exposure and urinary metabolite excretion in farmers repeatedly exposed to 2,4-D amine. Toxicol. Lett. 33:73–83.
- Grover, R., L. A. Kerr, K. Wallace, K. Yoshida, and J. Maybank. 1976. Residues of 2, 4-D in air samples from Saskatchewan: 1966-1975. J. Environ. Sci. Health. Part. B 11:331–347. doi:10.1080/10934527609385775.
- Grover, R., S. Shewchuk, A. Cessna, A. Smith, and J. Hunter. 1985. Fate of 2,4-D iso-octylester after application to a wheat field. J. Environ. Qual. 14:3–210.
- Grover, R., D. T. Waite, A. J. Cessna, W. Nicholaichuk, D. G. Irvin, L. A. Kerr, and K. Best. 1997. Magnitude and persistence of herbicide residues in farm dugouts and ponds in the Canadian prairies. Environ. Toxicol. Chem. 16:638–643. doi:10.1897/1551-5028(1997)016<0638:mapohr>2.3.co;2.
- Hall, J. C., T. D. Van Deynze, J. Struger, and C. H. Chan. 1993. Enzyme immunoassay based survey of precipitation and surface water for the presence of atrazine, metolachlor and 2,4-D. J. Environ. Sci. Health. Part. B 28:577–598. doi:10.1080/03601239309372842.
- Hallberg, G. R. 1989. Pesticide pollution of groundwater in the humid United States. Agric. Ecosystems Environ. 26:299–367. doi:10.1016/0167-8809(89)90017-0.
- Harris, S. A., P. N. Corey, A. M. Sass-Kortsak, and J. T. Purdham. 2001. The development of a new method to estimate total daily dose of pesticides in professional turf applicators following multiple and varied exposures in occupational settings. Int. Arch. Occup. Environ. Health 74:345–358.
- Harris, S. A., A. M. Sass-Kortsak, P. N. Corey, and J. T. Purdham. 2002. Development of models to predict dose of pesticides in professional turf applicators. J. Expo Anal. Environ. Epidemiol. 12:130–144. doi:10.1038/sj.jea.7500208.
- Harris, S. A., K. R. Solomon, and G. R. Stephenson. 1992. Exposure of homeowners and bystanders to 2,4-dichlorophenoxyacetic acid (2,4-D). J. Environ. Sci. Health. Part. B 27:23–38. doi:10.1080/03601239209372765.
- Hawthorne, S. B., D. J. Miller, P. K. K. Louie, R. D. Butler, and G. G. Mayer. 1996. Vapor-phase and particulate-associated pesticides and PCB concentrations in Eastern North Dakota air samples. J. Environ. Qual. 25:594–600.
- Hee, S. S. Q., R. G. Sutherland, and M. Vetter. 1975. Glc [gas-liquid chromatographic] analysis of 2,4-D concentrations in air samples from central Saskatchewan in 1972. Environ. Sci. Technol. 9:62–66. doi:10.1021/es60099a003.
- Herrero-Hernandez, E., E. Rodriguez-Gonzalo, M. S. Andrades, S. Sanchez-Gonzalez, and R. Carabias-Martinez. 2013. Occurrence of phenols and phenoxyacid herbicides in environmental waters using an imprinted polymer as a selective sorbent. Sci. Total Environ. 454-455:299–306. doi:10.1016/j.scitotenv.2013.03.029.
- Hill, B. D., K. N. Harker, P. Hasselback, D. J. Inaba, S. D. Byers, and J. R. Moyer. 2002. Herbicides in Alberta rainfall as affected by location, use and season: 1999 to 2000. Water Qual. Res. J. Can. 37:515–542.
- Hill, B. D., D. J. Inaba, S. D. Byers, and C. A. Grant. 2003. Levels of “phenoxy” herbicides in prairie rainfall during 2000-2001. Can. J. Plant Sci. 83:467–470.
- Hill, R. H., S. L. Head, S. Baker, M. Gregg, D. B. Shealy, S. L. Bailey, C. C. Williams, E. J. Sampson, and L. L. Needham. 1995a. Pesticide residues in urine of adults living in the United States: Reference range concentrations. Environ. Res. 71:99–108. doi:10.1006/enrs.1995.1071.
- Hill, R. H., D. B. Shealy, S. L. Head, C. C. Williams, S. L. Bailey, M. Gregg, S. E. Baker, and L. L. Needham. 1995b. Determination of pesticide metabolites in human urine using an isotope-dilution technique and tandem mass-spectrometry. J. Anal. Toxicol. 19:323–329.
- Hill, R. H.Jr., T. To, J. S. Holler, D. M. Fast, S. J. Smith, L. L. Needham, and S. Binder. 1989. Residues of chlorinated phenols and phenoxy acid herbicides in the urine of Arkansas children. Arch. Environ. Contam. Toxicol. 18:469–474.
- Hines, C. J., J. A. Deddens, C. A. F. Striley, R. E. Biagini, D. A. Shoemaker, K. K. Brown, B. A. MacKenzie, and R. D. Hull. 2003. Biological monitoring for selected herbicide biomarkers in the urine of exposed custom applicators: Application of mixed-effect models. Ann. Occup. Hyg. 47:503–517. doi:10.1093/annhyg/meg067.
- Hines, C. J., J. A. Deddens, S. P. Tucker, and R. W. Hornung. 2001. Distributions and determinants of pre-emergent herbicide exposures among custom applicators. Ann. Occup. Hyg. 45:227–239.
- Holler, J. S., D. M. Fast, R. H. Hill, F. L. Cardinali, G. D. Todd, J. M. McCraw, S. L. Bailey, and L. L. Needham. 1989. Quantification of selected herbicides and chlorinated phenols in urine by using gas-chromatography mass-spectrometry. J. Anal. Toxicol. 13:152–157.
- Hu, Y. A., D. B. Barr, G. Akland, L. Melnyk, L. Needham, E. D. Pellizzari, J. H. Raymer, and J. M. Roberds. 2000. Collecting urine samples from young children using cotton gauze for pesticide studies. J. Expo. Anal. Environ. Epidemiol. 10:703–709.
- Jurewicz, J., W. Hanke, W. Sobala, and D. Ligocka. 2012. Exposure to phenoxyacetic acid herbicides and predictors of exposure among spouses of farmers. Ann. Agric. Environ. Med. 19:51–56.
- Kelly, T., R. Mukund, C. Spicer, and A. Pollack. 1994. Concentrations and transformations of hazardous air pollutants. Environ. Sci. Technol. 28:378A–387A. doi:10.1021/es00057a719.
- King, K. W., and J. C. Balogh. 2010. Chlorothalonil and 2,4-D losses in surface water discharge from a managed turf watershed. J. Environ. Monit. 12:1601–1612. doi:10.1039/c0em00030b.
- Knopp, D. 1994. Assessment of exposure to 2,4-dichlorophenoxyacetic acid in the chemical industry: Results of a five year biological monitoring study. Occup. Environ. Med. 51:152–159.
- Knopp, D., and S. Glass. 1991. Biological monitoring of 2,4-dichlorophenoxyacetic acid-exposed workers in agriculture and forestry. Int. Arch. Occup. Environ. Health 63:329–333.
- Knopp, D., M. Schmidt, and R. Niessner. 1993. Accidental bystander overexposure to the herbicide 2,4-dichlorophenoxyacetic acid. Fresenius Environ. Bull. 2:148–150.
- Kolmodin-Hedman, B., and K. Erne. 1980. Estimation of occupational exposure to phenoxy acids (2,4-D and 2,4,5-T). Arch. Toxicol. Suppl. 4:318–321.
- Kolmodin-Hedman, B., S. Hoglund, and M. Akerblom. 1983. Studies on phenoxy acid herbicides. I. Field study. Occupational exposure to phenoxy acid herbicides (MCPA, dichlorprop, mecoprop and 2,4-D) in agriculture. Arch. Toxicol. 54:257–265.
- Kutz, F. W., B. T. Cook, O. D. Carter-Pokras, D. Brody, and R. S. Murphy. 1992. Selected pesticide residues and metabolites in urine from a survey of the U.S. general population. J Toxicol Environ Health 37(2):277–291. doi: 10.1080/15287399209531670.
- LaKind, J. S., M. Goodman, D. B. Barr, C. P. Weisel, and G. Schoeters. 2015. Lessons learned from the application of BEES-C: Systematic assessment of study quality of epidemiologic research on BPA, neurodevelopment, and respiratory health. Environ. Int. 80:41–71. doi:10.1016/j.envint.2015.03.015.
- LaKind, J. S., and D. Q. Naiman. 2015. Temporal trends in bisphenol A exposure in the United States from 2003–2012 and factors associated with BPA exposure: Spot samples and urine dilution complicate data interpretation. Environ. Res. 142:84–95. doi:10.1016/j.envres.2015.06.013.
- LaKind, J. S., J. R. Sobus, M. Goodman, D. B. Barr, P. Fürst, R. J. Albertini, T. E. Arbuckle, G. Schoeters, Y. M. Tan, J. Teeguarden, R. Tornero-Velez, and C. P. Weisel. 2014. A proposal for assessing study quality: Biomonitoring, Environmental Epidemiology, and Short-lived Chemicals (BEES-C) instrument. Environ. Int. 73:195–207. doi:10.1016/j.envint.2014.07.011.
- Lancas, F. M., S. R. Rissato, and M. S. Galhiane. 1999. Determination of 2,4-D and dicamba in food crops by MEKC. Chromatographia 50:35–40. doi:10.1007/bf02493614.
- Lancas, F. M., S. R. Rissato, and A. A. Mozeto. 1996. Off-line SFE-CGC-ECD analysis of 2,4-D and dicamba residues in real sugar cane, rice and corn samples. HRC-J High Res. Chromatogr. 19:564–568.
- Lavy, T. L., J. D. Walstad, R. R. Flynn, and J. D. Mattice. 1982. (2,4-Dichlorophenoxy)acetic acid exposure received by aerial application crews during forest spray operations. J. Agric. Food Chem. 30:375–381. doi:10.1021/jf00110a042.
- Lewis, R. C., D. E. Cantonwine, L. V. Anzalota Del Toro, A. M. Calafat, L. Valentin-Blasini, M. D. Davis, S. E. Baker, A. N. Alshawabkeh, J. F. Cordero, and J. D. Meeker. 2014. Urinary biomarkers of exposure to insecticides, herbicides, and one insect repellent among pregnant women in Puerto Rico. Environ. Health 13:97. doi:10.1186/1476-069x-13-97.
- Libich, S., J. C. To, R. Frank, and G. J. Sirons. 1984. Occupational exposure of herbicide applicators to herbicides used along electric power transmission line right-of-way. Am. Ind. Hyg. Assoc. J. 45:56–62. doi:10.1080/15298668491399370.
- Loos, R., B. M. Gawlik, G. Locoro, E. Rimaviciute, S. Contini, and G. Bidoglio. 2009. EU-wide survey of polar organic persistent pollutants in European river waters. Environ. Pollut. 157:561–568. doi:10.1016/j.envpol.2008.09.020.
- Loos, R., G. Locoro, S. Comero, S. Contini, D. Schwesig, F. Werres, P. Balsaa, O. Gans, S. Weiss, L. Blaha, M. Bolchi, and B. M. Gawlik. 2010a. Pan-European survey on the occurrence of selected polar organic persistent pollutants in ground water. Water Res. 44:4115–4126. doi:http://dx.doi.org/10.1016/j.watres.2010.05.032.
- Loos, R., G. Locoro, and S. Contini. 2010b. Occurrence of polar organic contaminants in the dissolved water phase of the Danube River and its major tributaries using SPE-LC-MS(2) analysis. Water Res. 44:2325–2335. doi:10.1016/j.watres.2009.12.035.
- Matthiessen, P., C. Allchin, R. J. Williams, S. C. Bird, D. Brooke, and P. J. Glendinning. 1992. The translocation of some herbicides between soil and water in a small catchment. J. Inst. Water Environ. Manage 6:496–504.
- McManus, S. L., M. Moloney, K. G. Richards, C. E. Coxon, and M. Danaher. 2014. Determination and occurrence of phenoxyacetic acid herbicides and their transformation products in groundwater using ultra high performance liquid chromatography coupled to tandem mass spectrometry. Molecules 19:20627–20649. doi:10.3390/molecules191220627.
- Meeker, J. D., D. E. Cantonwine, L. O. Rivera-González, K. K. Ferguson, B. Mukherjee, A. M. Calafat, X. Ye, L. V. Anzalota Del Toro, N. Crespo-Hernández, B. Jiménez-Vélez, A. N. Alshawabkeh, and J. F. Cordero. 2013. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ. Sci. Technol. 47: 3439–3447. doi:10.1021/es400510g.
- Melnyk, L. J., M. R. Berry, and L. S. Sheldon. 1997. Dietary exposure from pesticide application on farms in the agricultural health pilot study. J. Expo. Anal. Environ. Epidemiol. 7:61–80.
- Meru, S., K. Liber, K. Stonefield, K. Solomon, and G. Stephenson. 1990. Persistence and lateral movement of 2,4-dichlorophenoxy acetic acid and picloram on power line rights-of-way. Arch. Environ. Contam. Toxicol. 19:572–577.
- Messing, P., A. Farenhorst, D. Waite, and J. Sproull. 2013. Influence of usage and chemical-physical properties on the atmospheric transport and deposition of pesticides to agricultural regions of Manitoba, Canada. Chemosphere 90:1997–2003. doi:10.1016/j.chemosphere.2012.10.071.
- Messing, P. G., A. Farenhorst, D. T. Waite, D. A. R. McQueen, J. F. Sproull, D. A. Humphries, and L. L. Thompson. 2011. Predicting wetland contamination from atmospheric deposition measurements of pesticides in the Canadian Prairie Pothole region. Atmos. Environ. 45:7227–7234. doi:10.1016/j.atmosenv.2011.08.074.
- Messing, P. G., A. Farenhorst, D. T. Waite, and J. F. Sproull. 2014. Current-use herbicides in air as influenced by their estimated agricultural use at various distances from six sampling locations. Water Air Soil Pollut. 225. doi:10.1007/s11270-014-2013-y.
- Metayer, C., J. S. Colt, P. A. Buffler, H. D. Reed, S. Selvin, V. Crouse, and M. H. Ward. 2013. Exposure to herbicides in house dust and risk of childhood acute lymphoblastic leukemia. J Expo Sci Environ Epidemiol 23(4):363–370. doi: 10.1038/jes.2012.115.
- Mitchell, C., J. Brodie, and I. White. 2005. Sediments, nutrients and pesticide residues in event flow conditions in streams of the Mackay Whitsunday Region, Australia. Mar. Pollut. Bull. 51:23–36. doi:10.1016/j.marpolbul.2004.10.036.
- Moher, D., and A. C. Tricco. 2008. Issues related to the conduct of systematic reviews: A focus on the nutrition field. Am. J. Clin. Nutr. 88:1191–1199.
- Morgan, M., L. Sheldon, C. Croghan, P. Jones, J. Chuang, and N. Wilson. 2006. Levels of 2,4-dichlorophenoxyacetic acid in the homes and urine of 135 preschool children and their adult caregivers in North Carolina and Ohio. Epidemiology 17:S167–S168. doi:10.1097/00001648-200611001-00421.
- Morgan, M. K. 2015. Predictors of urinary levels of 2,4-dichlorophenoxyacetic acid, 3,5,6-trichloro-2-pyridinol, 3-phenoxybenzoic acid, and pentachlorophenol in 121 adults in Ohio. Int. J. Hyg. Environ. Health 218:479–488. doi:10.1016/j.ijheh.2015.03.015.
- Morgan, M. K., and P. A. Jones. 2013. Dietary predictors of young children’s exposure to current-use pesticides using urinary biomonitoring. Food Chem. Toxicol. 62:131–141. doi:10.1016/j.fct.2013.08.029.
- Morgan, M. K., L. S. Sheldon, K. W. Thomas, P. P. Egeghy, C. W. Croghan, P. A. Jones, J. C. Chuang, and N. K. Wilson. 2008. Adult and children’s exposure to 2,4-D from multiple sources and pathways. J. Expo. Sci. Environ. Epidemiol. 18:486–494. doi:10.1038/sj.jes.7500641.
- Morgan, M. K., N. K. Wilson, and J. C. Chuang. 2014. Exposures of 129 preschool children to organochlorines, organophosphates, pyrethroids, and acid herbicides at their homes and daycares in North Carolina. Int. J. Environ. Res. Public Health 11:3743–3764. doi:10.3390/ijerph110403743.
- Muir, D. C., and N. P. Grift. 1987. Herbicide levels in rivers draining two prairie agricultural watersheds (1984). J. Environ. Sci. Health. Part. B 22:259–284. doi:10.1080/03601238709372557.
- Murphy, R. S., F. W. Kutz, and S. C. Strassman. 1983. Selected pesticide residues or metabolites in blood and urine specimens from a general population survey. Environ. Health Perspect. 48:81–86.
- Nagy, E., and D. S. Painter. 1985. Seasonal variations of 2,4-D in Buckhorn Lake. Water Pollut. Res. J. Can. 20:106–117.
- Nam, S. W., B. I. Jo, Y. Yoon, and K. D. Zoh. 2014. Occurrence and removal of selected micropollutants in a water treatment plant. Chemosphere 95:156–165. doi:10.1016/j.chemosphere.2013.08.055.
- Needham, L. L., A. M. Calafat, and D. B. Barr. 2007. Uses and issues of biomonitoring. Int. J. Hyg. Environ. Health 210:229–238. doi:10.1016/j.ijheh.2006.11.002.
- Nigg, H. N., and J. H. Stamper. 1983. Exposure of Florida airboat aquatic weed applicators to 2,4-dichlorophenoxyacetic acid (2,4-D). Chemosphere 12(2):209–215. doi: 10.1016/0045-6535(83)90163-7.
- NIOSH. 1994. NIOSH Manual of Analytical Methods (NMAM), 4th ed. Atlanta GA: National Institute for Occupational Safety and Health.
- Nishioka, M. G., H. M. Burkholder, M. C. Brinkman, and R. G. Lewis. 1999. Distribution of 2,4-dichlorophenoxyacetic acid in floor dust throughout homes following homeowner and commercial lawn applications: Quantitative effects of children, pets, and shoes. Environ Sci Technol 33(9):1359–1365. doi: 10.1021/es980580o.
- Nishioka, M. G., R. G. Lewis, M. C. Brinkman, H. M. Burkholder, C. E. Hines, and J. R. Menkedick. 2001. Distribution of 2,4-D in air and on surfaces inside residences after lawn applications: Comparing exposure estimates from various media for young children. Environ. Health Perspect. 109:1185–1191.
- Omidi, F., M. Behbahani, H. S. Abandansari, A. Sedighi, and S. J. Shahtaheri. 2014. Application of molecular imprinted polymer nanoparticles as a selective solid phase extraction for preconcentration and trace determination of 2,4-dichlorophenoxyacetic acid in the human urine and different water samples. J. Environ. Health Sci. Eng. 12. doi:10.1186/s40201-014-0137-z.
- Osborne, P. P., Z. Xu, K. D. Swanson, T. Walker, and D. K. Farmer. 2015. Dicamba and 2,4-D residues following applicator cleanout: A potential point source to the environment and worker exposure. J. Air Waste Manage. Assoc. 65:1153–1158. doi:10.1080/10962247.2015.1072593.
- Palma, G., A. Sanchez, Y. Olave, F. Encina, R. Palma, and R. Barra. 2004. Pesticide levels in surface waters in an agricultural-forestry basin in Southern Chile. Chemosphere 57:763–770. doi:10.1016/j.chemosphere.2004.08.047.
- Panuwet, P., T. Prapamontol, S. Chantara, and D. B. Barr. 2009. Urinary pesticide metabolites in school students from northern Thailand. Int. J. Hyg. Environ. Health 212:288–297. doi:10.1016/j.ijheh.2008.07.002.
- Panuwet, P., T. Prapamontol, S. Chantara, P. Thavornyuthikarn, M. A. Montesano, R. D. Whitehead Jr., and D. B. Barr. 2008. Concentrations of urinary pesticide metabolites in small-scale farmers in Chiang Mai Province, Thailand. Sci. Total Environ. 407:655–668. doi:10.1016/j.scitotenv.2008.08.044.
- Park, J. Y., J. H. Choi, A. M. Abd El-Aty, B. M. Kim, J. H. Park, W. J. Choi, and J. H. Shim. 2011. Development and validation of an analytical method for determination of endocrine disruptor, 2,4-D, in paddy field water. Biomed. Chromatogr. 25:1018–1024. doi:10.1002/bmc.1559.
- Peterson, M., S. McMaster, D. Riechers, J. Skelton, and P. Stahlman. 2016. 2,4-D past, present, and future: A review. Weed Technol. 30:303–345.
- Phillips, P. J., and R. W. Bode. 2004. Pesticides in surface water runoff in south-eastern New York State, USA: Seasonal and stormflow effects on concentrations. Pest Manag. Sci. 60:531–543. doi:10.1002/ps.879.
- Qin, W., H. Wei, and S. F. Li. 2002. Determination of acidic herbicides in surface water by solid-phase extraction followed by capillary zone electrophoresis. J. Chromatogr. Sci. 40:387–391.
- Quaghebeur, D., B. De Smet, E. De Wulf, and W. Steurbaut. 2004. Pesticides in rainwater in Flanders, Belgium: Results from the monitoring program 1997-2001. J. Environ. Monit. 6:182–190. doi:10.1039/b312558k.
- Raeppel, C., M. Fabritius, M. Nief, B. M. Appenzeller, O. Briand, L. Tuduri, and M. Millet. 2015. Analysis of airborne pesticides from different chemical classes adsorbed on Radiello(R) Tenax(R) passive tubes by thermal-desorption-GC/MS. Environ. Sci. Pollut. Res. Int. 22:2726–2734. doi:10.1007/s11356-014-3534-z.
- Rawn, D. F. K., T. H. J. Halldorson, W. N. Turner, R. N. Woychuk, J. G. Zakrevsky, and D. C. G. Muir. 1999a. A multi-year study of four herbicides in surface water of a small prairie watershed. J. Environ. Qual. 28:906–917.
- Rawn, D. F. K., T. H. J. Halldorson, R. N. Woychuk, and D. C. G. Muir. 1999b. Pesticides in the Red River and its tributaries in southern Manitoba: 1993-95. Water Qual. Res. J. Can. 34:183–219.
- Roberts, J. W., and D. E. Camann. 1989. Pilot study of a cotton glove press test for assessing exposure to pesticides in house dust. Bull. Environ. Contam. Toxicol. 43:717–724.
- Rodriguez, T., B. Van Wen De ldeJoode, C. H. Lindh, M. Rojas, I. Lundberg, and C. Wesseling. 2012. Assessment of long-term and recent pesticide exposure among rural school children in Nicaragua. Occup. Environ. Med. 69:119–125. doi:10.1136/oem.2010.062539.
- Ryals, S. C., M. B. Genter, and R. B. Leidy. 1998. Assessment of surface water quality on three eastern North Carolina golf courses. Environ. Toxicol. Chem. 17:1934–1942. doi:10.1897/1551-5028(1998)017<1934:aoswqo>2.3.co;2.
- Sandmann, E., P. R. Debeer, and L. P. Vandyk. 1991. Atmospheric-pollution by auxin-type herbicides in Tala Valley, Natal. Chemosphere 22:137–145. doi:10.1016/0045-6535(91)90271-e.
- Sauerhoff, M. W., W. H. Braun, G. E. Blau, and P. J. Gehring. 1977. The fate of 2,4-dichlorophenoxyacetic acid (2,4-D) following oral administration to man. Toxicology 8:3–11.
- Scher, D. P., B. H. Alexander, J. L. Adgate, L. E. Eberly, J. S. Mandel, J. F. Acquavella, M. J. Bartels, and K. A. Brzak. 2007. Agreement of pesticide biomarkers between morning void and 24-h urine samples from farmers and their children. J. Expo. Sci. Environ. Epidemiol. 17:350–357. doi:10.1038/sj.jes.7500505.
- Schultz, D. P., and P. D. Harman. 1974. Residues of 2,4-D in pond waters, mud, and fish, 1971. Pest Monit. J. 8:173–179.
- Schultz, D. P., and E. W. Whitney. 1974. Monitoring 2,4-D residues at Loxahatchee National Wildlife Refuge. Pest Monit. J. 7:146–152.
- Semchuk, K., H. McDuffie, A. Senthilselvan, A. Cessna, and D. Irvine. 2004. Body mass index and bromoxynil exposure in a sample of rural residents during spring herbicide application. J. Toxicol. Environ. Health Part A 67:1321–1352. doi:10.1080/15287390490471424.
- Semchuk, K. M., H. H. McDuffie, A. Senthilselvan, J. A. Dosman, A. J. Cessna, and D. G. Irvine. 2003. Factors associated with detection of bromoxynil in a sample of rural residents. J. Toxicol. Environ. Health Part A 66:103–132. doi:10.1080/15287390306401.
- Shafik, M. T., H. C. Sullivan, and H. F. Enos. 1971. A method for determination of low levels of exposure to 2,4-D and 2,4,5-T. Int. J. Environ. Anal. Chem. 1:23–33. doi:10.1080/03067317108076364.
- Shea, B. J., J. M. Grimshaw, G. A. Wells, M. Boers, N. Andersson, C. Hamel, A. C. Porter, P. Tugwell, D. Moher, and L. M. Bouter. 2007. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 7:10. doi:10.1186/1471-2288-7-10.
- Shealy, D. B., M. A. Bonin, J. V. Wooten, D. L. Ashley, L. L. Needham, and A. E. Bond. 1996. Application of an improved method for the analysis of pesticides and their metabolites in the urine of farmer applicators and their families. Environ Int 22(6): 661–675. doi: 10.1016/s0160-4120(96)00058-x.
- Shin, E. H., J. H. Choi, A. M. Abd El-Aty, S. Khay, S. J. Kim, M. H. Im, C. H. Kwon, and J. H. Shim. 2011. Simultaneous determination of three acidic herbicide residues in food crops using HPLC and confirmation via LC-MS/MS. Biomed. Chromatogr. 25:124–135. doi:10.1002/bmc.1513.
- Siemering, G. S., J. D. Hayworth, and B. K. Greenfield. 2008. Assessment of potential aquatic herbicide impacts to California aquatic ecosystems. Arch. Environ. Contam. Toxicol. 55:415–431. doi:10.1007/s00244-008-9137-2.
- Spalding, R. F., G. A. Junk, and J. J. Richard. 1980. Pesticides in ground water beneath irrigated farmland in Nebraska, August 1978. Pest Monit. J. 14:70–73.
- Struger, J., and T. Fletcher. 2007. Occurrence of lawn care and agricultural pesticides in the Don River and Humber River watersheds (1998-2002). J. Great Lakes Res. 33:887–905. doi:10.3394/0380-1330(2007)33[887:oolcaa]2.0.co;2.
- Sutton, A. J., K. R. Abrams, D. R. Jones, T. A. Sheldon, and F. Song. 1998. Systematic reviews of trials and other studies. Health Technol Assess (Rockv) 2:1–276.
- Taskar, P. K., Y. T. Das, J. R. Trout, S. K. Chattopadhyay, and H. D. Brown. 1982. Measurement of 2,4-dichlorophenoxy acid (2,4-D) after occupational exposure. Bull. Environ. Contam. Toxicol. 29:586–591.
- Thomas, K. W., M. Dosemeci, J. A. Hoppin, L. S. Sheldon, C. W. Croghan, S. M. Gordon, M. L. Jones, S. J. Reynolds, J. H. Raymer, G. G. Akland, C. F. Lynch, C. E. Knott, D. P. Sandler, A. E. Blair, and M. C. Alavanja. 2010. Urinary biomarker, dermal, and air measurement results for 2,4-D and chlorpyrifos farm applicators in the Agricultural Health Study. J. Expo. Sci. Environ. Epidemiol. 20:119–134. doi:10.1038/jes.2009.6.
- Trevisan, M., C. Montepiani, L. Ragozza, C. Bartoletti, E. Ioannilli, and A. A. Del Re. 1993. Pesticides in rainfall and air in Italy. Environ. Pollut. 80:31–39.
- USDA. 2004. Pesticide Data Program. Annual summary calendar year 2002. US Department of Agriculture. Science and Technology Programs. https://www.ams.usda.gov/about-ams/programs-offices/science-technology-program
- USDA. 2015. Pesticide Data Program. Annual summary calendar year 2014. US Department of Agriculture. Science and Technology Programs. Available: https://www.ams.usda.gov/datasets/pdp
- USEPA. 2005. Reregistration eligibility decision for 2,4-D. Washington, DC: Prevention, Pesticides and Toxic Substances.
- USEPA. 2008. National functional guidelines for Superfund organic methods data review. Washington, DC: Office of Superfund Remediation and Technology Innovation.
- USEPA. 2009. Region III Fact Sheet quality control tools: Blanks. https://www.epa.gov/sites/production/files/2015-06/documents/blanks.pdf
- Vdovenko, M. M., A. S. Stepanova, S. A. Eremin, N. V. Cuong, N. A. Uskova, and I. Y. Sakharov. 2013. Quantification of 2,4-dichlorophenoxyacetic acid in oranges and mandarins by chemiluminescent ELISA. Food Chem. 141:865–868. doi:10.1016/j.foodchem.2013.04.060.
- Voos, G., and P. M. Groffman. 1997. Relationships between microbial biomass and dissipation of 2,4-D and dicamba in soil. Biol. Fertil. Soils 24:106–110. doi:10.1007/bf01420229.
- Waite, D. T., A. J. Cessna, R. Grover, L. A. Kerr, and A. D. Snihura. 2002a. Environmental concentrations of agricultural herbicides: 2,4-D and triallate. J. Environ. Qual. 31:129–144.
- Waite, D. T., R. Grover, N. D. Westcott, D. G. Irvine, L. A. Kerr, and H. Sommerstad. 1995. Atmospheric deposition of pesticides in a small southern Saskatchewan watershed. Environ. Toxicol. Chem. 14:1171–1175. doi:10.1897/1552-8618(1995)14[1171:adopia]2.0.co;2.
- Waite, D. T., R. Grover, N. D. Westcott, H. Sommerstad, and L. Kerr. 1992. Pesticides in ground-water, surface-water and spring runoff in a small Saskatchewan watershed. Environ. Toxicol. Chem. 11:741–748. doi:10.1897/1552-8618(1992)11[741:pigwsw]2.0.co;2.
- Waite, D. T., J. F. Sproull, D. V. Quiring, and A. J. Cessna. 2002b. Dry atmospheric deposition and deposition velocities of dicamba, 2,4-dichlorophenoxyacetic acid and gamma-1,2,3,4,5,6-hexachlorocyclohexane. Anal. Chim. Acta 467:245–252. doi:10.1016/s0003-2670(02)00073-9.
- Walters, J. 1999. Environmental fate of 2,4-dichlorophenoxyacetic acid. In C.D.O.P. Regulations, Edited by, 95814–93510. Sacramento, CA.
- Watson, J. R. 1977. Seasonal variation in the biodegradation of 2,4-D in river water. Water Res. 11:153–157. doi:http://dx.doi.org/10.1016/0043-1354(77)90120-8.
- Whitmore, R., F. Immerman, D. Camann, A. Bond, R. Lewis, and J. Schaum. 1994. Non-occupational exposures to pesticides for residents of two U.S. cities. Arch. Environ. Contam. Toxicol. 26:47–59.
- Wiergowski, M., L. Dabrowski, K. Galer, B. Makuch, and M. Biziuk. 2000. Evaluation of pollution degree of the Odra River basin with organic compounds after the 97 summer flood. Part I. Determination of pesticides and phenols in post-flood sediments and water. Chem. Anal. 45:281–290.
- Wijnja, H., J. J. Doherty, and S. A. Safie. 2014. Changes in pesticide occurrence in suburban surface waters in Massachusetts, USA, 1999-2010. Bull. Environ. Contam. Toxicol. 93:228–232. doi:10.1007/s00128-014-1251-4.
- Wilson, N. K., J. C. Chuang, and C. Lyu. 2001. Levels of persistent organic pollutants in several child day care centers. J. Expo Anal. Environ. Epidemiol. 11:449–458. doi:10.1038/sj.jea.7500190.
- Wilson, N. K., J. C. Chuang, C. Lyu, R. Menton, and M. K. Morgan. 2003. Aggregate exposures of nine preschool children to persistent organic pollutants at day care and at home. J. Expo. Anal. Environ. Epidemiol. 13:187–202. doi:10.1038/sj.jea.7500270.
- Wilson, N. K., W. J. Strauss, N. Iroz-Elardo, and J. C. Chuang. 2010. Exposures of preschool children to chlorpyrifos, diazinon, pentachlorophenol, and 2,4-dichlorophenoxyacetic acid over 3 years from 2003 to 2005: A longitudinal model. J. Expo. Sci. Environ. Epidemiol. 20:546–558. doi:10.1038/jes.2009.45.
- Wilson, R. D., J. Geronimo, and J. A. Armbruster. 1997. 2,4-D dissipation in field soils after applications of 2,4-D dimethylamine salt and 2,4-D 2-ethylhexyl ester. Environ. Toxicol. Chem. 16:1239–1246. doi:10.1897/1551-5028(1997)016<1239:ddifsa>2.3.co;2.
- Wojtalik, T. A., T. F. Hall, and L. O. Hill. 1971. Monitoring ecological conditions associated with wide-scale applications of DMA 2,4-D to aquatic environments. Pest Monit. J. 4:184–203.
- Woudneh, M. B., M. Sekela, T. Tuominen, and M. Gledhill. 2006. Isotope dilution high-resolution gas chromatography/high-resolution mass spectrometry method for analysis of selected acidic herbicides in surface water. J. Chromatogr. A. 1133:293–299. doi:10.1016/j.chroma.2006.08.013.
- Woudneh, M. B., M. Sekela, T. Tuominen, and M. Gledhill. 2007. Acidic herbicides in surface waters of Lower Fraser Valley, British Columbia, Canada. J. Chromatogr. A. 1139:121–129. doi:10.1016/j.chroma.2006.10.081.
- Yamini, Y., and A. Saleh. 2013. Ultrasound-assisted emulsification microextraction combined with injection-port derivatization for the determination of some chlorophenoxyacetic acids in water samples. J. Sep. Sci. 36:2330–2338. doi:10.1002/jssc.201300340.
- Yao, Y., L. Tuduri, T. Harner, P. Blanchard, D. Waite, L. Poissant, C. Murphy, W. Belzer, F. Aulagnier, Y.-F. Li, and E. Sverko. 2006. Spatial and temporal distribution of pesticide air concentrations in Canadian agricultural regions. Atmos. Environ. 40:4339–4351. doi:10.1016/j.atmosenv.2006.03.039.
- Zhang, X. F., S. Acevedo, Y. F. Chao, Z. S. Chen, T. Dinoff, J. Driver, J. Ross, R. Williams, and R. Krieger. 2011. Concurrent 2,4-D and triclopyr biomonitoring of backpack applicators, mixer/loader and field supervisor in forestry. J. Environ. Sci. Health Part. B 46:281–293. doi:10.1080/03601234.2011.559424.