Abstract
Experimental studies on desulfurization and denitrification were carried out using activated carbon irradiated by microwave. The influences of the concentrations of nitric oxide (NO) and sulfur dioxide (SO2), the flue gas coexisting compositions, on adsorption properties of activated carbon and efficiencies of desulfurization and denitrification were investigated. The results show that adsorption capacity and removal efficiency of NO decrease with the increasing of SO2 concentrations in flue gas; adsorption capacity of NO increases slightly first and drops to 12.79 mg/g, and desulfurization efficiency descends with the increasing SO2 concentrations. Adsorption capacity of SO2 declines with the increasing of O2 content in flue gas, but adsorption capacity of NO increases, and removal efficiencies of NO and SO2 could be larger than 99%. Adsorption capacity of NO declines with the increase of moisture in the flue gas, but adsorption capacity of SO2 increases and removal efficiencies of NO and SO2 would be relatively stable. Adsorption capacities of both NO and SO2 decrease with the increasing of CO2 content; efficiencies of desulfurization and denitrification augment at the beginning stage, then start to fall when CO2 content exceeds 12.4%. The mechanisms of this process are also discussed.
The prominent SO2 and NOx treatment techniques in power plants are wet flue gas desulfurization (FGD) and the catalytic decomposition method like selective catalytic reduction (SCR) or nonselective catalytic reduction (NSCR). However, these processes would have some difficulties in commercial application due to their high investment, requirement of expensive catalysts and large-scale equipment, and so on. A simple SO2 and NOx reduction utilizing decomposition by microwave energy method can be used. The pollutants control of flue gas in the power plants by the method of microwave-induced decomposition using adsorption of activated carbon/microwave desorption can meet the requirements of environmental protection, which will be stricter in the future.
Introduction
Sulfur dioxide (SO2) and nitric oxide (NO) are the main precursors of acid precipitation; strict environmental standards for the emission controls of SO2 and NO are being implemented around the world. Though existing technologies of flue gas desulfurization and denitrification are mature and have high removal efficiency, many problem s still exist (CitationGao et al. 2011; CitationGutiérrez et al. 2006; CitationRoy et al. 2009), such as technologic complexity, high investment, secondary pollution and so on. It is necessary, therefore, to study and develop new pollutants control technologies of flue gas with greater development prospects and environmental friendly.
The microwave is defined as a kind of electromagnetic wave with 300 MHz to 300 GHz frequency, l nm to 1 m wavelength, and 1.02 × 10−5 eV energy, lying between infrared rays and radiowaves, possessing all characteristics of waves, that is, wave–particle dualism, superposition of wave, energy distribution in space, high-frequency transmission, and so on. As a thermal energy technology, microwave heating has been applied to numerous research fields, such as environmental monitoring, waste gas control, wastewater treatment and waste solids handling, and so on (CitationAndrzej, 2006; CitationGedye, 1986; CitationJou, 1998; CitationKappe, 2004; CitationMa et al. 2011a; CitationWang et al. 2003).
Activated carbon is widely used in flue gas pollution control due to its properties of large surface area, good pore structure, abundant surface functional group, and high effectiveness related to in situ deoxidizing ability. Meanwhile, it also has certain effects on the removal of the dust, hydrocarbons, and toxic substances (HCl, HF, As, Se, Hg, etc.) from flue gas, because of its loading and reducing characteristics (CitationGiguere et al. 1986; CitationJones et al. 2002; CitationWigmans 1989). The use of combing microwave irradiation and activated carbon adsorption to realize catalytic reduction on desulfurization and denitrification has attracted many scholars' attentions. For example, Jiang et al. studied microwave irradiation over activated carbon for desulfurization and found the adsorption and catalytic properties of the activated carbon increased significantly after chemical modification, with SO2 adsorption capacity rising from 49.45 mg/g to 85.31 mg/g (CitationJiang et al. 2004). Zhang et al. reported a method that microwave could be used to reduce SO2 and NOx into harmless pure sulfur and N2 after the activated carbon adsorption process. They also found that the removals of NO and SO2 and the reaction temperature increased with the increasing of the microwave power (CitationZhang et al. 1997). Tang et al. used the method of microwave discharging under atmospheric pressure and reached high removal efficiency of NO and about 80% conversion rate of NO when NO, O2, exist in the flue gas (CitationTang et al. 2000). The earlier studies showed that a big shortage of microwave irradiating activated carbon for desulfurization and denitrification is that high temperature is needed (530°C or above) for reduction reactions on activated carbon, leading to high carbon mass loss and energy consumption (CitationMa 2011b). Therefore, it is necessary to reduce the reduction temperature for the industrial application of this technology. In this paper, we report the study of adsorbing SO2 and NO using activated carbon, which was desorbed by microwave radiation, showing how SO2 and NO could be reduced and decomposed by activated carbon at high temperature, and activated carbon could be regenerated in situ, to archive a high removal efficiency at low temperature, overcoming the defects of continuous microwave irradiation.
Experimental
Experimental apparatus and materials
A microwave reactor (WLD1S-03) was used in this study. Its working frequency and maximum output power are 2.45 GHz and 1100 W, respectively. A quartz tube packed with activated carbon (diameter 35 × 500 mm) was installed in the microwave cavity. An infrared temperature monitoring system was installed inside too. Composition concentrations in simulated flue gas were measured accurately by portable flue gas analyzer made by the RBR, Germany. The scheme of the experiment is shown in
Figure 1. Sketch of experimental device: 1, high-purity nitrogen; 2, sulfur dioxide; 3, nitrogen oxide; 4, oxygen; 5, mixing tank; 6, flow meter; 7, valve; 8 and 11, anti-leaking belt of microwave irradiation; 9, quartz tube; 10, activated carbon; 12, circulation pipe; 13, temperature display; 14, power adjustment button; 15, electric supply control system; 16, flue gas analyzer; 17, tail gas adsorption bottle; 18, emission; and 19, evaporation device.
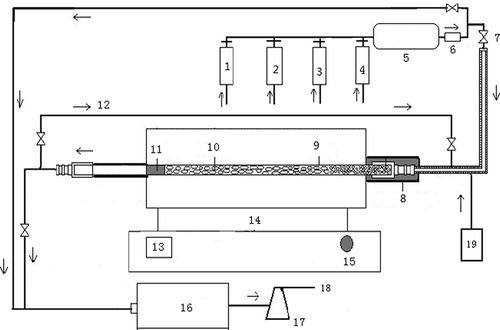
In order to obtain the base carbon for this study, the activated carbon was washed with deionization water to remove impurities and adhering particles first, then fully immersed in deionization water for 12 hr to remove the ionic impurities adsorbed in the activated carbon surface, and finally dried to constant weight under constant temperature at 120°C in an oven. lists the characteristics of fresh activated carbon particles used in the study. Activated carbon was treated at 420W power and nitrogen gas was used as protection gas. The activated carbon was activated during the process of further removing the ash and volatile matter on its surface.
Table 1. Characteristics of fresh particle activated carbon used in this study
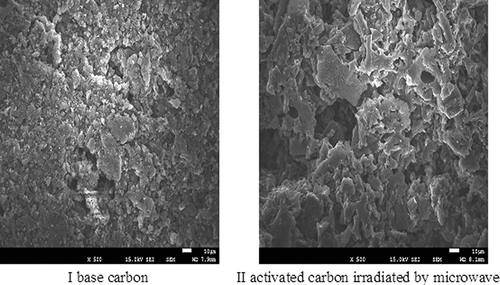
Scanning electron microscopy (Hitachi S-2500 SEM) was used and pictured the base carbon and activated carbon irradiated by microwave, to investigate the changes of pore structure on the carbon surface before and after the experiment. shows the SEM images for the base (I) and the microwave-activated (II) carbon. The surface of the base carbon is smooth and has many shallow holes of various sizes. The holes are small and not many holes with open structure are observed. In addition, the wall of the holes is smooth and fragments attach inside the holes and around. In contrast, the surface of the activated carbon (II) is rough, showing a bumpy form and many open holes. There are also slits that extend toward the inside. These features are beneficial to the adsorption for SO2 and NO, although the specific surface area is slightly smaller than that of the base carbon (CitationMa et al. 2010).
Experimental method
The simulated flue gas was prepared by mixing SO2, NO, O2, and N2 based on specific ratios. Water vapor content is controlled by a self-made steam generator. The processes of the experiment are described as follows: (1) Simulated flue gas passes into the quartz tube packed with activated carbon in the microwave reactors (i.e., activated carbon adsorbs sulfur and nitrogen oxides; saturated adsorption time is roughly 1.5 hr every time) via the mixing tank and the flowmeter; (2) when the concentrations of SO2 and NO in the outlet of the activated carbon bed are beyond the air pollutants control standard, the microwave generator was opened for irradiating for 25 min (with the rising of the temperature on the carbon bed, part of the SO2 and NO resolving from the activated carbon will desorb, and it returns to the entrance of the activated carbon bed via the circulating pipe installed in the outlet of the microwave reactors, which can be seen from ); SO2 and NO are reduced and decomposed on the activated carbon under the microwave irradiation during the process of rising temperature; (3) nitrogen gas was used to purge out SO2 and NO remained in activated carbon, and finally, the concentrations are measured by the flue gas analyzer and sulfur as a reduction product is converted into sulfur vapor for recovering by a condensation method.
The temperature monitored by an infrared temperature monitor has certain errors compared with the actual temperature inside the activated carbon bed, and the errors were corrected by measurements with a refitted thermocouple under the same environment temperature; the correlation was given as a linear function,
The expression for the adsorption capacity of activated carbon is
Reductive decomposition efficiencies of NO and SO2 (i.e., removal efficiency) can be calculated as
where η is desulfurization efficiency, %; AC' is the amounts of NO and SO2 purging out by nitrogen gas, mg; c' is the concentrations of NO and SO2 purging out via nitrogen gas at a certain time, mg/m3; V' is purging flow rate of nitrogen gas, m3/hr; and t' is purging time, hr.
Results and Discussion
Condition experiments
The influences of the concentrations of SO2 in the flue gas on the adsorption of NO and denitrification efficiency
In this experiment, 15 g activated carbon was used; we adjusted the flow rate of the flue gas to 0.08 m3/hr, kept 420 W constant microwave power, fixed the concentration of NO as 1100 mg/m3, and then changed the concentrations of SO2 to 465, 1097, 2036, 2502, and 3031 mg/m3, respectively. The influences of the concentrations of SO2 in the flue gas on the adsorption capacity of NO and denitrification efficiency are displayed in The adsorption capacity of NO is 6.27 mg/g when the concentration of SO2 is 0, and it declines to 3.76 mg/g rapidly when the concentration of SO2 reaches 465 mg/m3, indicating that the addition of SO2 has a greater influence on the adsorption of NO. In other words, there exists competitive adsorption between SO2 and NO, and activated carbon adsorbs SO2 first. The process can be shown by the following equations:
Figure 3. The influences of the concentrations of SO2 on adsorption capacity of NO and denitrification efficiency.
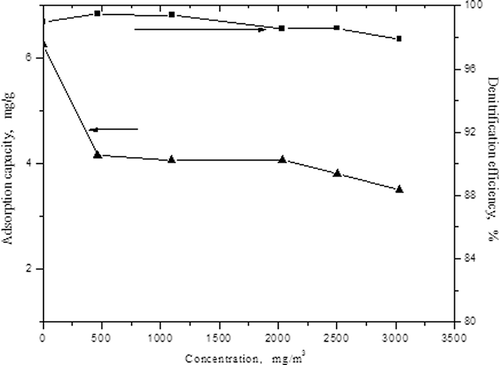
also shows that low concentrations of SO2 can promote removal of NO, but high concentrations of SO2 suppress denitrification slightly. The denitrification efficiency will decline when SO2 concentration is more than 465 mg/m3, with the increase of SO2 concentration; this shows that high concentrations of SO2 have a slightly inhibition effect on denitrification. This is related to the bond energy; the bond energies of NO and SO2 are 630.57 kJ/mol and 551.1 kJ/mol, respectively (CitationLuo, 2005). For example, the NO bond has higher energy; it is easier for carbon to compete with SO2 to make the reduction reaction take place, leading to the decline of denitrification efficiency. Zhu et al. had similar conclusions in their study (CitationZhu et al. 2005).
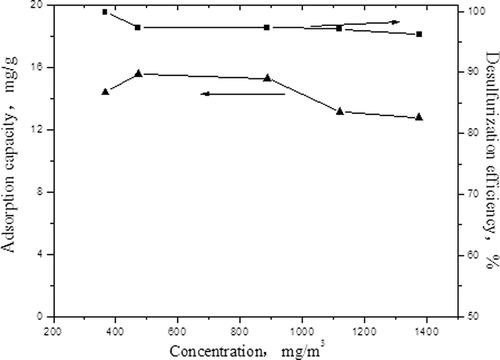
The influences of the concentrations of NO in flue gas on the adsorption capacity of SO2 and desulfurization efficiency
With the same experimental conditions as described earlier (15 g activated carbon, flow rate of the flue gas to 0.08 m3/r,h and 420 W constant microwave power, same hereafter), this investigationfixed the SO2 concentration at 1800 mg/m3 . Then we adjusted the concentrations of NO to 366, 472, 887, 1120, and 1375 mg/m3, respectively. The influences of the concentrations of NO in flue gas on the adsorption capacity of SO2 and desulfurization efficiency are shown in One can see that the adsorption capacity of SO2 will increase slightly with the increasing of NO concentrations first, and then drops to 12.79 mg/g. This is due to the competitive adsorption effect in the adsorption process for SO2 and NO; the principle is same as in the already-mentioned reactions ( Equationeqs 4 Equation–6). From , the removal efficiency of SO2 decreases with the increasing of NO concentration, and this is related to a carbon thermal reaction between carbon and SO2, and NOX in the flue gas. The active sites on the carbon surface will be consumed certainly with the increasing of NO concentrations, leading to the depletion of active sites. Furthermore, this will lead to a decline in the redox reaction rate.
The influence of O2 content on desulfurization and denitrification
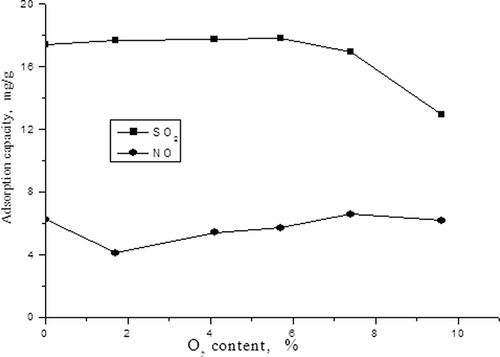
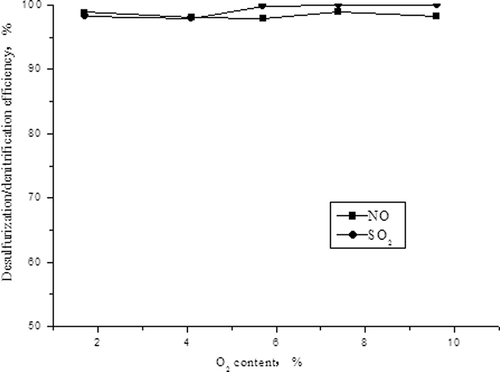
Similar to the first described experiment, the concentrations of NO and SO2 were adjusted to 500 mg/m3 and 1500 mg/m3, and the volume fraction of O2 in simulated flue gas was adjusted to 1.7%, 4.1%, 5.7%, 7.4%, and 9.6%, respectively. The effects of O2 content on the adsorption capacities of SO2 and NO and desulfurization and denitrification efficiencies were investigated, as shown in and
From we can see that the adsorption capacity of SO2 reduces with the increasing of O2 content, but adsorption capacity of NO decreases first and then rises. The adsorption capacity of NO increases, which should be due to the reaction: NO + 1/2 O2 + * → NO2 *. It's easy to form the adsorption state of NO2* in the presence of O2, which makes the adsorption capacity of NO increase. From and we can still see that although the adsorption of SO2 has some inhibition for that of NO, the presence of O2 is more apparent in its promoting effect.
shows that the desulfurization efficiency of activated carbon increases after adding O2 into the simulated flue gas, which stays at 99% or more when O2 content is higher than 5.9%; the influence of O2 on denitrification efficiency is not obvious, and the efficiency maintains at about 97%. The denitrification effect is in synchronous growth with high O2 content. This may be because O2 can oxidize NO into NO2 at the surface of activated carbon, and ; gas-phase CO will produce gas-phase reduction reactions with NO and SO2, and the reaction is more faster than that for the gas–solid, resulting in promoting the reduction decompositions of NO and SO2.
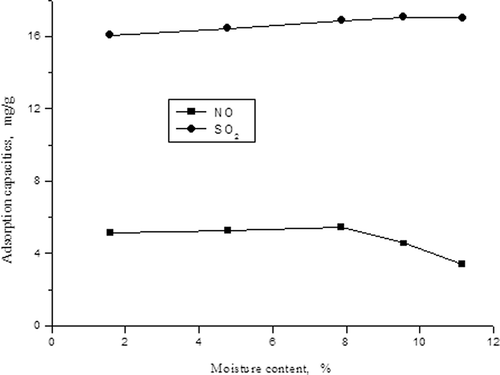
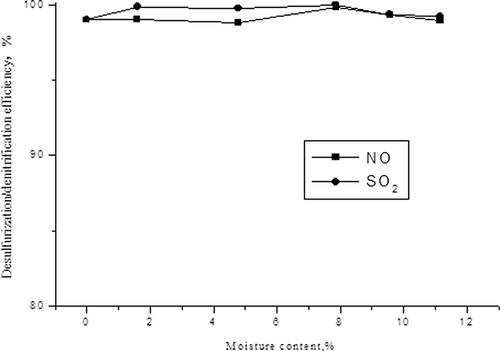
The influences of water vapor content on desulfurization and denitrification
The same as in the first experiment, we added a certain volume of water into simulated flue gas with evaporator, adjusted the concentrations of NO and SO2 at 500 mg/m3 and 1500 mg/m3, and adjusted the water vapor content in simulated flue gas to 1.59%, 4.78%, 7.87%, 9.56%, and 11.16% respectively. The influence of water vapor on desulfurization and denitrification is shown in and
It can be seen clearly from that the adsorption capacity of SO2 increases with the increasing of the water vapor content, but the adsorption capacity of NO has the opposite change. When SO2 spreads to the carbon surface through physical adsorption, it will be oxidized to SO3, and when water vapor is present, it will become sulfuric acid, and SO2, steam, and sulfuric acid all can compete with NO to be adsorbed onto the surface of activated carbon, causing the activity sites of the adsorption of NO to decrease and the adsorption capacity of NO declines.
shows that the changes of desulfurization efficiency and denitrification efficiency have almost the same trend with increasing moisture. They reach the highest value when the moisture is 7.87%, and then they drop. The reason may be because when the water vapor content is relative low, it can promote denitrification in a certain extent. However, the microwave-absorbing properties will increase when water enters into flue gas, causing the reaction temperature to rise faster, because water is a good wave-absorbing material. Therefore, moisture denitrification can be promoted, even at low moisture circumstance. However, denitrification efficiency will be reduced if the moisture ratio is too high. Desulfurization and denitrification efficiencies decrease obviously, when the moisture is higher than 7.87%. If moisture is high, the water adsorbed on the surface of the carbon increases dramatically, which would block the reaction between activated carbon and SO2, NOX. In addition, activated carbon could react with sulfuric acid, that is, . All of these can lead to the decrease of desulfurization and denitrification efficiencies, obviously. Richter et al. did a similar study and also found that high moisture is harmful to removal of pollutants using activated carbon (CitationRichtera et al. 1985).
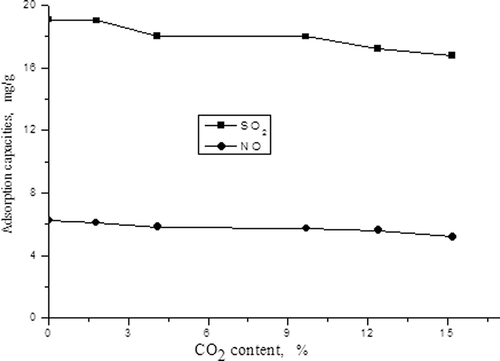
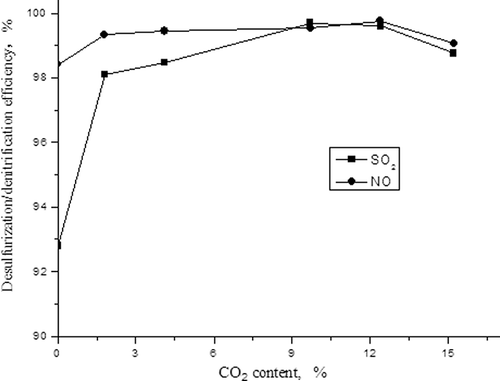
The influences of CO2 content on desulfurization and denitrification
Similar to the first experiment setup described, we kept the concentrations of NO and SO2 to 700 and 1900 mg/m3, respectively, and adjusted CO2 content in simulated flue gas to 0%, 1.7%, 4.1%, 9.7%, 12.4%, and 15.2%, respectively. The effects of CO2 content on the adsorption capacities of NO and SO2 and on desulfurization and denitrification efficiencies were studied and are displayed in and
From , the adsorption capacities of SO2 and NO are seen to reduce with the increasing of CO2 content. The reasons may be because of the competitive adsorptions between CO2 and SO2 with NO, which are more evident with the increasing CO2 content, leading to the decline in the adsorption capacities for SO2 and NO.
It can be concluded from that the desulfurization and denitrification efficiencies go up first with the increasing of CO2 content, and then the efficiency descends when the CO2 content is higher than 12.4%. This may be because CO2 and activated carbon could react with each other, leading to the increase of contact area between SO2, NO, and carbon, and enabling the reactions and
to go faster. It will, however, lead the preceding two reactions of the chemical equilibrium toward a reversed direction inevitably with the continuing increasing of CO2 content, resulting in decreasing of the removal efficiencies.
Mechanism of Activated Carbon Adsorption/microwave Reduction Decomposition
Mechanism of activated carbon adsorption
The adsorption of gas molecules on activated carbon is due to van der Waals forces mainly. The charge of activated carbon is very weak, and it cannot form any apparent electric field and electric potential gradient on carbon surface when the positive and negative charges are too close to each other. In addition, not only do both the small size of activated carbon pore and the area size of specific surface have important influences on gas adsorption, but also the surfaces of the polar groups have obvious influences on gas adsorption (CitationFigueir et al. 1999); both SO2 and NO are polar molecules. Activated carbon irradiated by microwave has more alkaline groups (CitationValente Nabais et al. 2004), which becomes more beneficial to the adsorbability for harmful gas molecules (SO2, NO). The adsorption process of harmful gas molecules (SO2, NO) on the activated carbon bed is listed as following these steps:
| 1. | Harmful gas molecules (SO2, NO) go onto the carbon surface from gas-phase bulk through the external diffusion effect. | ||||
| 2. | Harmful gas molecules (SO2, NO) enter into micropores (or holes) of activated carbon through the internal diffusion effect. | ||||
| 3. | Harmful gas molecules (SO2, NO) are adsorbed in the sorption sites of activated carbon and generate sorption states of SO2 and NO, that is, | ||||
The mechanism of desorption–reduction: Surface chemical reaction
First, part of the sorption states of and
can react with activated carbon directly—that is,
Second, part of the sorption states of SO2 and NO would resolve in the process of microwave heating, that is, ,
. SO2 and NO return to the carbon bed by the circulation piping of the carbon bed, leading to the following reactions under high temperature:
The preceding reactions and resolved products (CO2, CO, N2, S, SO2, and NO) spread to the bulk gas from the surface of activated carbon. Elemental sulfur exists from the gas phase under high temperature, and is recovered by an elemental sulfur recovery device after leaving the activated carbon bed.
Conclusions
The removal of NO and SO2 from flue gas using activated carbon adsorption-microwave radiation was carried out in this experimental study. The removal process includes four steps, including (1) activated carbon adsorbing SO2 and NO, (2) SO2 and NO desorbing under microwave heating, (3) reduction decompositions of SO2 and NO under microwave irradiating over activated carbon, and (4) activated carbon regeneration. The results of the study are listed as follows:
| 1. | NO adsorption capacity decreases with the increase of SO2 concentrations, and denitrification efficiency is lower; the SO2 adsorption capacity increases slightly with the increasing of NO concentrations, and then drops down to 12.79 mg/g, and desulfurization efficiency descends. | ||||
| 2. | NO adsorption capacity goes up, and adsorption capacity of SO2 decreases with the increasing of O2 content, but the desulfurization and denitrification efficiencies always stay over 99%. | ||||
| 3. | SO2 adsorption capacity increases but NO adsorption capacity reduces with the increasing of the water vapor content. Meanwhile, the desulfurization and denitrification efficiencies maintain stable. | ||||
| 4. | Adsorption capacities of SO2 and NO reduce and removing efficiencies increase first when CO2 content increases. Desulfurization efficiency drops, however, if CO2 content is larger than 15%. | ||||
| 5. | The mechanisms of SO2 and NO adsorbed onto activated carbon and reduction decomposition can be summarized as following six steps: | ||||
Acknowledgments
This work was partially supported by the National Natural Science Foundation of China (NSFC) (50976035). The authors greatly appreciate the help from the three anonymous reviewers. Their insightful comments resulted in a number of improvements to the original article.
References
- Andrzej , Z. 2006 . The application of microwave radiation to analytical and environmental chemistry . Crit. Rev. Anal. Chem. , 25 : 43 – 76 .
- Figueir , J.L. , Pereira , M.F.R. , Freitas , M.M.A. and Órfão , J.J.M. 1999 . Modification of the surface chemistry of activated carbons . Carbon , 37 : 1379 – 1389 .
- Gao , H.L. , Li , C.T. , Zeng , G.M. , Zhang , W. , Shi , L. , Fan , X.P. , Zeng , Y.N. , Wen , Q.B. and Shu , X. 2011 . experimental study of wet flue gas desulphurization with a novel type PCF device . Chem. Eng. Processing Process Intens. , 50 : 189 – 195 .
- Giguere , R.J. , Bray , T.L. and Duncan , S.M. 1986 . Application of commercial microwave oven to organic synthesis . Tetrahedron Lett. , 27 : 4945 – 4948 .
- Gedye , R. , Smith , F. , Westaway , K. , Ali , H. , Baldisera , L. , Laberge , L. and Rousell , J. 1986 . The use of microwave ovens for rapid organic synthesis . Tetrahedron Lett. , 27 : 279 – 282 .
- Gutiérrez Ortiz , F.J. , Vidal , F. , Ollero , P. , Salvador , L. and Cortés , V. 2006 . Pilot-plant technical assessment of wet flue gas desulfurization using limestone . Ind. Eng. Chem. Res. , 45 : 1466 – 1477 .
- Jiang , X. , Jiang , W.J. , Zhu , X.F. and Jin , Y. 2004 . Application of microwave technology in desulfurization of flue gas by activated carbon . Acta Sci. Circumst. , 24 : 1098 – 1103 .
- Jones , D.A. , Lelyveld , T.P. , Mavrofidis , S.D. , Kingman , S.W. and Miles , N.J. 2002 . Microwave heating application in environmental engineering . Resources Conserv. Recycling , 34 : 75 – 90 .
- Jou , G.C.J. 1998 . Application of activated carbon in a microwave radiation field to treat trichloroethylene . Carbon , 36 : 1643 – 1648 .
- Kappe , C.O. 2004 . Controlled microwave heating in modern organic synthesis . Angew. Chem. Int. Ed. , 43 : 6250 – 6284 .
- Luo , Y.R. 2005 . Chemical Bond Energy Data Handbook , Beijing : Sciences Publishing House .
- Ma , S.C. , Yao , J.J. , Jin , X. and Zhang , B. 2011 . Kinetic study on desulfurization and denitrification using microwave irradiation over activated carbon . Sci. China Tech. Sci. , 54 : 2321 – 2326 .
- Ma , S.C. , Jin , Y.J. and Jin , X. 2011 . Influences of co-existing components in flue gas on simultaneous desulfurization and denitrification using microwave irradiation over activated carbon . J. Fuel Chem. Technol. , 39 : 460 – 464 .
- Ma , S.C. , Jin , X. , Yao , J.J. and Cai , H.N. December 5–6 2010 . “ Characteristic change of activated carbon under microwave irradiation ” . In Paper presented at ICEET2010 December 5–6 , Changsha
- Richtera , E. , Kleinschmidta , R. , Pilarczyka , E. , Knoblauch , K. and Jontgen , H. 1985 . Thermal desorption of nitrogen oxides from activated carbon . Thermochim. Acta , 85 : 315 – 318 .
- Roy , S. , Hegde , M.S. and Madras , G. 2009 . Catalysis for NOx abatement . Appl. Energy , 86 : 2283 – 2297 .
- Tang , J. , Zhang , T. , Liang , D. , Xu , C.H. , Sun , X.Y. and Lin , L.W. 2000 . Microwave discharge-assisted catalytic conversion of NO to N2 . Chem. Commun. , 19 : 1861 – 1862 .
- Valente Nabais , J.M. , Carrott , P.J.M. , Ribeiro Carrott , M.M.L. and Menendez , J.A. 2004 . Preparation and modification of activated carbon fibers by microwave heating . Carbon , 42 : 1315 – 1320 .
- Wang , S.M. , Li , Q. , Xie , B. , Ji , W. and Qian , Y.T. 2003 . The synthesis of CdS/ZnO and CdS/Pb3O4 composite materials via microwave irradiation . Mater. Chem. Phys. , 78 : 288 – 291 .
- Wang , Y.L. , Huang , Z.G. , Liu , Z.Y. and Liu , Q.Y. 2004 . A novel activated carbon honeycomb catalyst for simultaneous SO2 and NO removal at low temperature . Carbon , 42 : 445 – 448 .
- Wigmans , T. 1989 . Industrial aspects of production and use of activated carbons . Carbon , 27 : 13 – 22 .
- Zhang , D.X. , Yu , A.M. and Jin , Q.H. 1997 . Studies on microwave carbon reduction method for the treatment of nitric oxide . Chemical Journal of Chinese Universities , 18 : 1271 – 1274 .
- Zhu , J.L. , Wang , Y.H. , Zhang , J.C. and Ma , R.Y. 2005 . Experimental investigation of adsorption of NO and SO2 on modified activated carbon sorbent from flue gases . Energy Conversion Manage , 46 : 2173 – 2184 .