Abstract
TiO2-supported manganese oxide catalysts formed using different calcination temperatures were prepared by using the wet-impregnation method and were investigated for their activity in the low-temperature selective catalytic reduction (SCR) of NO by NH3 with respect to the Mn valence and lattice oxygen behavior. The surface and bulk properties of these catalysts were examined using Brunauer-Emmett-Teller (BET) surface area, X-ray diffraction (XRD), temperature-programmed reduction (TPR), and temperature-programmed desorption (TPD). Catalysts prepared using lower calcination temperatures, which contained Mn4+, displayed high SCR activity at low temperatures and possessed several acid sites and active oxygen. The TPD analysis determined that the Brönsted and Lewis acid sites in the Mn/TiO2 catalysts were important for the low-temperature SCR at 80∼160 and 200∼350 °C, respectively. In addition, the available lattice oxygen was important for attaining high NO to NO2 oxidation at low temperatures.
Recently, various Mn catalysts have been evaluated as SCR catalysts. However, there have been no studies on the relationship of adsorption and desorption properties and behavior of lattice oxygen according to the valence state for manganese oxides (MnOx). Therefore, in this study, the catalysts were prepared by the wet-impregnation method at different calcination temperatures in order to show the difference of manganese oxidation state. These catalysts were then characterized using various physicochemical techniques, including BET, XRD, TPR, and TPD, to understand the structure, oxidation state, redox properties, and adsorption and desorption properties of the Mn/TiO2 catalysts.
Introduction
Selective catalytic reduction (SCR) using NH3 is the most widely used method of removing nitrogen oxides (NOx) emitted from stationary sources (CitationBosch and Janssen, 1988; CitationParvulescu et al., 1998). V/TiO2 or V/W/TiO2 catalysts have been commercialized for SCR and operate under moderate temperatures ranging from about 300 to 400 °C (CitationAlemany et al., 1995; CitationCenti and Perathoner, 1995). SCR reactors at power plants and gas turbines can be located in the later portions of the dust collection and desulfurization devices to avoid deactivation from dust or SO2. At this point in the process, the temperature is less than 200 °C, which is where the conventional V/W/TiO2 catalysts used in the NH3-SCR process cannot be used (CitationKuo et al., 1997; CitationLi et al., 2008). Accordingly, a denitrification catalyst is required for successful SCR operation at temperatures under 200 °C. In this temperature range, MnOx-based catalysts are suitable for the NH3-SCR system. Supported manganese oxides such as MnOx/Al2O3 (CitationStoilova et al., 1998) MnOx/TiO2 (CitationLi et al., 2007), MnOx/CeO2 (CitationQi and Yang, 2003), and MnOx/active carbon (CitationMarbán et al., 2004) possess excellent activity in low-temperature SCR. These catalysts have been shown to affect the SCR activity of Mn-based catalysts through changes to the Mn oxidation state. This result has been previously demonstrated by many researchers. We have also previous reported that natural manganese oxides readily undergo the redox cycle and the oxidation state of manganese ions can be easily changed (CitationKim et al., 2011). CitationKapteijn et al. (1994) investigated the reduction of NO by NH3 over pure manganese oxides and found that the NO conversions decreased in the order of MnO2 > Mn5O8 > Mn2O3 > Mn3O4. CitationTang et al. (2006) prepared a Mn/CeO2 catalyst using a modified co-precipitation method and tested for its HCHO oxidation activity. They reported that the MnOx-CeO2 catalyst that was calcined at 500 °C possessed more Mn4+ and richer lattice oxygen species, which resulted in significantly higher catalytic activity. CitationPeña et al. (2004) reported that Mn/TiO2 catalysts prepared at lower calcination temperatures provided the best catalytic performance, and the lower reduction temperature produced the MnO2 and Mn2O3 phases. However, the effect of the manganese oxidation state on the acid site and lattice oxygen has not been clearly demonstrated. CitationRoy et al. (2008) reported that NH3 can be adsorbed onto the surface of the catalysts as NH4 + and molecular NH3 at the Brönsted and Lewis acid sites, respectively. The adsorbed NH3 and NH4 + can form an NH2NO adduct with adsorbed NO, which dissociates into N2 and H2O. CitationPeña et al. (2004) reported that the Lewis acid sites of the catalyst appear to play an important role in activating NH3 in low-temperature SCR involving TiO2, such as Mn/TiO2 and Cu/TiO2. The purpose of the present study is to better understand the relationship between the acid sites, valence of manganese, and lattice oxygen. To achieve this goal, the catalysts were prepared by the wet-impregnation method at different calcination temperatures to produce manganese catalysts with different oxidation states. These catalysts were characterized using various physicochemical techniques, including Brunauer-Emmett-Teller (BET) surface area, X-ray diffraction (XRD), temperature-programmed reduction (TPR), and temperature-programmed desorption (TPD), to understand the structure, oxidation state, as well as redox, adsorption, and desorption properties of the Mn/TiO2 catalysts.
Experimental
Catalyst preparation
Mn/TiO2 catalysts were prepared using wet impregnation at different calcination temperatures with an MC-50 support. MC-50 is a commercial anatase TiO2 provided by Ishihara Sangyo Kaisha (Ltd., Japan). The required amount (20 wt% Mn w/w) of manganese(II) nitrate hydrate (Mn(NO3)2∙6H2O; Aldrich Chemical; Co. Inc., Korea) was dissolved in distilled water at 80 °C. After impregnation, the moisture was evaporated at 70 °C using a rotary evaporator and dried at 110 °C overnight. The catalysts were calcined for 4 hr at temperatures ranging from 300 to 700 °C. The obtained samples were ground and sieved through 40–50 mesh. The following naming convention was used to indicate the prepared catalysts: Mn/TiO2 (300) denotes 20 wt% Mn/TiO2 (MC-50) calcined at 300 °C.
Characterization
The surface areas of the TiO2 and manganese-loaded TiO2 catalyst powders were measured by physical nitrogen adsorption at −196 °C using an ASAP 2010 (Micrometrics Inc., USA). Approximately 0.1∼0.3 g of the catalysts were degassed at 300 °C for 2 hr.
X-ray diffraction (XRD) measurements were conducted using Cu Kα (λ = 1.5056 Å) radiation. The catalysts were scanned over a 2θ range of 20°∼90° with a step size of 0.1° at 1.0-sec intervals using X'Pert PRO MRD (PANalytical Spectrics Korea Ltd., Korea).
The temperature-programmed reduction (TPR) of H2 in 10% H2/Ar was measured using 0.3 g of catalyst with a total flow rate of 50 cc/min. The catalyst was pretreated in a 5% O2/Ar flow at 300 °C for 0.5 hr and cooled to 50 °C before measuring the H2-TPR. The catalyst was placed in dilute hydrogen, and the hydrogen consumption was monitored using an Autochem 2920 (Micrometrics) by increasing the temperature to 800 °C at a rate of 10 °C/min. NH3 temperature-programmed desorption (TPD) measurements were performed using 30 mg of the sample with a total flow of 50 cc/min. Before the TPD measurements, the catalyst was pretreated under a 5% O2/Ar flow at 300 °C for 0.5 hr and cooled to 50 °C. The samples were treated with 1% NH3/Ar for 0.5 hr. Adsorbed NH3 was removed using Ar flow for at least 1.5 hr before starting the TPD experiments. The NO-TPD (1% NO/Ar) experimental conditions were identical to the NH3-TPD experiment. During the TPD experiments, NH3 (m/z 15), NO (m/z 30), N2O (m/z 44), N2 (m/z 28), NO2 (m/z 46) were continuously monitored using a quadrupole mass spectrometer (QMS 422; Pfeiffer-vaccum., Germary) while increasing the temperature to 600 °C at a rate of 10 °C/min.
Catalytic activity measurement
The SCR apparatus consisted of a continuous-flow-type fixed-bed reaction system made of a quartz tube (inner diameter: 8 mm and height: 650 mm) and a catalytic bed that was fixed using quartz wool. The catalysts were made using a hydraulic press to pellet with 5,000 pounds of force and a 40–50-mesh sieve. It was then charged in a quartz tube. The temperature of the reactor was controlled using a proportional-integral-derivative (PID) controller and a K-type thermocouple fixed on the bottom of the bed. In order to measure the gas inlet temperature, another K-type thermocouple was installed on the top of the catalytic bed. The feeding gases consisted of 200 ppm NOx, 220 ppm NH3, 8% O2, and 8% moisture. Two hundred and eighty milligrams of sample was used in each run. The total flow through the reactor was 500 cc/min and a gas hourly space velocity (GHSV) of 60,000 hr−1 was obtained. The premixed gases were supplied by a mass flow controller (MFC; MKS Co. Ltd., Korea). Moisture was fed into the reactor by injecting N2-containing water vapor through the bubbler, and this level of moisture was maintained constant by circulating water at a fixed temperature of 45 °C using a circulator outside the jacket-type bubbler. The entire gas supply pipe was made of stainless steel and was wrapped with a heating band set at 180 °C so that the moisture would not condense. The concentrations of the reactants and products were measured as follows: inlet and outlet NO and N2O concentrations were analyzed using a NOx analyzer (Fuji Electric Co., Ltd., Japan) and N2O analyzer (Ultramat 6; Siemens Co., Germany). The concentration of NO2 and NH3 was measured using two different detector tubes: 9L (GASTEC Co., Japan) and 3M, 3La, 3L (GASTEC Co., Japan), respectively.
Results and Discussions
Characterization of catalysts
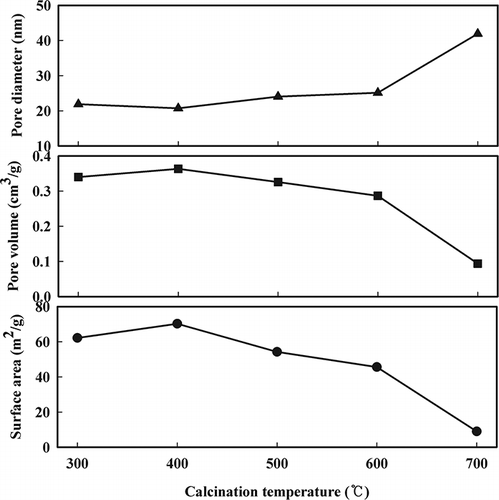
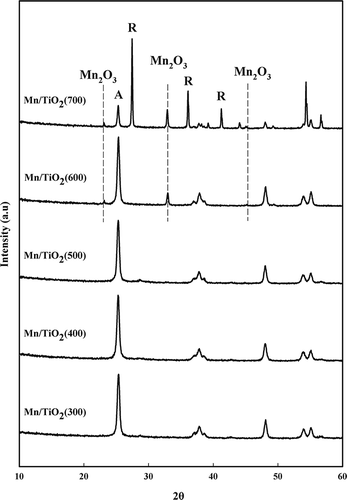
The surface area, pore volume, and pore size of the Mn/TiO2 catalysts that were prepared using different calcination temperatures are shown in . The surface area of the TiO2 support was 66.19 m2/g (not shown). As the calcination temperature increased from 300 to 700 °C, the surface area and pore volume decreased from 60 to 8 m2/g and 0.35∼0.1 cm3/g, respectively. In contrast, the pore diameter increased from 21 to 40 nm, which was expected. The XRD patterns for these catalysts are shown in . MnO2 peaks were not observed at 28.9°, 37.7°, and 57.1° in any catalyst. However, when the calcination temperature was above 600 °C, Mn2O3 peaks were observed at 23.0°, 32.9°, and 45.1°. In addition, the anatase TiO2 phase was partially transformed into the rutile TiO2 structure for the Mn/TiO2 catalyst calcined at 700 °C. CitationQi et al. (2004) reported that Mn2O3 and MnO2 were detected by XRD when pure manganese was calcined at 700 °C. In this study, amorphous MnO2 was formed because of the high surface energy for manganese in the TiO2 support. Sharp peaks were observed for the crystallite Mn2O3 structure when the catalyst was calcined at a temperature above 600 °C. For catalysts calcined at temperatures between 300 and 500 °C, no manganese structures were detected by XRD analysis, which may suggest that either the manganese was in a highly dispersed state or that the manganese ions had been inserted into the TiO2 lattice (CitationPechi et al., 2002). However, it is thought that the catalysts calcined at temperatures over 600 °C plugged the micropores of the support materials and either sintered or agglomerated as crystallite Mn2O3 because of the thermal effect. These results are in agreement with previous studies (CitationPeña et al., 2004; CitationSmirniotis et al., 2006).
TPR analysis
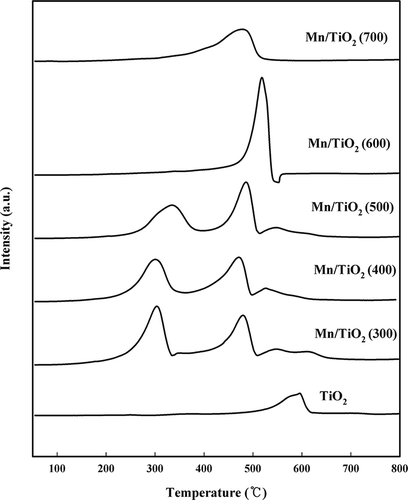
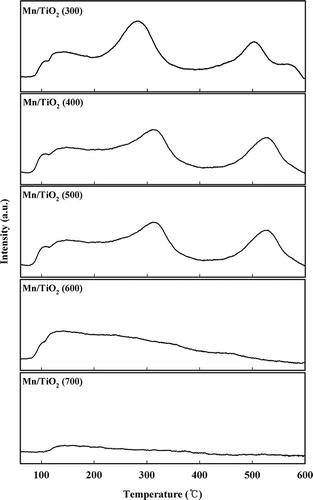
The XPS and H2-TPR analysis were mainly used to evaluate the oxidation state of manganese oxide. In recent literature, the SCR performance of all the Mn/TiO2 catalysts is accurately associated with the surface Mn4+ concentrations by XPS analysis (CitationThirupathi and Smirniotis, 2012). Also, these authors reported that the MnO2 phase is extremely dominant over the Mn2O3 phase (Mn4+/Mn3+ = 22.31, 96%) in the Mn-Ni(0.4)/TiO2 anatase catalyst and H2-TPR results are in good accordance with XPS results (CitationThirupathi and Smirniotis, 2011). In this paper, H2-TPR experiments were performed to investigate the oxidation state of manganese (). CitationEttireddy et al. (2007) reported that peak reductions for the 11.1 wt% Mn/TiO2 catalyst occurred at 215, 331, 421, and 468 °C. The first peak change at 215 °C was from the reduction of Ti4+ to Ti3+. In the second reduction step, which occurred at 331 °C, MnO2 was reduced to Mn2O3, which was further reduced to Mn3O4 and finally to MnO at 421 and 468 °C, respectively. In this study, the calcined TiO2 contained only one reduction peak near 600°C, which was very low relative to the TiO2-supported manganese oxide catalysts. The reduction of Ti4+ to Ti3+ did not occur for any of the catalysts at 215 °C. For the Mn/TiO2 (300) catalyst, the first reduction of MnO2 to Mn2O3 occurred at 303 °C, and the second reduction step of Mn2O3 to Mn3O4 occurred at 477 °C. The last reduction step, which converted Mn3O4 to MnO, occurred at 546 °C. However, unlike the other catalysts, the Mn/TiO2 (300) catalyst possessed overlapping reduction peaks in the area of the Mn2O3 to Mn3O4 transition at 615 °C. This extra peak may be due to the slight reduction of Ti4+ to Ti3+ in the bulk TiO2 after the surface manganese species had been reduced. CitationPeña et al. (2004) reported that higher reduction temperatures were required for catalysts synthesized with lower Mn loading or calcined at higher temperatures. It was also reported that a catalyst calcined at higher temperatures (>400 °C) could not be easily reduced because of the stronger metal-support interactions (CitationPeña et al., 2004). Manganese catalysts calcined at 400 °C possessed a similar reduction peak as catalysts calcined at 300 °C. However, the overlapping peak at approximately 615 °C was not observed. Increasing the calcination temperature from 400 to 600 °C shifted the reduction peak to lower temperatures, and the MnO2 to Mn2O3 reduction peak was not observed in catalysts calcined above 600 °C. These results indicate that the primary Mn species in the Mn/TiO2 (600) and Mn/TiO2 (700) catalysts consisted of Mn3+. The Mn/TiO2 (700) catalyst only displayed a single, low-intensity peak for the reduction of Mn2O3 to Mn3O4, which occurred at 477 °C. This peak was shifted to a lower temperature compared with the other catalysts. The XRD results confirmed that the transition from the anatase phase to the rutile phase occurred in the Mn/TiO2 (700) catalyst because of the thermal effect. The interaction between the MnOx and anatase-phase TiO2 was stronger than in the rutile phase (CitationEttireddy et al., 2007), and the reduction transition occurred at a different temperature can be explained by the presence of different TiO2 phases (CitationEttireddy et al., 2007). It indicated that the interaction of manganese with rutile TiO2 is weaker than the anatase TiO2. Therefore, the reduction of Mn2O3 to Mn3O4 in the Mn/TiO2 (700) catalyst occurred at a lower temperature compared with the other catalysts.
In addition, as shown by the BET result, severe induration was caused by the plugging of small pores, which left only large pores and reduced diffusion. Thus, the Mn/TiO2 (700) catalyst has a greater difficulty removing oxygen, and the last reduction step of Mn3O4 to MnO did not occur. The TPR and XRD results indicated that most of the manganese was stabilized in the Mn4+ oxidation state for the Mn/TiO2 (300–500) catalysts. In contrast, most of the manganese was stabilized in the Mn3+ oxidation state for the Mn/TiO2 (600–700) catalysts.
TPD analysis
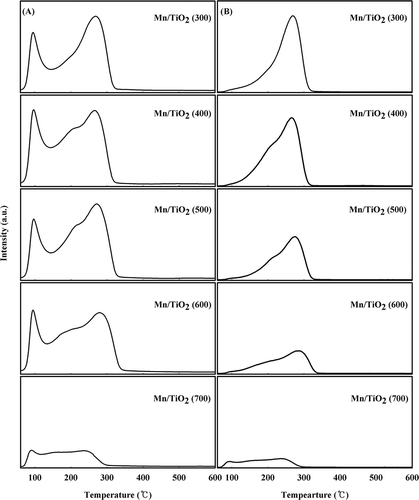
NH3-TPD experiments using TCD were performed to investigate the adsorption properties of NH3 (). Three desorption peaks were observed between about 80 and 600 °C for the Mn/TiO2 (300–500) catalysts. In contrast, broad desorption peaks were observed between about 80 and 500 °C for the Mn/TiO2 (600–700) catalysts. Several studies on the Brönsted and Lewis acid sites in Mn/TiO2 catalysts have been reported, and NH3 adsorbed onto the Brönsted acid sites desorbed at a lower temperature than the Lewis acid–coordinated NH3. Many researchers have reported that Lewis acidity is important in low-temperature SCR; however, the reported NH3 desorption temperatures are quite different when using a TCD detector. For example, CitationChmielarz et al. (2004) reported that NH3 was desorbed from the strong and broad Lewis acid sites at approximately 650 °C, and CitationJin et al. (2010) reported that NH3 desorbed from a Mn-Ce/TiO2 catalyst at 570∼680 °C and was most strongly linked to the Lewis acid sites. CitationWu et al. (2008) reported that NH3 adsorbed at the weak and medium acid sites desorbed at 100∼350 °C, whereas the NH3 adsorbed at strong Lewis acid sites desorbed between 564 and 665 °C. The Mn/TiO2 catalyst is one of the best catalysts for oxidizing NH3 (CitationWu et al., 2008). Therefore, it is possible that the strongly chemisorbed NH3 was oxidized by lattice oxygen during the NH3-TPD experiment.
Figure 4. NH3-TPD profiles of the Mn/TiO2 catalysts with different calcination temperatures using TCD.
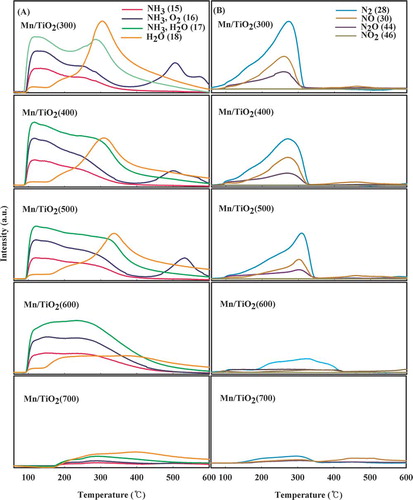
NH3-TPD experiments that use TCD are capable of measuring all materials in addition to NH3. Therefore, NH3-TPD experiments using a mass spectrometer were performed to investigate NH3 and other by-products (). NH3 was recorded using the molecular weights of 15, 16, 17, and 18 and was measured simultaneously with oxygen (m/z 16) and moisture (m/z 17 and 18). With the exception of catalyst calcined at 700 °C, all of the catalysts contained two broad NH3 desorption peaks at 80∼160 and 200∼350 °C. These two peaks were attributed to the Brönsted and Lewis acid sites. As shown in , NO, NO2, and N2O were generated through the oxidation of NH3, which was attributed to the desorption of NH3 chemisorbed to strong Lewis acid sites at approximately 200∼350 °C. The lower the calcination temperature, the greater the amount of oxidized material, which could only be from the lattice oxygen present in the catalyst because gaseous oxygen was not provided. The relative quantity of materials oxidized by the lattice oxygen in the catalyst was very low relative to NH3, and the lower calcination temperatures produced more lattice oxygen. In addition, the peak formed at 500 °C in the mass analysis corresponded to oxygen (m/z 16) and not NH3 (m/z 15). Because of the TPR test, the reduction of MnO2 to Mn2O3 in the Mn/TiO2 (300–500) catalysts was shown to occur at a temperature of 303 °C. Accordingly, the activation of oxygen plays a role in partially oxidizing the adsorbed NH3 through the reduction of MnO2 to Mn2O3, which occurred at approximately 300 °C. In addition, the structure of Mn–O–Ti is unstable and provides further lattice oxygen molecules for the oxidation of NH3. As this structure is unstable, Mn2O3 might be reduced to Mn3O4 when the temperature is over 500 °C because of the thermal effect, and the oxygen would not participate in reactions within the developed catalysts. Mn/TiO2 (600–700) catalysts were already calcined at temperatures over 600 °C, and their manganese oxidation state on the catalyst surface does not form an unstable structure with deficient lattice oxygen and mostly exists as Mn3+. Therefore, the generation of oxygen was not observed for the Mn/TiO2 (600–700) catalysts at temperatures over 500 °C during the NH3-TPD test. For such catalysts, the reduction of MnO2 to Mn2O3 at approximately 300 °C did not occur. As the amount of oxygen available was insignificant because of the reduction of MnO2 to Mn2O3, NH3 oxidation using lattice oxygen was incomplete. Because the NH3-TPD test used Mn/TiO2 catalysts prepared at different calcination temperatures, the chemisorptions at the Lewis acid sites for temperatures over 200 °C were shown to be more important than desorption from the Brönsted acid sites at approximately 100 °C in the low-temperature SCR. In addition, for catalysts calcined at lower temperatures, the acidity of the acid site might change to allow for the use of the lattice oxygen, which had a higher catalytic activity due to the effective activation of molecular oxygen through the oxygen transfer mechanism.
Figure 5. NH3-TPD profiles of the Mn/TiO2 catalysts with different calcination temperatures using mass spectrum. (A) Investigation of NH3, O2 and H2O; (B) investigation of N2, NO, N2O, and NO2 (color figure available online).
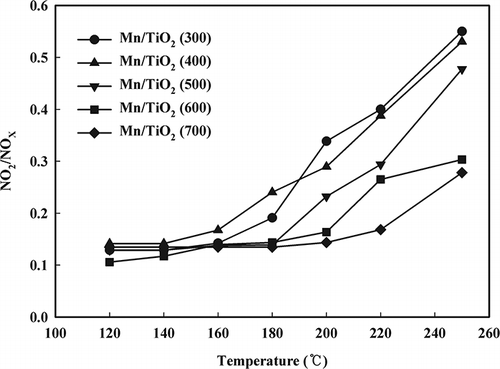
NO-TPD experiments using a mass spectrometer were also performed to investigate the adsorption properties of nitrogen oxide. All of the catalysts contained two desorption peaks (). The first peak corresponded to either a weakly or physically adsorbed NO and appeared at 100 °C, whereas a broader second peak, which corresponded to desorption of the more strongly bound nitrate species, occurred between 200 and 300 °C. No desorbed NO species were observed above 300 °C. A similar NO desorption trend was observed for all of the catalysts. The relative quantity of NO desorbed from the Mn/TiO2 (600–700) catalysts was relatively small. As shown in , NO2 began to desorb at approximately 150 °C, with the maximum desorption occurring between 250 and 300 °C. The results of the NO-TPD test were similar to the NH3-TPD test. Most of the NO2 was oxidized from NO through chemically adsorbed oxygen, and the higher the oxidation state of the Mn catalyst, the more NO2 was produced. In addition, unlike the catalysts calcined at 300 and 400 °C, which reached a maximum peak at 270 °C, the peak maximum for catalysts calcined at 500 °C was 278 °C, and the maximum peak for catalysts calcined at 600 °C was 288 °C. As the calcination temperature decreased, the Mn4+ content of the catalyst increased, which could be easily identified using the lattice oxygen. The low-temperature SCR continued once NH3 and NO were adsorbed. Because of the TPD test, both adsorbed compounds (NH3 and NO) were shown to produce lattice oxygen. The oxidation of NH3, a reducing agent in the SCR, negatively affected SCR activity. However, the materials formed from this oxidation were primarily N2 and NO2, whereas during the fast SCR process, only some of the NO was oxidized to NO2, the reducing agent was oxidized, and the oxidation process did not have a profound effect on SCR activity. Therefore, the capability to oxidize NO to NO2 plays a greater role than NH3 in the SCR.
NO oxidation activity
In the previous TPD test, the NH3 and NO adsorption characteristics of Mn/TiO2 catalysts prepared at different calcination temperatures were evaluated. A similar trend of desorption was observed for all of the catalysts, except the catalyst calcined at 700 °C, where a higher calcination temperature resulted in a reduced production of the oxidizing material. When both NO and NO2 were included into the feed, a fast SCR may have occurred when compared with the standard SCR of NO (CitationCiardelli et al., 2007; CitationKoebel et al., 2002a, Citation2002b; CitationTronconi et al., 2007). The oxidation activity tests for the conversion of NO to NO2 by O2 on the Mn/TiO2 catalysts prepared at different calcination temperatures were performed (). The lower the calcination temperature, the higher the NO oxidation activity, and a conversion rate of 76% was obtained at 250 °C. The adsorptive capabilities of NO in the region of low temperatures were similar to each other; the cause for the difference in NO oxidation activity with the same amount of gaseous oxygen was due to the difference in the amount of lattice oxygen that participated in the reaction inside the catalyst. The Mn/TiO2 catalysts can be reduced from MnO2 to Mn2O3, and the released oxygen species from MnO2 participated in the oxidation of NO to NO2. The reduced Mn2O3 can then be reoxidized by gaseous oxygen. That is to say, gaseous oxygen cannot be expected to participate in the reaction at extremely low temperatures, but it can participate in reoxidation.
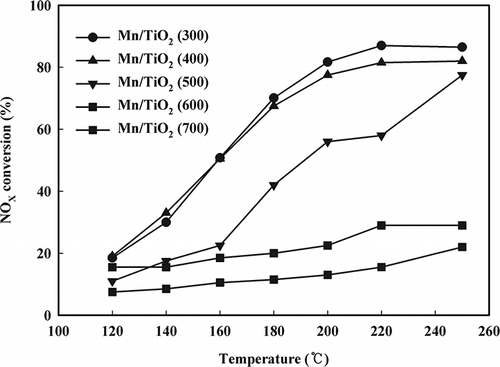
SCR activity
shows the NOx activity tests for the Mn/TiO2 catalysts with respect to their calcination temperatures. Conversion of NOx decreased with increasing calcination temperature for all of the catalysts. At low temperatures, the Mn/TiO2 catalyst calcined at 300 °C showed the highest NOx removal efficiency, which was approximately 90% of the conversion rate at 180 °C. However, the crystalline Mn2O3 catalyst did not convert NOx at any temperature.
Conclusions
The oxidation state and lattice oxygen behavior of the Mn/TiO2 catalysts strongly affected their SCR activity at low temperatures. The structure and oxidation state analyses by XRD and TPR confirmed that the manganese oxidation state (Mn4+) was higher for the Mn/TiO2 (300–500) catalysts than for the Mn/TiO2 (600–700) catalysts. TPD analysis revealed the presence of Brönsted and Lewis acid sites at 80∼160 and 200∼350 °C, respectively. The Lewis acid sites, which chemisorbed at temperatures above 200 °C, were important to the low-temperature SCR. However, regardless of the manganese oxidation state on the catalyst surface, the adsorption and adsorptive strength of NH3 and NO did not change. Catalysts calcined at low temperatures existed as amorphous MnO2 and contained active lattice oxygen in more abundance than catalysts containing Mn2O3. As indicated by the NO oxidation test, the oxygen released from the MnO2 structure can more readily oxidize NO to NO2 than the Mn2O3 structure, which resulted in a higher NOx conversion rate because of this excellent redox characteristic. The manganese oxidation state and availability of lattice oxygen were shown to play important roles in low-temperature SCR activity.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0023963).
References
- Alemany , J.L. , Lietti , L. , Ferlazzo , N. , Forzatti , P. , Busca , G. , Ramis , G. , Giamello , E. and Bregani , F. 1995 . Reactivity and physicochemical characterization of V2O5-WO3/TiO2 De-NOx catalysts . J. Catal. , 155 : 117 – 130 . doi: 10.1006/jcat.1995.1193
- Bosch , H. and Janssen , F. 1988 . Formation and control of nitrogen oxide . Catal. Today , 2 : 369 – 379 . doi: 10.1016/0920-5861(88)80002-6
- Centi , G. and Perathoner , S. 1995 . Nature of active species in copper-based catalysts and their chemistry of transformation of nitrogen oxides . Appl. Catal. A Gen. , 132 : 179 – 259 . doi: 10.1016/0926-860X(95)00154-9
- Chmielarz , L. , Kustrowski , P. , Zbroja , M. , Knap , B.G. , Datka , J. and Dziembaj , R. 2004 . SCR of NO by NH3 on alumina or titania pillared montmorillonite modified with Cu or Co: Part II. Temperature programmed studies . Appl. Catal. B Environ , 53 : 47 – 61 . doi: 10.1016/j.apcatb.2004.04.019
- Ciardelli , C. , Nova , I. , Tronconi , E. , Chatterjee , D. , Burkhardt , T. and Weibel , M. 2007 . NH3 SCR of NOx for diesel exhausts aftertreatment: Role of NO2 in catalytic mechanism, unsteady kinetics and monolith converter modeling . Chem. Eng. Sci. , 62 : 5001 – 5006 . doi: 10.1016/j.ces.2006.11.031
- Ettireddy , P.R. , Ettireddy , N. , Mamedov , S. , Boolchand , P. and Smirniotis , P.G. 2007 . Surface characterization studies of TiO2 supported manganese oxide catalysts for low temperature SCR of NO with NH3 . Appl. Catal. B Environ. , 76 : 123 – 134 . doi: 10.1016/j.apcatb.2007.05.010
- Jin , R. , Liu , Y. , Wu , Z. , Wang , H. and Gu , T. 2010 . Low-temperature selective catalytic reduction of NO with NH3 over Mn–Ce oxides supported on TiO2 and Al2O3: A comparative study . Chemosphere , 78 : 1160 – 1166 . doi: 10.1016/j.chemosphere.2009.11.049
- Kapteijn , F. , Singoredjo , L. and Andreini , A. 1994 . Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia . Appl. Catal. B Environ. , 3 : 173 – 189 . doi: 10.1016/0926-3373(93)E0034-9
- Kim , S.S. , Lee , S.M. , Park , K.H. , Kwon , D.W. and Hong , S.C. 2011 . A study on the reaction characteristics of vanadium-impregnated natural manganese oxide in ammonia selective catalytic reduction . J. Air Waste Manage. Assoc. , 61 : 552 – 558 . doi: 10.3155/1047-3289.61.5.552
- Koebel , M. , Madia , G. and Elsener , M. 2002a . Selective catalytic reduction of NO and NO2 at low temperatures . Catal. Today , 73 : 239 – 247 . doi: 10.1016/S0920-58610200006-8
- Koebel , M. , Madia , G. , Raimondi , F. and Wokaun , A. 2002b . Enhanced reoxidation of vanadia by NO2 in the fast SCR Reaction . J. Catal. , 209 : 159 – 165 . doi: 10.1006/jcat.2002.3624
- Kuo , M.Y. , Gitschier , J. and Packman , S. 1997 . Developmental expression of the mouse mottled and toxic milk genes suggests distinct functions for the Menkes and Wilson disease copper transporters . Hum. Mol. Genet. , 6 : 1043 – 1049 . doi: 10.1093/hmg/6.7.1043
- Li , J.H. , Chen , J.J. , Ke , R. , Luo , C.K. and Hao , J.M. 2007 . Effects of precursors on the surface Mn species and the activities for NO reduction over MnOx/TiO2 catalysts . Catal. Commum. , 8 : 1896 – 1900 . doi: 10.1016/j.catcom.2007.03.007
- Li , Y. , Cheng , H. , Li , D. , Qin , Y. , Xie , Y. and Wang , S. 2008 . WO3/CeO2-ZrO2, a promising catalyst for selective catalytic reduction (SCR) of NOx with NH3 in diesel exhaust . Chem. Commun. , 12 : 1470 – 1472 . doi: 10.1039/B717873E
- Marbán , G. , Valdes-Solis , T. and Fuertes , A.B. 2004 . Mechanism of low-temperature selective catalytic reduction of NO with NH3 over carbon-supported Mn3O4: Role of surface NH3 species: SCR mechanism . J. Catal. , 26 : 138 – 155 . doi: 10.1016/j.jcat.2004.05.022
- Parvulescu , V.I. , Grange , P. and Delmon , B. 1998 . Catalytic removal of NO . Catal. Commum. , 46 : 233 – 316 . doi: 10.1016/S0920-5861(98)00399-X
- Pechi , G. , Reyes , P. , Lopez , T. , Gomez , R. , Moreno , A. and Fierro , J.L.G. 2002 . Effect of precursors on surface and catalytic properties of Fe/TiO2 catalysts . J. Chem. Technol. Biotechnol. , 77 : 944 – 951 . doi: 10.1002/jctb.660
- Peña , D.A. , Uphade , B.S. and Smirniotis , P.G. 2004 . TiO2-supported metal oxide catalysts for low-temperature selective catalytic reduction of NO with NH3: I. Evaluation and characterization of first row transition metals . J. Catal. , 221 : 421 – 431 . doi: 10.1016/j.jcat.2003.09.003
- Qi , G. and Yang , R.T. 2003 . Performance and kinetics study for low-temperature SCR of NO with NH3 over MnOx–CeO2 catalyst . J. Catal. , 217 : 434 – 441 . doi: 10.1016/S0021-9517(03)00081-2
- Qi , G. , Yang , R.T. and Chang , R. 2004 . MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures . Appl. Catal. B Environ. , 51 : 93 – 106 . doi: 10.1016/j.apcatb.2004.01.023
- Roy , S. , Viswanath , B. , Hegde , M.S. and Madras , G. 2008 . Low-temperature selective catalytic reduction of NO with NH3 over Ti0.9M0.1O2-δ (M = Cr, Mn, Fe, Co, Cu) . J. Phys. Chem. C , 112 : 6002 – 6012 . doi: 10.1021/jp7117086
- Smirniotis , P.G. , Sreekanth , P.M. , Peña , D.A. and Jinkins , R.G. 2006 . Manganese Oxide catalysts supported on TiO2, Al2O3, and SiO2: A comparison for low-temperature SCR of NO with NH3 . Ind. Eng. Chem. Res. , 45 : 6444 – 6449 . doi: 10.1021/ie060484t
- Stoilova , D. , Cheshkova , K. and Nickolov , R. 1998 . FTIR spectroscopic study of NH3 and no adsorption on alumina-supported manganese oxide catalyst . React. Kinet. Catal. Lett. , 65 : 265 – 270 . doi: 10.1007/BF02475263
- Tang , X. , Li , Y. , Huang , X. , Xu , Y. , Zhu , Y. , Wang , J. and Shen , W. 2006 . MnOx–CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: Effect of preparation method and calcination temperature . Appl. Catal. B Environ. , 62 : 265 – 273 . doi: 10.1016/j.apcatb.2005.08.004
- Thirupathi , B. and Smirniotis , P.G. 2011 . Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures . Appl. Catal. B Environ. , 110 : 195 – 206 . doi: 10.1016/j.apcatb.2011.09.001
- Thirupathi , B. and Smirniotis , P.G. 2012 . Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations . J. Catal. , 288 : 74 – 83 . doi: 10.1016/j.jcat.2012.01.003
- Tronconi , E. , Nova , I. , Ciardelli , C. , Chatterjee , D. and Weibel , M. 2007 . Redox features in the catalytic mechanism of the “standard” and “fast” NH3-SCR of NOx over a V-based catalyst investigated by dynamic methods . J. Catal. , 245 : 1 – 10 . doi: 10.1016/j.jcat.2006.09.012
- Wu , Z. , Jin , R. , Liu , Y. and Wang , H. 2008 . Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature . Catal. Commum. , 9 : 2217 – 2220 . doi: 10.1016/j.catcom.2008.05.001