Abstract
Oxyfuel combustion is a promising technology that may greatly facilitate carbon capture and sequestration by increasing the relative CO2 content of the combustion emission stream. However, the potential effect of enhanced oxygen combustion conditions on emissions of criteria and hazardous air pollutants (e.g., acid gases, particulates, metals and organics) is not well studied. It is possible that combustion under oxyfuel conditions could produce emissions posing different risks than those currently being managed by the power industry (e.g., by changing the valence state of metals). The data available for addressing these concerns are quite limited and are typically derived from laboratory-scale or pilot-scale tests. A review of the available data does suggest that oxyfuel combustion may decrease the air emissions of some pollutants (e.g., SO2, NOx, particulates) whereas data for other pollutants are too limited to draw any conclusions. The oxy-combustion systems that have been proposed to date do not have a conventional “stack” and combustion flue gas is treated in such a way that solid or liquid waste streams are the major outputs. Use of this technology will therefore shift emissions from air to solid or liquid waste streams, but the risk management implications of this potential change have yet to be assessed. Truly useful studies of the potential effects of oxyfuel combustion on power plant emissions will require construction of integrated systems containing a combustion system coupled to a CO2 processing unit. Sampling and analysis to assess potential emission effects should be an essential part of integrated system tests.
Implications: Oxyfuel combustion may facilitate carbon capture and sequestration by increasing the relative CO2 content of the combustion emission stream. However, the potential effect of enhanced oxygen combustion conditions on emissions of criteria and hazardous air pollutants has not been well studied. Combustion under oxyfuel conditions could produce emissions posing different risks than those currently being managed by the power industry. Therefore, before moving further with oxyfuel combustion as a new technology, it is appropriate to summarize the current understanding of potential emissions risk and to identify data gaps as priorities for future research.
Introduction
Coal-fired power plants and their emissions are found in all parts of the United States. These emissions include pollutants associated directly with the incoming coals themselves (e.g., mercury, arsenic, and sulfur) and those formed during combustion of the fuel (e.g., acid gases, dioxins, formaldehyde). Unlike many other industrial sectors where emissions tend to be dominated by a small number of pollutants, emissions from coal-fired power plants can be highly complex and variable mixtures. The nature of the emissions and their environmental fate may vary based on the type of coal being used, the control technologies in place, and terrain and atmospheric factors. This poses a challenge to estimating the effects of these emissions on human and ecological health. Although air emissions from coal-fired power plants receive the most attention, discharges to surface water or land disposal of ash residues may also pose risks to human health. For example, coal fly ash may be highly enriched in heavy metals and other compounds relative to feed coal, although the leachability and mobility of these chemicals may be limited.
Coal is primarily composed of compounds of hydrogen and carbon. When coal is burned, the resulting heat is used to produce steam that is, in turn, used to generate electricity via turbine generators. The carbon component of coal is oxidized primarily to carbon dioxide (CO2), essentially an undesirable by-product that when emitted contributes to the greenhouse gas effect. The quantities of CO2 released via fossil fuel combustion are substantial; CO2 emissions due to fossil fuel combustion were estimated at 5387 metric tons in the United States in 2010 (CitationU.S. Environmental Protection Agency [EPA], 2011). Coal-fired power plants represent the largest source of CO2 emissions in the United States, although releases due to transportation are not far behind (CitationEPA, 2011). In an effort to address concerns about global climate change, a number of technologies are being studied that would remove CO2 from the emissions stream so that it can subsequently be sequestered, for example, in voids in deep rock formations. A key challenge for these carbon capture approaches is the need to treat enormous volumes of flue gas (i.e., the gases and particulate matter produced by coal combustion) to separate the CO2 from other gases (e.g., nitrogen and oxygen) in the emission stream. One promising solution to this problem involves oxyfuel combustion, a process in which the coal is combusted not with ambient air (containing roughly 21% oxygen by volume) but with an oxygen-enriched mixture. This results in a flue gas that is much richer in CO2 than that derived from air-fired combustion, and from which the CO2 may be more readily and efficiently separated.
There are a number of technical challenges to be solved before oxyfuel combustion becomes a viable method for addressing CO2 emissions. For example, the technologies currently available for producing oxygen from ambient air involve the use of either selectively permeable membranes or cryogenic distillation. Both processes are energy-intensive and result in a large efficiency penalty if employed as part of the oxyfuel combustion process. Beyond these technological challenges, the use of this new technology might also result in changes in the process chemistry of coal combustion that could lead to the formation of chemical species that pose different health risks relative to those currently observed (CitationAndersen et al., 2010). How this possible difference may be manifested is unclear and requires greater study (e.g., greater or reduced risks overall, differences in routes of exposure, differences in health endpoints or risk drivers, etc.). The effect of the new technology on health risks may be particularly relevant for metals (e.g., mercury, arsenic, chromium), which constitute a significant portion of the human health and environmental risk currently associated with coal-fired power plants (CitationBoffetta et al., 1991; CitationFrench et al., 1997; CitationKhillare et al., 2012; CitationEPA, 2012). Valence state is critical to the transport of many metals through the environment (notably elemental versus inorganic species of mercury, which have very different deposition patterns). Valence state may also be an important determinant of toxicity, although the situation is complex; a higher oxidation state can confer greater toxicity for some metals (e.g., hexavalent versus trivalent chromium) via some exposure routes, but lesser toxicity for others (e.g., pentavalent versus trivalent arsenic) (CitationGibb and Chen, 1989; CitationYokel et al., 2006). It is therefore a valid question whether the valence state of metals derived from coal in oxyfuel combustion units would be altered substantially from that in conventional units. Oxyfuel combustion might in theory also have significant effects on emissions of criteria pollutants (sulfur oxides [SOx], nitrogen oxides [NOx]), particularly given that far less nitrogen will be present in the system due to oxygen enrichment. The potential effect on formation of complex organic molecules (e.g., dioxins, formaldehyde) is also uncertain. Beyond alterations in the combustion process itself, consideration needs to be given to how oxyfuel combustion would interact with potential control technologies, including new methods for carbon sequestration. It may be that emissions may shift among different media (e.g., from air to solid waste), leading to differences in potential exposure pathways and/or affected populations.
The determination of whether oxyfuel combustion as a technology can be adopted to help ameliorate carbon accumulation in the atmosphere and its consequences for climate change will depend in part on whether any new risks posed by the technology are judged to be acceptable to the public. It will therefore be necessary to quantify the emissions of all chemicals of concern from oxyfuel combustion to evaluate the potential impacts on human health and environment. Regulators considering any future power plant technology will want to know whether such technologies reduce CO2 emissions in an energy-efficient and cost-effective manner but also whether they pose an acceptable level of risk to human health and the environment (where acceptable is typically defined using benchmarks established by regulatory agencies, e.g., a one in one million excess cancer risk) (CitationHunter and Fewtrell, 2001; CitationInternational Risk Governance Council [IRGC], 2009). The situation with amine-based postcombustion carbon capture may serve as a worthwhile lesson on the need to be forward-thinking. Substantial work has been done to address the technological and cost-effectiveness of using this technology, yet basic questions remain concerning the exposure potential and toxicity of some of the by-products of this process, and such questions have created major hurdles in terms of advancing this carbon capture process (Norwegian Institute for Public Health, 2011). Thus, before moving further with oxyfuel combustion as a new technology, it is reasonable to ask what we currently know about potential changes in emissions and discharges (in terms of chemical form and environmental media) and to identify data gaps as priorities for future research.
In the remainder of this paper, we will first discuss the basic principles of oxyfuel combustion and describe the features of different oxyfuel combustion/carbon capture systems that have been proposed. We then summarize the state of the science concerning the effects of an enriched oxygen environment on pollutant formation during the combustion process. We subsequently discuss the potential of pollutants generated during oxyfuel combustion to be emitted to the environment in light of anticipated pollution control technology. We conclude by identifying important data gaps and areas where additional research is needed.
CO2 Capture with Oxyfuel Combustion Systems
Oxyfuel combustion was originally conceived as a retrofit option for coal-fired boilers that would allow relatively easy separation of CO2 from the flue gas. As noted previously, oxyfuel combustion requires large quantities of oxygen, which is produced on-site from ambient air through a separation process; the typical output of the separation is a gas with a minimum oxygen concentration of 95% by volume (CitationMatuszewski, 2010). When fuel is burned in a high-oxygen environment, the flame temperature is higher than when fuel is burned in air. In boiler retrofit applications of oxyfuel combustion, the materials of construction in the boiler are not compatible with the high temperatures of a pure-oxygen flame, and therefore some CO2-rich flue gas is recycled to the combustor to control the flame temperature, such that it is similar to that in a normal air-fired system. Oxyfuel combustion relies on up-front physical separation processes for oxygen (cryogenic or membrane separation) to produce a relatively pure CO2 stream, thus eliminating the need for the reagents or solvents that are used in postcombustion CO2 scrubbers. The main drawback to oxyfuel combustion is the cost associated with the production of large quantities of oxygen, including capital and energy costs. Calculations of the output of a 550 MW (net) supercritical combustor have shown that the auxiliary power requirements as a percentage of gross power output are 28% for an oxy-fired combustion unit versus 5% for air-fired combustion (CitationMatuszewski, 2010).
The CO2 produced from the combustion process is intended to be sequestered or used for an application such as enhanced oil recovery. The large volumes of CO2 produced from coal combustion must be compressed for transport. Prior to, or during, compression, the CO2 in the flue gas stream needs to be separated from impurities such as SOx, NOx, oxygen, nitrogen, argon, and various trace contaminants such as mercury (CitationWhite et al., 2011; CitationStanger and Wall, 2011). SOx and NOx are expected to form acids during the early stages of compression, which can be separated from the gas stream (CitationWhite et al., 2011). Mercury may be dissolved in the nitric acid formed by the NOx or removed in sorbent beds (CitationWhite et al., 2011; CitationMitsui, et al., 2011). Low-temperature flash evaporation, membrane separation, or distillation during later stages of compression is used to reduce the amounts of oxygen and inert gas impurities (CitationWhite et al., 2011).
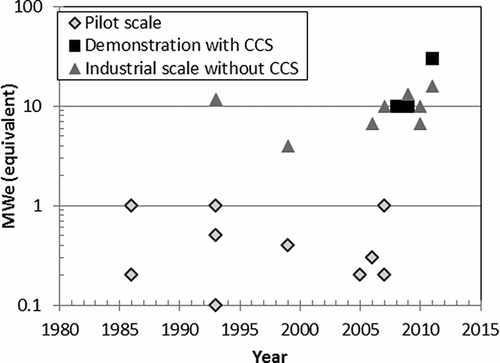
At this point, there is considerable uncertainty about certain aspects of the process flow diagram for an oxy-combustion system, including recovery of CO2. The uncertainty is related, in part, to the lack of full-scale experience with oxy-combustion systems for generation of power and sequestration of CO2. Pilot-scale systems have been designed, built, and operated. CitationWall et al. (2011) summarized the chronological evolution of pilot- and industrial-scale oxyfuel combustion demonstration projects, as shown in The largest demonstration to date (30 MWe) is the Callide demonstration project, currently in the early stages of testing in Australia. At the present time, there are no full-scale, commercial oxyfuel combustion units in operation or under construction.
Figure 1. Progress in initiation of oxyfuel combustion pilot-scale and demonstration units, as summarized by CitationWall et al. (2011).
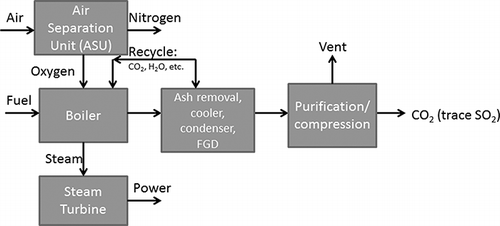
Various process diagrams have been proposed. For example, CitationWall et al. (2009) presented a high-level diagram of an oxyfuel retrofit system including CO2 compression, which is shown in Oxygen is produced in the air separation unit (ASU), resulting in a waste stream of nitrogen. The oxygen combines with fuel and a recycled flue gas stream in the boiler. Steam produced in the boiler generates electricity in a steam turbine. An integral feature of a coal-fired oxy-combustion system is the recycling of flue gas and the treatment of the entire flue gas stream to produce a CO2 stream. Before the CO2 stream can be compressed, it goes through a series of cleanup steps, which might include filtration to remove particles (fly ash), a flue gas desulfurization (FGD) step, and a condenser to remove water. Further purification might take place in the CO2 processing unit (CPU). As shown in , there is a “vent” stream in the system, which is the only gaseous emission from the system. The details of this vent stream depend on the specific system for flue gas treatment and CO2 compression.
Fiugre 2. Flowsheet of oxyfuel technology for power production (from CitationWall et al., 2009).
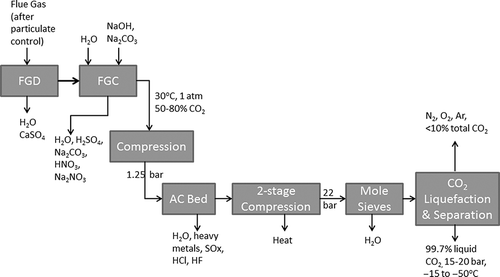
Two different flue gas treatment schemes have been described in the literature (CitationWall et al., 2013), one for the Schwarze Pumpe pilot facility operated by Vattenfall in Germany and one under construction at the Callide Demonstration Plant in Australia. shows the details of the Schwarze Pumpe treatment system. Successive modules remove sulfur species, NOx, mercury, and water. The FGD module removes most sulfur species and other acid gases. A flue gas conditioning (FGC) module removes water, NOx, and additional SOx. Compression of the flue gas (after desulfurization and cooling) was integrated with an activated carbon (AC) bed for removal of mercury, SOx, hydrochloric acid (HCl), etc. A refrigeration unit was used to separate CO2 from noncondensable gases. The only gaseous emission is the vent stream in the CO2 liquefaction process. As discussed below, the vent stream in an oxyfuel combustion system can be an order of magnitude smaller in volume than the flue gas stream in a conventional coal combustor. CitationStromberg (2011) presented some general results from the Vattenfall Schwarze Pumpe plant. More than 90% of the flue gas CO2 was captured and compressed for sequestration. The vent gas was composed of 35–40% unrecoverable CO2, 30–35% oxygen, and 20–25% nitrogen. The vent gas could also contain CO, SO2, and NOx but concentrations were not specified.
Figure 3. Flowchart of Vattenfall's pilot plant flue gas treatment (from CitationWall et al., 2013). Abbreviations used are defined at the end of the article.
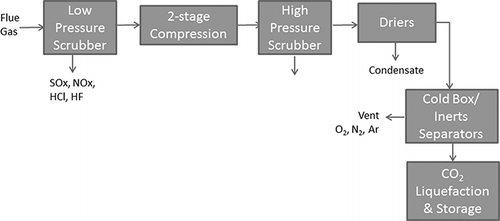
shows the details of the Callide treatment system (CitationSpero, 2012). Particulate matter is removed from the flue gas in a fabric filter (not shown). The flue gas enters a low-pressure scrubber where acid gases are removed (further description of the scrubber was not provided in the reference). The gas is compressed, then passes through a high-pressure scrubber and a drier to remove moisture. The final process module before CO2 liquefaction is a stripper for noncondensable gases, which emits nitrogen, oxygen, and argon to the atmosphere.
Figure 4. Flowchart of Callide OxyFuel Project flue gas treatment (from CitationSpero, 2012).
In addition to these two treatment schemes related to specific facilities, a number of early-stage designs have also been discussed in the literature. For example, Praxair has proposed a CO2 processing unit (CPU) concept (CitationShah et al., 2011). The Praxair design uses an activated carbon process to oxidize SO2 and NO and then collect these gases on carbon beds. Praxair reported 94–98% NOx removal efficiency and 99.8% or greater SOx removal efficiency in preliminary testing. A vacuum pressure-swing absorption unit was used to purify CO2. They reported a vent stream from this unit with a composition of ∼7% CO2, 0 ppm SOx, 9 ppm NOx, and <10 ppm CO.
Air Products has proposed removing NOx, SOx, and mercury via a “sour” compression system (CitationWhite et al., 2011). A temperature-swing system removes inerts, producing a vent gas that is approximately 25% CO2, approximately 25% oxygen, and approximately 50% nitrogen. The vent gas flow is a small percentage of the flue gas processed in the CPU; one estimate was that this stream represented 4% of the flue gas volume leaving the combustor (CitationTranier and Perrin, 2011).
B&W and Air Liquide carried out a design study (CitationMcDonald et al., 2011), which assumed a Powder River Basin (PRB) coal and a plant capacity of 700 MWe (gross). For the specific plant configuration studied, B&W predicted <1 ppmv emissions of NOx, SO2, total particulate matter (PM), and mercury. They also predicted 25.8 mg/MJ CO emissions. A study was carried by the Department of Energy National Energy Technology Laboratory (DOE-NETL) using the ASPEN model featuring performance and cost calculations for power plants fueled by low-rank coals (CitationMatuszewski, 2010). Three cases in this study for subbituminous-fired boilers are illustrative and will be discussed here. The net power output for all cases was 550 MW, but the gross output varied. Broadly, these three cases can be described as follows:
| • | Case 12A—Conventional air-fired supercritical boiler with selective catalytic reduction (SCR), dry FGD, and fabric filter; activated carbon is injected for mercury control. | ||||
| • | Case 12D—Supercritical boiler with cryogenic air separation unit and flue gas recycling; the flue gas is treated with a dry FGD-baghouse combination and the flue gas is compressed, which removes water, but only results in a CO2 purity of 85%. | ||||
| • | Case 12E—Supercritical boiler with cryogenic air separation unit and flue gas recycling; the flue gas is treated with a dry FGD-baghouse combination and is flash-distilled to remove noncondensable gases (which are emitted in the vent stream). The process results in a CO2 purity of 98%. | ||||
The plant output and efficiency, as well as the mass balance of output and input streams, are shown in . The different emissions patterns produced by these systems are presented in In the conventional (12A) case, air emissions through the stack are the single largest emission. There are no water discharges, owing to the use of the dry scrubber. In both oxyfuel combustion cases, the immediate postcombustion air emissions are eliminated but both have a substantial nitrogen emission stream from the air separation unit. Fly ash also increases in the oxyfuel combustion cases by about 25% relative to the air-fired baseline, because of the larger amount of coal that must be burned to produce the same net megawatts. In the compression-only (12D) case, there are water discharge streams from cooling of the flue gas (after the dry FGD-FF) and from a polishing SO2 scrubber (in addition to the dry FGD). The water discharge stream from the polishing scrubber contains sulfur compounds, according to the NETL analysis. This stream might also contain mercury and other metals, although this is not taken into account in the NETL analysis. In the flash distillation (12E) case, there are water discharge streams (as in 12D) and a vent stream from the CO2 distillation module. It should be noted that the total stack emission that has to be managed in the conventional case is over 18 times as great (on a mass basis) as the vent stream in oxyfuel combustion case 12E (largely due to atmospheric nitrogen which is removed prior to combustion in the oxyfuel case).
Table 1. Plant performance and mass balance for convention andxyfuel combustion plants CitationMatuszewski (2010)
Figure 5. Relative emissions (mass basis) for selected cases in CitationMatuszewski (2010).
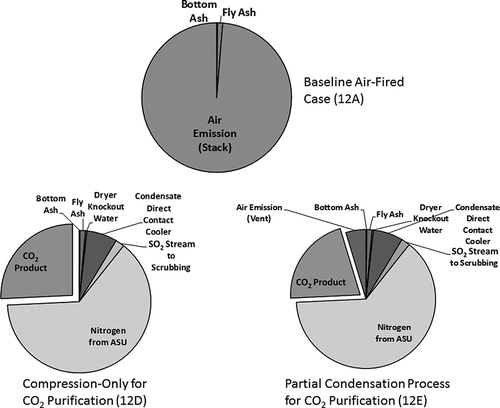
gives the calculated percent compositions of the air emissions from these cases. Again, note that the total mass of air emissions will be reduced in case 12E relative to case 12A. The NETL analysis did not include modeling of trace species or the partitioning of particulate matter, NOx, SO2, mercury, and other metals between gas and liquid streams in the condensation and distillation modules or in the polishing scrubber. This analysis is not detailed enough to estimate emissions of trace species.
Table 2. Composition of air emissions, conventional and oxyfuel combustion plants (CitationMatuszewski, 2010)
Production of a compressed CO2 stream in an oxy-combustion plant means that air emissions are eliminated or reduced to a large extent. However, there are significant new water discharge streams. The trace emissions have not been modeled to date in either air or water discharges from oxyfuel combustion plants.
Information on the impact of oxyfuel combustion on smaller volume emission constituents is available from a recent review by CitationThimsen and Wheeldon (2011), which focused on the 2010 Electric Power Research Institute (EPRI) analysis of oxyfuel plant performance. The annual estimate of CO emissions for a 700 MW oxy-fired pulverized coal plant is given as 1753 metric tons/year. CO will largely end up in the vent stream (which is assumed to be ∼30% O2). Air Products has suggested CO in the vent stream could be oxidized to CO2 over a suitable catalyst. Volatile organic carbon (VOC) emissions are estimated to be below 90.7 metric tons/year. Residual traces of NO are projected to be present in the vent gas from the CPU. No PM is expected to be found in the vent gas. The presence of mercury as a contaminant of the CO2 stream prior to compression is a technological issue due to its corrosive effect on the CO2 compressor (CitationMitsui et al., 2011). Corrosion by elemental mercury in the heat exchangers used in liquefied natural gas (LNG) plants has been known for decades (CitationLeeper, 1980). Mercury in the high-pressure gas amalgamates with aluminum components in these systems and causes corrosion. Mercury can also amalgamate with other metals. Based on industrial experience in natural gas compression systems, the assumption is that mercury will be removed to below detectable levels at the inlet to the CPU. Start-up and shutdown were identified as times when emissions could be higher.
As suggested in Figures , the oxy-combustion systems that have been proposed to date do not have a conventional “stack,” in that all the combustion flue gas is treated in such a way that solid or liquid by-product streams are the major outputs. Gaseous emissions should consist mainly of relatively pure nitrogen, oxygen, argon, and CO2 near the end of the process. If flue gas is vented instead of being treated (on start-up, for example), then there may be some transient emissions of flue gas. However, the overall designs described to date suggest that use of oxyfuel combustion will result in shifts of chemical emissions to nonatmospheric waste streams. This may provide an improved opportunity for emissions control given that solid and liquid waste streams are typically more concentrated and therefore easier to control. We next consider the available data on how oxyfuel combustion may affect emissions of particular pollutants.
Emissions in Oxy-Combustion Systems
Pollutant formation
NOx . Pilot-scale oxy-combustion studies were carried out on three Australian coals at IHI Corporation's 150 kg/hr combustion test facility in Japan (CitationWall et al., 2009). For the oxy-firing tests, flue gas was recycled to the burner, and oxygen was added to 27%. The flue gas oxygen concentration was 3%. Compared with air-firing, there was a 45% increase in NOx concentration in the flue gas during oxy-firing. However, oxy-firing represents a reduction in total flue gas flow with respect to air-firing. The NOx production rate (in mg/MJ) during oxy-firing was one-third of that during air-firing.
CitationAxelbaum and Biswas (2009) also carried out experiments involving combustion of a PRB subbituminous coal in a 35 kWth pilot combustor at the Energy and Environmental Research Center (EERC) at the University of North Dakota. Experiments were carried out under air- and oxy-firing conditions. It was reported that oxy-firing conditions resulted in a 20% reduction in NOx. More recent pilot-scale combustion at the EERC (CitationZhuang and Pavlish, 2012) showed a 90% reduction of NOx emitted per MJ of fuel burned, when compared with air-fired combustion.
Alstom carried out air- and oxy-firing testing at a 15-MWth tangentially fired boiler (CitationLevasseur et al., 2011). Test campaigns were carried out on four coals: a North Dakota lignite, a PRB subbituminous coal, a low-sulfur bituminous coal, and a high-sulfur bituminous coal. Details were not reported, but Alstom concluded that “NOx emissions during oxy-firing were typically less than 50% of the NOx levels during air firing at comparable firing conditions.” Significantly lower NOx emissions were observed under oxy-firing conditions as compared with air-firing conditions in Babcock and Wilcox's (B&W's) Combustion Emissions Development Facility (CEDF) pilot-scale operation, which fired 5 to 6 tons/hr of coal (CitationMcCauley et al., 2009). This facility had several different flue gas cleaning devices: an electrostatic precipitator (ESP), a dry SO2 scrubber, and a wet SO2 scrubber/cooler. The latter cooled the flue gas for recycle back to the burners. There was no CO2 capture and compression. A portion of the flue gas was vented to a stack, whereas the rest was recycled back to the combustor. Thus, the “emissions” from the CEDF might be representative of flue gas composition entering a compression unit in a full-scale carbon capture and sequestration (CCS) plant. Combustion and emission tests were carried out in the CEDF using three coals: an eastern bituminous coal, a PRB subbituminous coal, and a North Dakota lignite. NOx emissions in the stack were 50–70% of the corresponding concentrations during air combustion.
A review of many different oxy-combustion studies by CitationWall et al. (2011) concluded the following: “Consensus is building amongst the literature that the low nitrogen environment and recycling of flue gas produces a lower emission level of NOx prior to compression in the oxyfuel process.” Absolute NOx emissions will depend on the specific details of the fuel and the combustion system. However, decreased NOx emissions, in the range of 20–70%, can be expected leaving the combustor in an oxy-fired boiler, as compared with a comparable air-fired boiler. It is important to note that emissions from the combustor are not the same as emissions from the entire system.
SOx . In the pilot-scale oxyfuel combustion studies carried out on three Australian coals (CitationWall et al., 2009), SO2 concentrations in the flue gas were 3 times higher under oxy-firing conditions as compared with air-firing conditions. Flue gas was recycled to the burner under oxy-firing conditions, after a baghouse but without any desulfurization. Therefore, the concentration of SO2 in the oxidant stream going to the burner was significant. The mass emission rate of SO2 (in mg/MJ) during oxy-firing was two-thirds that during air-firing. The fly ash and furnace deposits produced during oxy-firing contained more sulfur than during air-firing, which accounts for the lower mass emission rate of sulfur compounds in the flue gas. In contrast, research from B&W reported (CitationMcCauley et al., 2009) that SO2 emissions from the CEDF, which included SO2 capture, were the same under oxy-firing as under air-firing. The coal sulfur content and ash composition probably affect how much SO2 is removed with the fly ash under oxy-firing conditions.
In the Australian study, concentrations of SO3 in the flue gas were 2.5–3 times higher in the oxy-firing case (CitationWall et al., 2009). Alstom also reported high SO3 concentrations during oxy-firing as compared with air-firing (CitationLevasseur et al., 2011). Sulfur trioxide is a trace constituent in flue gas, but it can condense under certain conditions to form ultrafine sulfuric acid particles. Sulfur trioxide/sulfuric acid are removed to some extent by wet flue gas desulfurization (FGD) units, but some sulfuric acid in aerosol form does escape the FGD. The fate of sulfuric acid particles in the CPU is not known at present.
The largest pilot-scale oxy-firing systems, such as the CEDF or the Schwarze Pumpe plant, include wet FGD modules to reduce SO2 emissions before compression. A pilot-scale study of FGD applied to oxy-combustion flue gas (CitationHansen et al., 2011) demonstrated that high levels of SO2 removal were achieved in a wet FGD on oxy-combustion flue gas.
Pilot-scale testing of air-fired and oxyfuel combustion of a bituminous coal at EERC (CitationZhuang and Pavlish, 2012) was carried out in a system with an ESP and wet FGD. Flue gas was recycled to the burner either after the ESP or after the ESP/FGD. SO2 concentrations were higher at the combustor exit under the oxyfuel combustion conditions. SO3 concentrations at the ESP outlet were doubled under oxyfuel combustion conditions as compared with air-fired combustion, because of the much higher SO2 concentrations. The amount of sulfur retained in the fly ash increased in the oxyfuel combustion tests relative to air-fired combustion. Although the concentrations of SO2 were higher in the flue gas in the oxyfuel tests, the total amount of flue gas was lower and the FGD performed more efficiently owing to the lower flue gas volume. In these tests, the stack emissions of SO2 (per MJ of fuel burned) were reduced by more than 95% relative to the air-fired tests.
Further SO2 reduction after the conventional FGD has been included in the various system designs discussed earlier. Thus, we can anticipate that SO2 concentrations in the vent gas from the CPU will be very low.
Particulate matter. Ultrafine particulate matter (UPM) is one potential emission of concern from coal combustion systems that have state-of-the-art particulate control devices. UPM is composed of three major components:
| • | metal oxides, the result of vaporization of coal minerals in the flame, followed by nucleation and condensation; | ||||
| • | soot or black carbon, the result of nucleation of organic molecules released during coal pyrolysis; and | ||||
| • | sulfates, the result of postcombustion reaction and condensation of SO2 and SO3. | ||||
There are no full-scale data on the effect of oxy-combustion on UPM formation and composition. Drop-tube and pilot-scale studies contain the only data available.
Small-scale studies suggest that combustion temperature is the primary factor in determining how much of the inorganic elements vaporize during combustion (CitationSheng et al., 2007a, Citation2007b; CitationAxelbaum and Biswas, 2009; CitationCarbone et al., 2010; CitationJia, 2011). The higher concentration of CO2 in oxy-combustion was shown to have a secondary effect and suppressed vaporization in a study by CitationJia (2011). The high CO2 concentration around the burning char particle might reduce the vaporization of the more volatile metal suboxides, because their formation depends on the relative amounts of CO and CO2.
CitationCarbone et al. (2010) carried out laboratory testing of particle formation mechanisms using a flat-flame burner into which coal particles were injected. The results showed that oxygen concentration in the oxidant stream influenced the size of the ultrafine, or nucleating particles, and the preferential vaporization of some inorganic compounds with respect to others. The authors concluded that enhanced oxygen concentration promoted the formation of ultrafine particles.
In the previously discussed CitationAxelbaum and Biswas (2009) pilot-scale study, the authors also studied the effects of different oxidant gases on particulate formation. When nitrogen in air was replaced with CO2, the amount of submicron particles in the ash decreased, and the geometric mean size of the submicron ash particles was 28% smaller. Using higher concentrations of oxygen in an O2/CO2 oxidant gas increased the geometric mean size of the submicron mode, because of increased vaporization of inorganic elements at the higher combustion temperature arising from the higher oxygen content.
Soot formation under air- and oxy-firing conditions has been studied in single-particle combustion experiments. Laboratory-scale combustion experiments by CitationShaddix et al. (2009) showed that substituting CO2 for nitrogen in the oxidant stream decreased volatilization of organics, which means less material to form soot. Increased furnace residence time and a higher concentration of oxygen in the oxidant stream (27–32% O2 in oxy-combustion) could further decrease the amount of soot formed. Pilot-scale coal combustion studies by CitationMorris et al. (2011) showed lower amounts of soot produced during combustion of two different bituminous coals under oxy-fired combustion conditions as compared with air-fired conditions.
The largest pilot-scale oxy-firing systems, the CEDF and the Schwarze Pumpe plant, include a baghouse followed by an FGD. These two units will serve to reduce the ash concentration in the flue gas to very low levels before the gas enters the CO2 compression section of the plant. Pilot-scale combustion studies have not shown significant differences in the size distribution or the oxide composition of the fly ash between air- and oxy-firing (CitationWall et al., 2009; CitationMorris et al., 2011; CitationYu et al., 2011), but have shown lower concentrations of ultrafine soot particles (CitationMorris et al., 2011). The sulfur content of the fly ash is increased by oxy-combustion, as noted above, because of increased SOx concentration in the flue gas, which might cause an increase in submicron sulfate aerosol.
The submicron aerosol is captured least efficiently in particulate control devices and is of the greatest concern from the perspective of human health, once emitted into the atmosphere. The submicron aerosol from combustion consists of organic (soot), inorganic (metal oxides), and sulfate fractions. Each of these fractions responds differently to the shift from air-firing to oxy-firing. Limited laboratory- and pilot-scale studies have been carried out to date. Soot aerosol concentrations appear to decrease, whereas submicron oxide particles have not shown significant differences between the two combustion systems. Submicron sulfate aerosol particles have the potential to increase as the SO2 and SO3 concentrations increase under oxy-firing conditions. Finally, the design of the combustion system also affects the processes that form submicron particles. The limited laboratory- and pilot-scale data to date should be extrapolated to full-scale systems with caution.
Mercury and other trace hazardous air pollutants (HAPs). Nearly all of the research investigating the effects of oxyfuel combustion on metals emissions has been focused on mercury, presumably because it can interact with system equipment and adversely affect performance. Studies regarding the relative effects of oxyfuel combustion on mercury have suggested variable results. For example, in the pilot-scale studies at the 150 kg/hr IHI combustion test facilities in Aioi, Japan, the fraction of mercury in the ash increased during oxy-firing for two of the three coals tested (CitationWall et al., 2009). However, the mercury was reported to be nonleachable in the ash from oxy-firing. In contrast, the previously discussed CitationAxelbaum and Biswas (2009) study involving combustion with different mixtures of oxidant gas reported that the fractions of oxidized mercury in the flue gas were similar for oxy- and air-fired combustion.
A recent study reported on mercury behavior in three pilot-scale combustors, including two scales of pulverized coal furnaces and a circulating fluidized bed while air- and oxy-firing a western bituminous coal (CitationVan Otten et al., 2011). Results from the circulating fluidized bed showed slight increases in native mercury capture under oxy-firing conditions, as compared with air-firing conditions. Results from a small pulverized coal unit show that the native mercury removal increased by 16% compared with air-firing conditions. In a third system, a 3.17 kJ/hr pulverized coal unit, native mercury removal increased by 68% compared with air-firing conditions, which the researchers attributed to increased carbon in ash due to instabilities in the coal feeding system during oxy-combustion. Mercury speciation at the outlet of the particulate control device did not change between air- and oxy-firing conditions.
CitationKikkawa et al. (2011) from Babcock-Hitachi reported results from a pilot combustion test facility capable of firing 110–200 kg coal per hour and operated under air- or oxy-firing conditions. The pilot facility had an SCR, which was optimized for mercury oxidation, a specialized gas cooler, an ESP, a wet FGD, and a CPU. Flue gas recycle was taken between the ESP and the FGD. Results were not reported on mercury emissions under air-firing conditions for comparison with oxy-firing conditions. Under oxy-firing operation, mercury concentrations at the outlet of the wet FGD (inlet to the CPU) were on the order of 0.5 μg/Nm3, representing 90–99% reduction in mercury relative to the SCR inlet. Babcock-Hitachi (CitationKikkawa et al., 2011) proposed, but did not test at the pilot scale, an additional mercury absorber between the wet FGD and the CPU, presumably as a polishing device for mercury, if needed.
CitationZhuang and Pavlish (2012) found higher relative amounts of particulate-bound mercury and a higher fraction of oxidized mercury in the gas phase at the ESP inlet under oxyfuel combustion conditions when compared with air-fired combustion conditions. These changes were attributed to higher unburned carbon in fly ash and higher concentrations of HCl in the flue gas under oxyfuel combustion conditions. Particulate-bound mercury is removed in ESPs and oxidized gaseous mercury is removed in FGDs. Mercury emissions at the outlet of the FGD in the pilot system of Zhuang and Pavlish were reduced from about 7 μg/dNm3 under air-firing conditions to about 3.5 μg/dNm3 under oxy-firing conditions, because of higher removal in the upstream ESP and FGD.
Comparatively little work has been done on the behavior of trace elements other than mercury in oxy-combustion flames. CitationZhang et al. (2011) conducted drop-tube furnace experiments in air- and oxy-firing conditions using a bituminous coal. Various gases were doped in the furnace, including SO2, HCl, and steam. Using O2/CO2 mixtures for combustion, the researchers found that organically bound chromium was preferentially vaporized when compared with chromium trichloride (CrCl3). The vaporized chromium appeared to be quickly scavenged by iron oxide in ash particles to form stable chromite (Cr[III]). Addition of SO2 was also found to potentially promote the formation of hexavalent chromium (Cr VI), although the quantities were difficult to detect because they were so low.
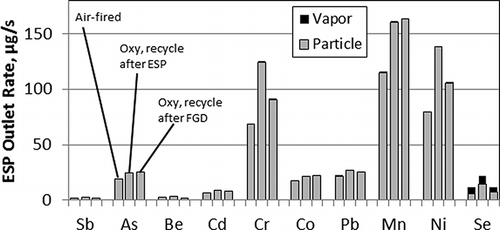
CitationZhuang and Pavlish (2012) measured emissions and enrichment factors of 10 other trace elements (antimony, arsenic, beryllium, cadmium, chromium, cobalt, lead, manganese, nickel, and selenium) at the outlet of the pilot ESP. With the exception of selenium, the trace elements in the flue gas were found predominantly in the fly ash. Selenium was found in both the gas phase and particulate phase of the flue gas. The concentration of the trace elements was higher in the flue gas under oxyfuel combustion as compared with air-fired combustion, both because of the recycle of ultrafine particles in the flue gas back to the combustor and because of the smaller volume of flue gas in the latter conditions. Some trace elements (antimony, arsenic, beryllium, cadmium, cobalt, and lead) were less enriched in the fly ash (i.e., more of the element was retained in the bottom ash) under oxyfuel combustion conditions. On the other hand, chromium, nickel, selenium, and possibly manganese were more enriched in the fly ash under oxyfuel combustion conditions. Emission rates at the ESP outlet were significantly higher for chromium, manganese, nickel, and selenium under oxyfuel conditions when the flue gas was recycled back to the burner after the ESP, as illustrated in Under oxyfuel conditions when the flue gas was recycled after the FGD, the emission rate of selenium at the ESP outlet was lower in the oxyfuel combustion case as compared with air-fired combustion, because of removal of vapor-phase selenium across the FGD.
Figure 6. Emission rates of trace elements at the ESP outlet in pilot-scale combustion experiments of CitationZhuang and Pavlish (2012).
CitationWall et al. (2009) in pilot-scale combustion studies noted that the leachability of some trace metals in fly ash changed as a result of oxy-firing, although there were variations among the three coals studied. The results illustrate that there is the potential to change the leachability of some trace elements as a result of oxy-firing, but systematic research is needed to better understand this effect.
Emissions of organic HAPs have not been reported from oxy-combustion systems. CitationShaddix et al. (2009) have speculated that less organic volatile matter from coal is released during oxyfuel combustion. The lower amounts of soot formed during oxyfuel combustion tend to support this speculation (CitationShaddix et al., 2009; CitationMorris et al., 2011).
In summary, the limited work done to date on release of inorganic HAPs during oxyfuel combustion suggests that emissions and speciation of mercury are not always changed significantly by oxy-combustion. In some cases, more mercury is captured by the fly ash in oxy-combustion systems than in air-fired systems and the increased HCl concentration in the boiler, because of flue gas recycle, can result in more oxidized mercury in the flue gas. Greater removal of mercury can take place under oxy-firing as compared with air-firing for these reasons, assuming that the pollution control system includes a wet FGD. If mercury concentrations are still too high at the exit of the FGD, a polishing absorber has been proposed by one research group.
Limited work on differences of chromium speciation under oxy-combustion conditions highlights the need for more data on metal speciation in fly ash from oxy-combustion boilers. Even less is known about the production and emissions of organic HAPs in oxy-combustion boilers.
Estimated air emissions
In a draft report for EPRI, URS Corporation (URS) made assumptions regarding the operation of a future oxy-fired pulverized coal unit. The assumptions and references used were not included or were marked “industry data” without further citation, so it is difficult to know exactly how the numbers were calculated (CitationURS, 2011). Furthermore, the URS report used an SCR in their model, whereas others such as CitationKoornneeff et al. (2010) have said in their model that SCR is unnecessary due to the specific conditions of the oxy-combustion process. In the Koornneef et al. model, NOx was also removed in the compression and drying stage, which may create a nitric acid liquid waste stream, which is not found at current air-fired units (CitationKoornneeff et al., 2010).
Despite some differences and ambiguities in assumptions, there is reasonable agreement between the URS and Koornneef et al. models. However, Koornneef et al. also acknowledged that depending upon the exact technology or system implemented, a wide range of emissions could be vented to the atmosphere. A comparison of the two models as well as the ranges of potential emissions cited in the literature is presented in .
Table 3. Comparison of oxy-fired and air-fired plant emissions
These data suggest that overall atmospheric emissions will be considerably reduced when compared with air-fired pulverized coal boilers. The ranges are very broad for the oxy-fired emissions because many technologies are currently being evaluated, but a full-scale unit has yet to be built. The ranges also reflect different fuels as well as different potential flue gas processing technologies.
The overall conclusion is that oxy-firing will reduce atmospheric emissions of important pollutants on a mass per kWhr of electricity generated basis. This indicates that the implementation of oxy-fired power plants will not decrease local air quality conditions as a side effect of greenhouse gas reductions. Data relating to the formation and emission of organic compounds are absent at present from the literature.
Nonair emissions
As noted previously, a review of proposed oxyfuel plant designs indicates that emissions will largely be shifted from the vent stack to solid or liquid waste streams (in addition to CO2 sequestration). The altered combustion environment may result in alterations in the composition of fly or bottom ash, boiler slag, FGD material, or other miscellaneous wastes. For example, CitationWall et al. (2009) in pilot-scale combustion studies noted that the leachability of some trace metals in fly ash changed as a result of oxy-firing, although there were variations among the three coals studied. The results illustrate that there is the potential to change the leachability of some trace elements as a result of oxy-firing, but systematic research is needed to better understand this effect. At the current time, it must be concluded that data relating to nonair waste streams are too limited to draw any meaningful conclusions.
Data Gaps
Due to the wide variety of gas processing equipment and various assumptions that can be made about the construction of future plants, there is a wide range of potential atmospheric emissions depending on which designs are built full scale. Some estimates have been put forth; however, different assumptions were made in each case.
The air emissions from oxy-combustion systems come from the CPU in which flue gas that has been cleaned, dried, and compressed. More information is needed to predict the air emissions from CPUs. Problems that arise in making comparisons between oxyfuel and air-fired emissions include the following:
| 1. | First, the composition of the flue gas entering the CPU depends on the fuel being burned and the specific air pollution control devices (APCDs) used to clean the flue gas. There have been suggestions in the literature that conventional ACPDs might not perform the same in oxy-fired systems as they do in air-fired systems. At this point, APCDs have not been built at full scale, so there are no operating data on their efficiencies under oxy-firing conditions. | ||||
| 2. | Second, full-scale CPUs are not currently operating. Different designs have been proposed for CPUs, with different efficiencies for removal of residual pollutants (e.g., NOx or mercury). As the components of CPUs are built and operated, data must be collected (and made available) on the efficiency for removal of trace pollutants. | ||||
| 3. | Little is known about the formation of organic HAPs in oxy-combustion boilers. The complexity of the combustion process makes it difficult to draw inferences from conventional combustion systems. Data on dioxin/furans and other organic air emissions should be collected on the largest scale combustion system possible. The fate of these compounds in CPUs associated with oxyfuel combustion systems should also be investigated. | ||||
| 4. | Finally, most of the data characterizing emissions associated with oxyfuel combustion concern changes in emissions to air. However, these systems will generate solid and liquid waste streams as well. These streams must be characterized in terms of their composition and potential environmental impacts. | ||||
The current state of knowledge and indications from available data are indicated in . As indicated in this table, the potential risks associated with emissions are receiving only limited attention. At best, data are available from a limited number of pilot-scale studies that reflect only limited conditions and do not encompass the variability in conditions that would be encountered at commercial scale. In some instances, even pilot-scale data are lacking. Most of the attention is being focused on the impact of the emission products on feasibility and efficiency. As a result, the suite of chemicals being evaluated in current studies is relatively limited (e.g., SOx, NOx, mercury). Although this may be appropriate at the current state of the technology (i.e., it makes little sense to study potential health effects of a technology that may prove nonviable or one that may change substantially before commercialization), the relative lack of attention to potential health effects needs to be addressed. It is appropriate at this time to convene a symposium on this issue in the next year or two to gather data on the research that is currently underway and to develop strategies for addressing some of the issues listed above. The Department of Energy (DOE) is funding FutureGen 2.0, an oxy-combustion demonstration project in Illinois, and this would be an ideal opportunity to gather data on these topics.
Table 4. State of knowledge concerning impacts of oxy-fired combustion on plant emissions
Questions may be addressed to Connie Senior at [email protected].
Abbreviations
| AC | = |
Activated carbon |
| APCDs | = |
Air pollution control devices |
| ASU | = |
Air separation unit |
| B&W | = |
Babcock and Wilcox |
| CCS | = |
Carbon capture and sequestration |
| CEDF | = |
Combustion emissions development facility |
| CPU | = |
CO2 processing unit |
| dNm3 | = |
Dry standard cubic meter |
| DOE | = |
Department of Energy |
| EPA | = |
U.S. Environmental Protection Agency |
| EPRI | = |
Electric Power Research Institute |
| ESP | = |
Electrostatic precipitator |
| FF | = |
Fabric filter |
| FGC | = |
Flue gas conditioning |
| FGD | = |
Flue gas desulfurization |
| HAP | = |
Hazardous air pollutant |
| MWe | = |
Megawatt electrical |
| MWth | = |
Megawatt thermal |
| NETL | = |
National Energy Technology Laboratory |
| NOx | = |
Oxides of nitrogen |
| PM | = |
Particulate matter |
| PRB | = |
Powder River Basin |
| SCR | = |
Selective catalytic reduction |
| SOx | = |
Oxides of sulfur |
| UPM | = |
Ultrafine particulate matter |
| VOC | = |
Volatile organic carbon compounds |
References
- Andersen , M. , Roberts , W.L. , Wendt , J.O.L. , Lee , C.W. and Linak , W.P. Development of a bench-scale oxy-fuel combustion system for the study of pollutant behavior . American Institute of Chemical Engineers (AIChE) AICHe Proceedings, High temperature environmentally sustainable energy processes. Salt Lake City, UT, November 10, Paper 199310. pp. 106–108 .
- Axelbaum , R.L. and Biswas , P. December 12 2009 . Multi-Pollutant Control Through Novel Approaches to Oxygen Enhanced Combustion , December 12 , Final Report DE-FG26-05NT42531. St. Louis: Washington University in Saint Louis .
- Boffetta , P. , Cardis , E. , Vainio , H. , Coleman , M.P. , Kogevinas , M. , Nordberg , G. , Parkin , D.M. , Partensky , C. , Shuker , D. and Tomatis , L. 1991 . Cancer risks related to electricity production . Eur. J. Cancer , 27 : 1504 – 1519 . doi: 10.1016/0277-5379(91)90040-K
- Carbone , F. , Beretta , F. and Anna , A. D . 2010 . Factors influencing ultrafine particulate matter (PM0.1) formation under pulverized coal combustion and oxyfiring combustion . Energy Fuels , 24 : 6248 – 6256 . doi: 10.1021/ef100780c
- French , C. , Peters , W. , Maxwell , B. , Rice , G. , Colli , A. , Bullock , R. , Cole , J. , Heath , E. , Turner , J. , Hetes , B. , Brown , D.C. , Goldin , D. , Behling , H. , Loomis , D. and C. Nelson , C. 1997 . Assessment of health risks due to hazardous air pollutant emissions from electric utilities . Drug Chem. Toxicol. , 20 : 375 – 386 . doi: 10.3109/01480549709003894
- Gibb , H. and Chen , C. 1989 . Evaluation of issues relating to the carcinogen risk assessment of chromium . Sci. Total Environ. , 86 : 181 – 186 . doi: 10.1016/0048-9697(89)90204-0
- Hansen , B.B. , Fogh , F. , Knudsen , N.O. and Kül , S. 2011 . Performance of a wet flue gas desulfurization pilot plant under oxy-fuel conditions . Ind. Eng. Chem. Res. , 50 : 4238 – 4244 . doi: 10.1021/ie1022173
- Hunter , P.R. and Fewtrell , L. 2001 . “ Acceptable risk ” . In In Water Quality: Guidelines, Standards and Health. Assessment of Risk and Risk Management for Water-Related Infectious Disease , Edited by: Fewtrell , L. and Bartram , J. 79 – 109 . London : IWA Publishing .
- International Risk Governance Council . 2009 . Power Plant CO2 Capture Technologies: Risks and Risk Governance Deficits , Geneva : International Risk Governance Council .
- Jia , Y. 2011 . Particulate formation from pulverized coal under oxy-fuel combustion conditions , Utah : M.S. thesis, University of Utah, Salt Lake City .
- Khillare , P.S. , Jyethi , D.S. and Sarkar , S. 2012 . Health risk assessment of polycyclic aromatic hydrocarbons and heavy metals via dietary intake of vegetables grown in the vicinity of thermal power plants . Food Chem. Toxicol. , 50 : 1642 – 1652 . doi: 10.1016/j.fct.2012.01.032
- Kikkawa , H. , Imada , N. and Saito , T. January 25–26 2011 . Advanced AQCS for oxy-fuel combustion system; controlling mercury & SO3. Presented at Special Workshop on Oxyfuel Combustion Addressing SO2/SO3/Hg/Corrosion Issues in Oxyfuel Combustion Boiler and Flue Gas Processing Unit January 25–26 , 2011 London
- Koornneef , J. , Ramirez , A. , van Harmelen , T. , van Horssen , A. , Turkenburg , W. and Faaij , A. 2010 . The impact of CO2 capture in the power and heat sector on the emission of SO2, NOx, particulate matter, volatile organic compounds and NH3 in the European Union . Atmos. Environ. , 44 : 1369 – 1385 . doi: 10.1016/j.atmosenv.2010.01.022
- Leeper , J.E. 1980 . Mercury—LNG's problem . Hydrocarbon Processing , November 237–240.
- Levasseur , A. , Turek , D. , Kenney , J. , Chapman , P. , Edberg , C. , Kang , S. , Monckert , P. , Kluger , F. and Fout , T. Oxy-fired tangential boiler development and large-scale (15 MWth) validation . Presented at 2nd Oxyfuel Combustion Conference . September 12–16 2011 , Yeppoon , Australia.
- Matuszewski , M. September 2010 . Cost and Performance for Low-Rank Pulverized Coal Oxycombustion Energy Plants , September , Pittsburgh , PA : National Energy Technology Center . DOE/NETL-401/093010
- McCauley , K.J. , Farzan , H. , Alexander , K.C. , McDonald , D.K. , Varagani , R. , Prabhakar , R. , Tranier , J.-P. and Perrin , N. 2009 . Commercialization of oxy-coal combustion: Applying results of large 30MWth pilot project . Energy Procedia , 1 : 439 – 446 . doi: 10.1016/j.egypro.2009.01.059
- McDonald , D. , Dubettier , R. , Flanigan , T. and Thimsen , D. 700 MWe oxycombustion reference plant performance and costs . Presented at 2nd Oxyfuel Combustion Conference . September 12–16 2011 , Yeppoon , Australia.
- Mitsui , Y. , Imada , N. , Kikkawa , H. and Katagawa , A. Study of Hg and SO3 behavior in flue gas of oxy-fuel combustion system . Presented at 2nd Oxyfuel Combustion Conference . September 12–16 2011 , Yeppoon , Australia.
- Morris , W.J. , Yu , D. and Wendt , J.O.L. 2011 . Soot, unburned carbon and ultrafine particle emissions from air- and oxy-coal flames . Proc. Combust. Inst , 33 : 415 – 3421 . doi: 10.1016/j.proci.2010.05.059
- Norwegian Institute of Public Health . 2011 . Carbon dioxide capture: Health effects of amines and their derivatives. ScienceDaily , http://www.sciencedaily.com/releases/2011/04/110404084756.htm .
- Shaddix , C.R. and Molina , A. 2009 . Particle imaging of ignition and devolatilization of pulverized coal during oxy-fuel combustion . Proc. Combust. Inst. , 32 : 2091 – 2098 . doi: 10.1016/j.proci.2008.06.157
- Shah , M. , Degenstein , N. , Zanfir , M. , Solunke , R. , Kumar , R. , Bugayong , J. and Burgers , K. Purification of oxy-combustion flue gas for SOx/NOx removal and high CO2 recovery . Presented at 2nd Oxyfuel Combustion Conference . September 12–16 2011 , Yeppoon , Australia.
- Sheng , C. , Li , Y. , Liu , X. , Yao , H. and Xu , M. 2007a . Ash particle formation during O2/CO2 combustion of pulverized coals . Fuel Process. Technol. , 88 : 1021 – 1028 . doi: 10.1016/j.fuproc.2007.06.009
- Sheng , C. , Lu , Y. , Gao , X. and Yao , H. 2007b . Fine ash formation during pulverized coal combustion—A comparison of O2/CO2 combustion versus air combustion . Energy Fuels , 21 : 435 – 440 . doi: 10.1021/ef060420v
- Spero , C. September 2–3 2012 . Callide oxyfuel project development and progress , September 2–3 , Tokyo , , Japan : Presented at Oxyfuel Capacity Building Course, Tokyo Institute of Technology . 2012
- Stanger , R. and Wall , T. 2011 . Sulphur impacts during pulverised coal combustion in oxy-fuel technology for carbon capture and storage . Prog. Energy Combust. Sci. , 37 : 69 – 88 . doi: 10.1016/j.pecs.2010.04.001
- Stromberg , L. Oxyfuel combustion has come a long way, but . Presented at 2nd Oxyfuel Combustion Conference . September 12–16 2011 , Yeppoon , Australia.
- Thimsen , D. and Wheeldon , J. The prospects of achieving ultra-low emissions from oxy-coal power plants . Presented at 2nd Oxyfuel Combustion Conference . September 12–16 2011 , Yeppoon , Australia.
- Tranier , J.-P. and Perrin , N. Impurities management in an oxy-combustion power plant . Presented at 2nd Oxyfuel Combustion Conference . September 12–16 2011 , Yeppoon , Australia.
- Corporation , URS . March 24 2011 . Emission Estimates for Future Fossil- & Biomass-Fueled Power Generation System Configurations: Year 2030 , March 24 , EPRI Draft Report. San Francisco, CA: URS Corporation .
- U.S. Environmental Protection Agency . April 2011 . Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2009 , April , Washington , DC : U.S. Environmental Protection Agency . US EPA #430-R-11-005
- U.S. Environmental Protection Agency. 2012. Mercury and air toxics standards—Cleaner power plantsaccessed May 22, 2013 http://www.epa.gov/airquality/powerplant toxics/powerplants.html (http://www.epa.gov/airquality/powerplant toxics/powerplants.html)
- Van Otten , B. , Fry , A. , Adams , B. and Bool , L. Mercury speciation and emission from pilot-scale PC furnaces under air- and oxy-fired conditions . Presented at Air Quality VIII Conference . October 24–27 2011 , Crystal City , CA .
- Wall , T. , Liu , Y. , Spero , C. , Elliot , L. , Khare , S. , Rathnam , R. , Zeenathai , F. , Moghtaderi , B. , Buhre , B. , Sheng , C. , Gupta , R. , Yamada , T. , Makino , K. and Yu , J. 2009 . An overview on oxyfuel coal combustion—State of the art research and technology development . Chem. Eng. Res. Des. , 87 : 1003 – 1016 . doi: 10.1016/j.cherd.2009.02.005
- Wall , T. , Stanger , R. and Santos , S. 2011 . Demonstration of coal-fired oxy-fuel technology for carbon capture and storage and issues with commercial deployment . Int. J. Green. Gas Control , 55 : S5 – S15 . doi: 10.1016/j.ijggc.2011.03.014
- Wall , T. , Stanger , R. and Liu , Y. 2013 . Gas cleaning challenges for coal-fired oxy-fuel technology with carbon capture and storage . Fuel , 108 : 85 – 90 . doi: 10.1016/j.fuel.2011.03.037
- White , V. , Fogash , K. and Petrocelli , F. Air Products oxyfuel CO2 compression and purification developments . Presented at 2nd Oxyfuel Combustion Conference . September 12–16 2011 , Yeppoon , Australia.
- Yokel , R.A. , Lasley , S.M. and Dorman , D.C. 2006 . The speciation of metals in mammals influences their toxicokinetics and toxicodynamics and therefore human health risk assessment . J. Toxicol. Environ. Health B Crit. Rev. , 9 : 63 – 85 . doi: 10.1080/15287390500196230
- Yu , D. , Morris , W.J. , Erickson , R. , Wendt , J.O.L. and Fry , A. 2011 . Ash and deposit formation from oxy-coal combustion in a 100 kW test furnace . Int. J. Green. Gas Control , 5: : S159 – S167 . doi: 10.1016/j.ijggc.2011.04.003
- Zhang , L. , Jiao , F. , Ninimiya , Y. , Bhattacharya , S. and Li , C.-F. Ash formation and speciation of trace metals during oxy-fuel combustion . Presented at 2nd Oxyfuel Combustion Conference . September 12–16 2011 , Yeppoon , Australia.
- Zhuang , Y. and Pavlish , J.H. 2012 . Fate of hazardous air pollutants in oxygen-fired coal combustion with different flue gas recycling . Environ. Sci. Technol. , 46 : 4657 – 4665 . doi: 10.1021/es300143q