Abstract
Ammonia gas emission is a major concern in concentrated animal production operations. It not only reduces the manure value as fertilizer due to nitrogen loss, but also has considerable environmental consequences for both animals and ecosystem. In this work, a microalgae culture system was developed as an ammonia gas bioscrubber to reduce ammonia gas emission. The green algae Scenedesmus dimorphus was grown in a flat-panel photobioreactor aerated with ammonia-laden air. A continuous culture was performed at different operational conditions including dilution rate (D = 0.05, 0.1, 0.2, and 0.3 day−1), ammonia gas loading rate (9.4, 19.3, 28.9, 39.9, 55.6 mg/L-day), and medium pH (5, 6, 7, and 8). The alga culture at 0.1 day−1 dilution rate, 39.9 mg/L-day ammonia gas loading rate, and pH 7 resulted in the highest cell density and biomass productivity. In order to provide a wide spectrum evaluation of the algae-based ammonia mitigation system, four parameters were determined, including ammonia removal rate, overall ammonia gas removal efficiency, cellular ammonia consumption rate, and cell yield based on ammonia input. Depending on the operational conditions used, the maximum values of those four evaluative parameters were 50.92 ± 2.91 mg/L-day of ammonia removal rate, 94.90 ± 1.87% of ammonia removal efficiency, 0.0597 ± 0.0024 g NH3/g cell-day of cellular ammonia consumption rate, and 19.40 ± 2.52 g cell/g NH3 of cell yield based on ammonia. It was also found that the majority of nitrogen in the ammonia gas was assimilated by the algal cells. At D = 0.1 day−1, 39.9 mg/L-day of ammonia gas loading rate and pH 7, algal biomass assimilated 98.6% of nitrogen contained in the ammonia gas input, with less than 5% of inlet ammonia gas was exhausted after the algal treatment.
Implications: This study demonstrated the effectiveness of using microalgae for mitigating ammonia gas emission from animal production operations. The results enabled us to better understand the mechanisms of ammonia assimilation by microalgae, the engineering design parameters for the process scale up, and the economic viability of the system. Eventually, it will lead to a novel, alternative method for mitigating ammonia gas emission from concentrated animal operations while producing biomass as high-quality feed ingredient.
Introduction
Modern livestock production occurs primarily in confined, environmentally controlled buildings in order to increase productivity and to protect the animal welfare from outside environment. These large-scale animal houses commonly have manure accumulated inside the houses for an extended period prior to removal for land application. This type of manure handling practice results in a favorable environment for degradation of organic nitrogen in the manure into ammonia, such as prolonged contact of manure with bacterial enzymes, high manure moisture, and adequate pH and temperature for both bacterial growth and ammonia generation (CitationGroot Koerkamp, 1994).
Ammonia generation and emission of animal production facilities is an environmental concern in that ammonia can have negative effects on both ecosystem and animal health. The consequences of high ammonia concentrations include respiratory and retinal diseases, eutrophication of surface water bodies, soil and water acidification and leaching (Citationvan Breemen et al., 1982; CitationSommer, 2001), noxious effect on vegetation or ecosystem (CitationKrupa, 2003), and enhanced greenhouse gas generation, such as nitrous oxide (N2O) (CitationBlackmer et al., 1980). Ammonia volatilization from animal houses is also economically undesirable because the loss of nitrogen from the manure reduces its fertilizer value.
Various methods have been developed to reduce ammonia gas emissions from animal production facilities, including (1) optimizing nitrogen combination in animal feed to minimize the nitrogen excretion (CitationBussink and Oenema, 1998; CitationLiang et al., 2005; CitationLi et al., 2012); (2) changing the animal manure management system (CitationGroot Koerkamp, 1994; CitationXin et al., 2011); (3) adsorbing the ammonia to substances with high affinity for binding NH4 + ions such as zeolite and peat moss (CitationLi et al., 2008; CitationNdegwa et al., 2008); (4) absorbing ammonia by acids or acidifying salts (CitationNdegwa et al., 2008); and (5) using nitrifying bacteria in the form of biofilters (CitationPhilippe et al., 2011). In general, the ammonia reduction efficiency of each method varied widely depending on the operational conditions. For example, for dietary manipulation, each 1% reduction in dietary protein level generally leads to about 10% reduction in ammonia emissions from the manure (CitationLiang et al., 2005). Certain dietary additives such as EcoCal can reduce ammonia emissions by an average of 40% (CitationLi et al., 2012). Changing manure management from long-term storage inside the barns (i.e., high-rise laying hen houses) to frequent removal (i.e., manure-belt houses) significantly reduces ammonia emissions (as high as >90%), depending upon manure removal interval (CitationLiang et al., 2005). Application of chemical additives such as Al+ Clear can be very effective in reducing ammonia emission (>90%) (CitationLi et al., 2008). Biofilter with ammonia oxidizing bacteria can also lead to a very high ammonia removal efficiency (>95%) (CitationJun and Wenfeng, 2009). When considering the practical application of each method, however, factors such as the environmental effects, animal welfare, and economic feasibility should also be considered. For example, modification of dietary and manure management practices may be limited by high implementation cost (CitationGroot Koerkamp, 1994). The adsorption and absorption methods have disadvantages of negative environment due to salts accumulation and strong acidic discharge (CitationNdegwa et al., 2008). The biofilter method resulted in high ammonia removal but the installation and maintenance cost was high (CitationPhilippe et al., 2011).
Compared with the conventional methods, microalgae culture as an ammonia bioscrubber is a promising alternative for mitigating ammonia emission. Environmentally, this method improves air quality without exerting any environmental damage. Economically, microalgae can produce high-quality protein from ammonia, so the harvested algal biomass can be potentially used as animal feed, fertilizer, or dietary supplement. So far, microalgae use for treating wastewater has been widely studied (CitationAbeliovich and Azov, 1976; CitationAzov and Goldman, 1982; CitationGonzález et al., 1997; CitationPark et al., 2010; CitationXin et al., 2010); however, using microalgae for mitigating ammonia gas has remained unexploited. Therefore, the aim of this work was to investigate the potential of using microalgae for reducing emission of ammonia gas in a concentration range typically found in a concentrated poultry house operation.
Materials and Methods
Algae strain, medium, and subculture
The green algae Scenedesmus dimorphus (UTEX 1237) was used due to its efficient ammonia removing capability (CitationGonzalez et al., 1997), tolerance in wide pH range (CitationNalewajko et al., 1997), as well as the highest growth rate with ammonium being the nitrogen (CitationXin et al., 2010). The strain was maintained on agar slant at 4 °C. To prepare seed culture, the cells on agar slant were transferred to 250-mL Erlenmeyer flasks containing 50 mL modified Bold's basal medium (CitationAnderson, 2005). The medium consisted of (per liter) 175 mg KH2PO4, 25 mg CaCl2·2H2O, 75 mg MgSO4·7H2O, 250 mg NaNO3, 75 mg K2HPO4, 25 mg NaCl, 50 mg EDTA, 31 mg KOH, 4.98 mg FeSO4·7H2O, 1 μL H2SO4, 11.42 mg H3BO3, 1.42 mg MoO3, and 1 mL trace metal solution containing 8.82 g/L ZnSO4·7H2O, 1.44 g/L MnCl2·4H2O, 1.57 g/L CuSO4·5H2O, and 0.49 g/L Co(NO3)2·6H2O. The medium pH was approximately 6.8 before autoclaving at 121 °C for 15 min. The flasks were placed in an orbital shaker set at 200 rpm under 25 °C and continuous illumination at 110–120 μmol sec−1 m−2. The cultures were incubated for 5 days and then transferred to a photobioreactor for ammonia gas reduction study.
Photobioreactor setup and continuous algal culture
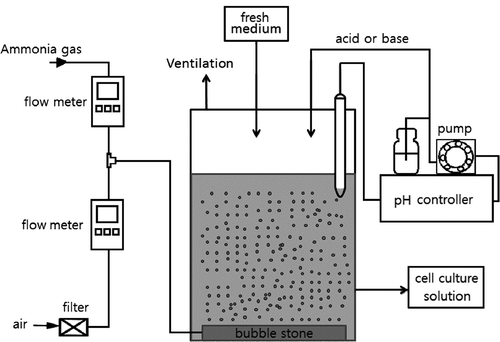
A flat panel photobioreactor system was used for algal culture. As shown in , the reactor was made of plexiglass with a dimension of 8.5 × 9.5 × 62 cm (L × W × H) and working volume of 5 L. Compressed air and ammonia gas were mixed at certain ratio and introduced to the reactor through a sintered gas diffuser to provide mixing in the liquid. The flow rate of ammonia gas and air were respectively regulated by a digital flow meter (MCS series for ammonia gas; MC series for air; Alicat Scientific, Tucson, AZ) at a preset level to achieve the ammonia concentration in the inlet gas. The aeration rate of inlet gas (air plus ammonia gas) was 2.77 L/min (0.56 vvm, vol gas/vol liquid/min). The atmospheric CO2 contained in the inlet gas was the carbon source for the algal growth. Stainless steel tubing was used to avoid corrosion by ammonia. The reactor was equipped with a pH control loop to maintain the algal culture at a preset pH. The bioreactor and ammonia gas tanks were all placed in a hood at 25 °C. The photobioreactor was continuously illuminated at 110–120 μmol sec−1 m−2 on one side of the flat panel.
The algal culture in the photobioreactor was run in a batch mode for the first 5 days, and switched to a continuous mode by withdrawing cell broth (spent medium + algal cells) culture medium and feeding the same volume of fresh medium on a daily basis. The ratio of the volume of daily medium exchanged to the work volume of the photobioreactor was defined as dilution rate. Bold's basal medium was used as the initial batch culture medium as well as fresh feed medium for continuous culture from days 5 to 12. From days 12 to 24, the fresh feed medium was modified by replacing NaNO3 of Bold's basal medium with NH4Cl to adapt the cells to ammonia-nitrogen so the cells would have less shock when ammonia gas was used for cells growth. During these first 24 days of operation, the inlet gas was a pure compressed air. From day 24, fresh feed medium was further modified by completely eliminating NH4Cl from of Bold's basal medium; at the same time, ammonia-laden air was introduced into the reactor to provide the sole source of nitrogen for the algae.
The continuous culture was done at different dilution rate settings (by varying the volume of daily medium exchange), inlet ammonia gas loading rates (by changing the ammonia gas concentration in the inlet air), and medium pH. The cell density and the ammonia concentration in the exhaust air were measured daily. Each operational condition was operated for a certain time period during which the volume of total medium change was up to 3-fold of the reactor working volume. For example, at D = 0.1 day−1, the daily medium change volume was 10% of the reactor working volume; thus, this dilution rate will be operated for 30 days to accumulate a total of medium change being 3-fold of reactor working volume. Because the cell density was measured daily, the cell density values from the last five consecutive days were averaged as the steady-state cell density at that operational condition. At steady state, the cell suspension withdrawn from the reactor was centrifuged at 3000 rpm for 5 min to collect both the supernatant and cell pellets, which were immediately freeze-dried. Both dried biomass and supernatant were stored in a −20 °C freezer for further analyses. The biomass productivity (P; g cell/L-day, dry basis) at each steady state was calculated as follows:
Evaluation of ammonia removal performance
The performance of ammonia removal by the algal culture was evaluated through the following four parameters:
| i. | Ammonia removal rate (R V; g NH3/L-day) | ||||
| ii. | Ammonia gas removal efficiency (E; %) | ||||
| iii. | Cellular ammonia consumption rate (R cell; g NH3/g cell-day) | ||||
| iv. | Biomass yield based on ammonia (Y X/N; g cell/g NH3) | ||||
Analyses of the cell growth and gas and liquid samples
The cell growth was determined by measuring optical density (OD) of the cell suspension at 750 nm (OD750) and then converted into cell density (X; g/L) using a calibration curve OD750 = 0.9627X + 0.0384 (R 2 = 0.995). Nitrogen content of algal biomass was determined by Combustion Method (AOAC 990.03) using a Vario MAX Carbon Nitrogen analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). The ammonia concentration in the inlet and exhaust air stream was measured by a gas analyzer (BW gas alert Micro5 electrochemical detector; BW technologies/Honeywell, Calgary, Canada). The nitrate and ammonia in the liquid phase were respectively determined through a HACH Nitrate TNT plus LR kit and HACH TNT AmVer LR kit using a spectrophotometer (Hach model DR 3900; Loveland, CO, USA).
Statistical analysis
One-way analysis of variance (ANOVA) was used to test the significance in the response variables among different operational regimens. Pairwise comparisons of the marginal means for each condition were computed at the 95% confidence level. The statistical software package SPSS (version 19.0.0; IBM SPSS Statistics, Chicago, IL) was used for the analysis.
Results and Discussion
Algal growth and ammonia removal at different dilution rates
Dilution rate (D) is an important operational parameter in the continuous cultures. As cell specific growth rate (μ) equals the dilution rate in continuous culture operations, the highest dilution rate should not exceed the maximal specific growth rate in order to avoid the cell washout. Here, a Monod equation with ammonia nitrogen as limiting substrate was used to determine the maximum specific growth rate (μmax), i.e.,
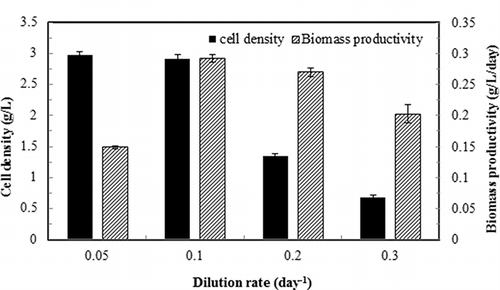
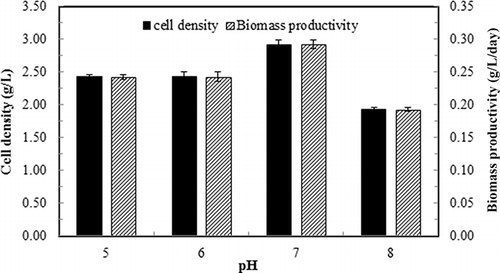
The cell density and biomass productivity as a function of dilution rate are shown in . As shown in the figure, at D = 0.1 day−1, both cell density and biomass productivity reached the highest levels. D = 0.05 day−1 also resulted in a high cell density, but a lower biomass productivity, probably due to the insufficient nutrients available for the algal growth with such a low medium exchange rate. When dilution rate exceeded 0.1 day−1, both the cell density and biomass productivity significantly decreased (P < 0.05).
Figure 2. Cell density and biomass productivity of continuous culture of S. dimorphus under different dilution rates. Ammonia gas loading rate 39.9 mg/L-day; medium pH 7. Data are means of five consecutive samples at the steady state (after at least three volume changes), and error bars show standard deviations.
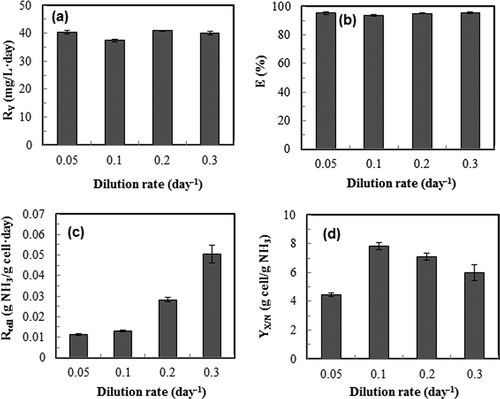
The ammonia removal performance by S. dimorphus is shown in . Within the range of dilution rate from 0.05 to 0.3 day−1, both the ammonia removal rate and ammonia removal efficiency were maintained at a constant level ( and b). However, cellular ammonia consumption rate increased significantly (P < 0.05) with the increase of dilution rate (), indicating that the actively growing cells (i.e., the cells with higher dilution rate) also had a higher ammonia consumption capability. The trend of biomass yield based on ammonia () was similar to that of biomass productivity () because of the constant ammonia removal rate (); thus, the proportional relationship between the cell yield and the biomass productivity ( Equationeq 5) throughout the dilution rates studied.
Figure 3. Effects of dilution rate of continuous culture of S. dimorphus on ammonia gas removal performance. Ammonia gas loading rate 39.9 mg/L-day; medium pH 7. (a) ammonia removal rate, R V; (b) overall ammonia gas removal efficiency, E; (c) cellular ammonia consumption rate, R cell; (d) cell yield based on ammonia input, Y X/N. Data are means of five consecutive samples at the steady state (after at least three volume changes), and error bars show standard deviations.
Algal growth and ammonia removal at different ammonia loading rates
Ammonia loading rate (mg NH3/L-day) is an important parameter influencing the algal growth performance. Here, the ammonia loading rate was determined as following,
Where [NH3]inlet is ammonia concentration in inlet gas (ppm), F inlet is the volumetric flow rate of inlet gas (air + ammonia) (2.77 L/min), and D NH3 is the ammonia density (0.697 g/L at operational conditions), and V is the reactor working volume (5 L). EquationEquation 7 indicates that the ammonia loading rate is proportional to the ammonia gas concentration, as all the other parameters are fixed. Therefore, in this work, we regulated ammonia gas concentration for adjusting the ammonia loading rate. To mimic the air quality in a real animal house operation, the ammonia gas concentration was adjusted within the range from 10 to 80 ppm, which closely represents ammonia concentration that may be encountered in the exhaust (not the bird-level) air of modern high-rise laying-hen houses during different seasons (H. Xin, personal communication). Based on the above setting, the effect of ammonia loading rate on cell growth performance was evaluated.
As shown in , the cell density and biomass productivity followed a similar trend. Both cell density and biomass were significantly (P < 0.05) low at an ammonia gas loading rate of 9.4 mg/L-day, and leveled off at relatively high levels when ammonia loading rate ranged from 19.3 to 55.6 mg/L-day (). It was also observed that the medium pH was 7.00–7.10 when ammonia gas loading rate ranged from 9.4 to 39.9 mg/L-day, with the minimal amount of acid needed to control the pH at the desired level (˜7.0). However, when the ammonia loading reached 55.6 mg/L-day, the medium pH tended to increase. As a result, significant amount of acid was added to the medium in order to keep the pH at neutral level. This pH increase indicates that the ammonia input to the reactor exceeded the cell capability of assimilation. This is confirmed by the high concentration of ammonia in the liquid at 55.6 mg/L-day of ammonia loading, which will be discussed later (). Therefore, for an effective ammonia gas removal using algal culture, the upper limit of ammonia loading should be carefully monitored through the adjustment of gas flow rate and the ammonia gas concentration in the inlet gas (per Equationeq 7) to prevent undesirable pH rise and probable inhibitory effect caused by high level of dissolved but un-ionized ammonia (CitationAbeliovich and Azov, 1976).
Figure 4. Cell density and biomass productivity of continuous culture of S. dimorphus under different inlet ammonia gas contents. Dilution rate: 0.1 day−1; medium pH 7. Data are means of five consecutive samples at the steady state (after at least three volume changes), and error bars show standard deviations.
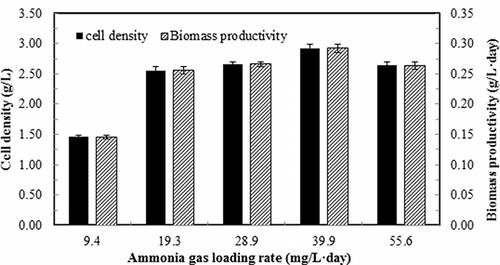
Table 1. Nitrogen distribution of inlet ammonia (Ninlet) among cell biomass (Ncell), liquid medium (Nliquid), and exhausted gas (Noutlet) at different dilution rates (D; day−1) of microalgal culture
Table 2. Nitrogen distribution of inlet ammonia (Ninlet) among cell biomass (Ncell), liquid medium (Nliquid), and exhausted gas (Noutlet) at different ammonia gas loading rates (mg/L-day) for microalgal culture
As shown in , the ammonia removal rate proportionally increased with the ammonia loading rate (R 2 = 0.99); similar trend was also reported in bacteria-based biofilter systems with four different packing materials for ammonia gas removal, in which a linear relationship between the input ammonia gas concentration and ammonia removal capacity was observed in the range of 42–290 ppm of ammonia concentration with a flow rate of 0.4–1.8 L/min (CitationKim et al., 2000).
Figure 5. Effects of ammonia gas loading rate of continuous culture of S. dimorphus on ammonia gas removal performance. Dilution rate: 0.1 day−1; medium pH 7. (a) ammonia removal rate, R v; (b) overall ammonia gas removal efficiency, E; (c) cellular ammonia consumption rate, R cell; (d) cell yield based on ammonia, Y X/N. Data are means of five consecutive samples at the steady state (after at least three volume changes), and error bars show standard deviations.
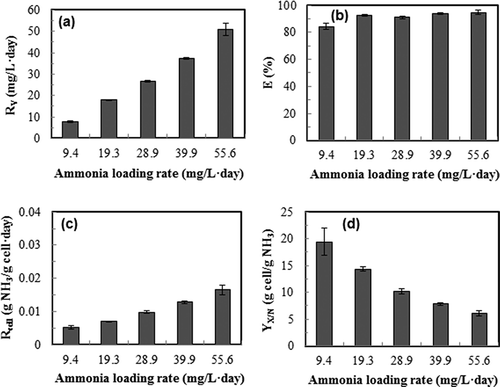
Within the range of ammonia loading rate studied, the overall ammonia removal efficiency maintained at high levels (), whereas cellular ammonia consumption rate increased with increasing ammonia loading rate (). CitationTam and Wong (1996) reported a similar trend of specific ammonium uptake (mg N uptake per cell) with increasing initial ammonium concentration. shows the relationship of biomass yield with ammonia loading rate. It can be seen that accumulation of biomass was more efficient when ammonia loading rate was low.
Algal growth and ammonia removal at different medium pH
pH is an important factor affecting both ammonia gas solubility and algal metabolic activities. The sensitivity of algal cells to the pH is determined by the proton permeability to the cells, which is controlled by both the passive inflow and active outflow of protons through the proton-translocating membrane adenosine triphosphatase (ATPase; CitationBender et al., 1986).
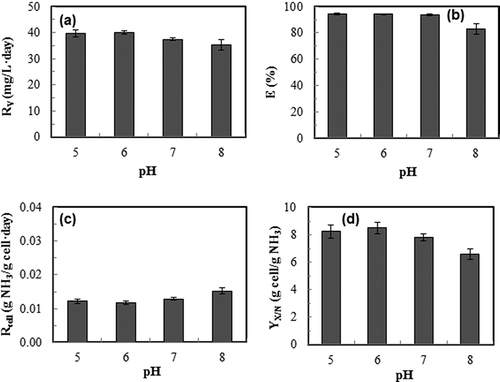
As shown in , the algae grew best at pH 7, which means the algal enzymatic metabolism is most favorable at neutral pH. The lowest cell growth performance at pH 8 may be explained by the cell membrane's selective penetration by different ammonia species. At pH 8, the majority of ammonia exists in un-ionized form (NH3), which can diffuse through cell membrane and elevate the cell's internal pH to an inhibitory level (CitationAbeliovich and Azov, 1976; CitationAzov and Goldman, 1982). On the contrary, at lower pH (e.g., pH 5 and 6), the majority of ammonia is in ionized form (NH4 +), which needs mediated transport though the algal cell membrane; thus, the internal pH is not affected by the surrounding ammonia.
Figure 6. Cell density and biomass productivity of continuous culture of S. dimorphus under different medium pH. Dilution rate: 0.1 day−1; ammonia gas loading rate: 39.9 mg/L-day. Data are means of five consecutive samples at the steady state (after at least three volume changes), and error bars show standard deviations.
In terms of ammonia removal performance at different pH, the ammonia removal rate did not change significantly with different pH used (). However, ammonia removal efficiency was constantly above 93% at pH 5–7 (). It was reduced significantly (P < 0.05) at pH 8 (), due to the poor ionization of ammonia in the medium at alkali condition, which in turn resulted in release of higher amount of ammonia in the exhaust gas. The cellular ammonia consumption rate () and the ammonia-based biomass yield () show an opposite trend, with pH 7 resulting in the lowest cellular consumption rate but highest biomass yield on ammonia.
Figure 7. Effects of medium pH of continuous culture of S. dimorphus on ammonia gas removal performance. Dilution rate: 0.1 day−1; ammonia gas loading rate: 39.9 mg/L-day. (a): ammonia removal rate, R v; (b): overall ammonia gas removal efficiency, E; (c): cellular ammonia consumption rate, R cell; (d): cell yield based on ammonia, Y X/N. Data are means of five consecutive samples at the steady state (after at least three volume changes), and error bars show standard deviations.
The fate of nitrogen in the inlet ammonia gas–nitrogen distribution
To further evaluate the performance of microalgae as an ammonia scrubber and to delineate the fate of nitrogen in the inlet gas, a mass balance of nitrogen in different streams, including input gas, liquid medium, algal biomass generated, and exhaust gas, was analyzed as follows:
To evaluate the distribution of nitrogen of inlet ammonia, the ratio of each portion of nitrogen to the Ninlet was determined. shows the nitrogen distribution at different dilution rates. As shown in the table, within the range of dilution rate from 0.1 to 0.3 day−1, the majority of inlet nitrogen was assimilated by the cells, which is evidenced by high Ncell values; D = 0.1 day−1 resulted in the highest value of cell assimilation. At D = 0.05 day−1, however, liquid medium contained a significant quantity of the nitrogen as evidenced by a higher Nliquid value, but low cell assimilation (Ncell value). This trend of cell assimilation of nitrogen is similar to that of the biomass productivity (), indicating the cell assimilation capability is directly related to cellular activity.
The nitrogen distribution as a function of ammonia loading rate is shown in . Within the range of ammonia loading rates studied, algal cells assimilated a majority of nitrogen in the inlet gas. However, at either low or high level of ammonia loading rate (9.4 and 55.6 mg/L-day), the portion of nitrogen assimilated by the cells was lower compared with other cases. At 9.4 mg/L-day of ammonia loading rate, the outlet nitrogen was significantly high, implying that the microalgae-based ammonia removal system is not effective at low ammonia loading; this is probably caused by the low cell activity/productivity at this level of ammonia loading (). When the inlet ammonia increased to 55.6 mg/L-day, the ammonia input exceeded the cells’ assimilation capability. At this level of ammonia loading rate, the medium pH tended to increase with acid being used to control the pH at the neutral level, most of the unassimilated ammonia gas was therefore trapped in liquid, which was reflected by the high Nliquid value (). Correspondingly, the ammonia gas from the exhaust outlet was low, resulting in a low Noutlet value ().
shows that the medium pH had a drastic effect on the nitrogen distribution. Algal cells assimilated majority of ammonia-nitrogen (98.6%) at pH 7, which is the optimal pH for cellular metabolism (). At pH 5 and 6, cell assimilation of nitrogen was markedly low; a significant portion of ammonia-N was dissolved in the liquid because ammonia tends to dissolve and ionize at lower pH. At pH 8, the cell assimilation of ammonia (Ncell) was also reduced; however, the ammonia-N distributed in the outlet exhaust gas (Noutlet) became significant compared with other cases because ammonia remained un-ionized at higher pH level (). In other words, at pH 8, the un-utilized ammonia by cells was mostly lost to the environment though the exhaust gas.
Table 3. Nitrogen distribution of inlet ammonia (Ninlet) among cell biomass (Ncell), liquid medium (Nliquid), and exhausted gas (Noutlet) at different medium pH in microalgal culture
Collectively, the nitrogen mass balance in , , and shows that algal cells assimilated significant amount of ammonia within the range of experimental parameters studied. In some cases (e.g., D = 0.1 day−1, ammonia loading rate of 39.9 mg/L-day, and pH 7), cells can assimilate as high as 98.6% of the inlet ammonia-nitrogen. The high algal ability to assimilate the nitrogen and its mass balance were also reported by CitationZimmo et al. (2004) using ammonium in algae pond for different seasonal periods. The authors concluded that the largest nitrogen flux was algae biomass sedimentation and the higher algae cell growth was the key to mitigating ammonia gas (CitationZimmo et al., 2004).
Summary and Conclusion
This study demonstrates that microalgae cultivation is a promising alternative for mitigating ammonia gas emissions at levels typically emitted from animal production facilities. Over a considerable range of the operational conditions, the algal culture demonstrated a high biomass productivity as well as ammonia removal performance. The following conclusions and observations were made:
| • | The algae Scenedesmus dimorphus was able to grow in a flat panel photobioreactor aerated with ammonia-laden air in a continuous cultivation mode. | ||||
| • | At the condition of dilution rate of 0.1 day−1, ammonia gas loading of 39.9 mg/L-day, and pH 7, the continuous algal culture achieved the highest cell density (2.92 ± 0.069 g/L, dry basis) and biomass productivity (0.292 ± 0.0069 g/L-day, dry basis). | ||||
| • | At operational condition of 0.1 day−1 dilution rate, 55.6 mg/L-day ammonia loading rate, and pH 7, the algal cultivation system resulted in the highest ammonia removal rate (50.92 ± 2.91 mg/L-day) and highest ammonia removal efficiency (94.90 ± 1.87%). | ||||
| • | At operational condition of 0.3 day−1 dilution rate, 39.9 mg/L-day ammonia loading rate, and pH 7, the algal cultivation system resulted in the highest cellular ammonia consumption rate (0.0505 ± 0.0044 g NH3/g cell-day). | ||||
| • | At operational condition of 0.1 day−1 dilution rate, 9.4 mg/L-day ammonia loading rate, and pH 7, the algal cultivation system resulted in the highest cell yield based on nitrogen (19.42 ± 2.53 g cell/g NH3). | ||||
| • | At 0.1 day−1 dilution rate, 55.6 mg/L-day ammonia loading rate, and pH 7, the algal culture demonstrated the highest ammonia assimilation capability, with 98.6% of the inlet ammonia-nitrogen assimilated by the algae. | ||||
Acknowledgment
Performance of the statistical analysis was consulted by Kent Kroeger of Department of Statistics in Iowa State University. Technical assistance by Tim Shepherd and Wade Sitzmann of the Department of Agricultural and Biosystems Engineering and Ben Huseman of the Department of Mechanical Engineering in Iowa State University are gratefully acknowledged.
Funding
This study was financially supported by Iowa Space Grant Consortium, Iowa State University Bailey Foundation Award. The first author is thankful to Korea Institute of Energy Technology Evaluation and Planning (KETEP) for providing scholarship toward her master's degree program.
References
- Abeliovich , A. and Azov , Y. 1976 . Toxicity of ammonia to algae in sewage oxidation ponds . Appl. Environ. Microbiol , 31 : 801 – 806 .
- Anderson , R.A. 2005 . “ Algal Culturing Techniques ” . In Official Methods of Analysis of AOAC International , 17th ed. , Gaithersburg , MD : AOAC International . Burlington, MA: Elsevier Academic Press. AOAC Official Method. 2000
- Azov , Y. and Goldman , J.C. 1982 . Free ammonia inhibition of algal photosynthesis in intensive cultures . Appl. Environ. Microbiol , 43 : 735 – 739 .
- Bender , G.R. , Sutton , S.V. and Marquis , R.E. 1986 . Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci . Infect. Immun , 53 : 331 – 338 .
- Blackmer , A.M. , Bremner , J.M. and Schmidt , E.L. 1980 . Production of nitrous oxide by ammonia-oxidizing chemoautotrophic microorganisms in soil . Appl. Environ. Microbiol , 40 : 1060 – 1066 .
- Bussink , D.W. and Oenema , O. 1998 . Ammonia volatilization from dairy farming systems in temperate areas: A review . Nutr. Cycl. Agroecosys , 51 : 19 – 33 . doi: 10.1023/A:1009747109538
- González , L.E. , Cañizares , R.O. and Baena , S. 1997 . Efficiency of ammonia and phosphorus removal from a colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and . Scenedesmus dimorphus. Bioresour. Technol , 60 : 259 – 262 .
- Groot Koerkamp , P.W.G. 1994 . Review on emissions of ammonia from housing systems for laying hens in relation to sources, processes, building design and manure handling . J. Agric. Eng. Res , 59 : 73 – 87 .
- Jun , Y. and Wenfeng , X. 2009 . Ammonia biofiltration and community analysis of ammonia-oxidizing bacteria in biofilters . Bioresour. Technol , 100 : 3869 – 3876 . doi: 10.1016/j.biortech.2009.03.021
- Kim , N.-J. , Hirai , M. and Shoda , M. 2000 . Comparison of organic and inorganic packing materials in the removal of ammonia gas in biofilters . J. Hazard. Mater , 72 : 77 – 90 . doi: 10.1016/S0304-3894(99)00160-0
- Krupa , S.V. 2003 . Effects of atmospheric ammonia (NH3) on terrestrial vegetation: A review . Environ. Pollut , 124 : 179 – 221 . doi: 10.1016/S0269-7491(02)00434-7
- Li , H. , Xin , H. , Burns , R.T. and Liang , Y. 2008 . Reduction of ammonia emission from stored poultry manure using additives: Zeolite, Al+Clear, Ferix-3 and PLT . J. Appl. Poult. Res , 17 : 421 – 431 . doi: 10.3382/japr.2007-00076
- Li , H. , Xin , H. , Burns , R.T. , Roberts , S.A. , Li , S. , Kliebenstein , J. and Bregendahl , K. 2012 . Reducing ammonia emissions from high-rise laying-hen houses through dietary manipulation . J. Air Waste Manage. Assoc , 62 : 160 – 169 . doi: 10.1080/10473289.2011.638414
- Liang , Y. , Xin , H. , Wheeler , E.F. , Gates , R.S. , Zajaczkowski , J.S. , Topper , P. , Li , H. and Casey , K.D. 2005 . Ammonia emissions from U.S. laying hen houses in Iowa and Pennsylvania . Trans. ASAE , 48 : 1927 – 1941 . doi: 10.13031/2013.20002
- Nalewajko , C. , Colman , B. and Olaveson , M. 1997 . Effects of pH on growth, photosynthesis, respiration, and copper tolerance of three Scenedesmus strains . Environ. Exp. Bot , 37 : 153 – 160 . doi: 10.1016/S0098-8472(96)01029-5
- Ndegwa , P.M. , Hristov , A.N. , Arogo , J. and Sheffield , R.E. 2008 . A review of ammonia emission mitigation techniques for concentrated animal feeding operations . Biosyst. Eng , 100 : 453 – 469 . doi: 10.1016/j.biosystemseng.2008.05.010
- Park , J. , Jin , H.F. , Lim , B.R. , Park , K.Y. and Lee , K. 2010 . Ammonia removal from anaerobic digestion effluent of livestock waste using green alga Scenedesmus sp . Bioresour. Technol , 101 : 8649 – 8657 .
- Philippe , F.-X. , Cabaraux , J.-F. and Nicks , B. 2011 . Ammonia emissions from pig houses: Influencing factors and mitigation techniques . Agric. Ecosyst. Environ , 141 : 245 – 260 . doi: 10.1016/j.agee.2011.03.012
- Sommer , S.G. and Hutchings , N.J. 2001 . Ammonia emission from field applied manure and its reduction—invited paper . Eur. J. Agron , 15 : 1 – 15 . doi: 10.1016/S1161-0301(01)00112-5
- Tam , N.F.Y. and Wong , Y.S. 1996 . Effect of ammonia concentrations on growth of Chlorella vulgaris and nitrogen removal from media . Bioresour. Technol , 57 : 45 – 50 . doi: 10.1016/0960-8524(96)00045-4
- van Breemen , N. , Burrough , P.A. , Velthorst , E.J. , Vandobben , H.F. , Wit , T.De , Ridder , T.B. and Reijnders , H.F.R. 1982 . Soil acidification from atmospheric ammonium sulphate in forest canopy throughfall . Nature , 299 : 548 – 550 . doi: 10.1038/299548a0
- Xin , H. , Gates , R.S. , Green , A.R. , Mitloehner , F.M. Jr. , Moore , P.A. and Wathes , C.M. 2011 . Environmental impacts and sustainability of egg production systems . Poult. Sci , 90 : 263 – 277 . doi: 10.3382/ps.2010-00877
- Xin , L. , Hong-ying , H. , Ke , G. and Jia , Y. 2010 . Growth and nutrient removal properties of a freshwater microalga Scenedesmus sp. LX1 under different kinds of nitrogen sources . Ecol. Eng , 36 : 379 – 381 . doi: 10.1016/j.ecoleng.2009.11.003
- Zimmo , O.R. , van der Steen , N.P. and Gijzen , H.J. 2004 . Nitrogen mass balance across pilot-scale algae and duckweed-based wastewater stabilisation ponds . Water Res , 38 : 913 – 920 . doi: 10.1016/j.watres.2003.10.044