Abstract
Direct-reading organic vapor monitors are often used to measure volatile organic compound concentrations in complex chemical gas mixtures. However, there is a paucity of data on the impact of multiple gases on monitor performance, even though it is known that monitor sensitivity may vary by chemical. This study investigated the effects of interferents on the performance of the MIRAN SapphIRe Portable Ambient Air Analyzer (SAP) and Century Portable Toxic Vapor Analyzer (TVA-1000) when sampling a specific agent of interest (cyclohexane). The TVA-1000 contained a dual detector: a photoionization detector (PID) and a flame ionization detector (FID). Three devices of each monitor were challenged with different combinations of cyclohexane and potential interferent vapors (hexane, methyl ethyl ketone, trichloroethylene, and toluene) at 21°C and 90% relative humidity (RH), an extreme environmental condition. Five replicates at four target concentrations were tested: 30, 150, 300, and 475 ppm. Multiple proportions of cyclohexane to interferent enabled the determination of the interferent effect on monitor performance. The monitor concentrations were compared to reference concentrations measured using NIOSH Method 1500. Three scenarios were investigated: no response factor, cyclohexane response factor, and weighted-mixed response factor applied. False negatives occurred more frequently for PID (21.1%), followed by FID (4.8%) and SAP (0.2%). Measurements from all monitors generally had a positive bias compared to the reference measurements. Some monitor measurements exceeded twice the reference concentrations: PID (36.8%), SAP (19.8%), and FID (6.3%). Evaluation of the 95% confidence intervals indicated that performance of all monitors varied by concentration. In addition, the performance of the PID and SAP varied by presence of an interfering compound, especially toluene and hexane for the PID and trichloroethylene for the SAP. Variability and bias associated with all these monitors preclude supplanting traditional sorbent-based tube methods for measuring volatile organic compounds (VOCs), especially for compliance monitoring.
Implications: Industrial hygienists need to use care when using any of the three monitor detection types to measure the concentration of unknown chemical mixtures. Monitor performance is affected by the presence of interferents. Application of manufacturer recommended response factors may not adequately scale measurements to minimize monitor bias when compared to standard reference methods. Users should calibrate their monitors to a known reference method prior to use, if possible. Each of the monitors has its own limitations, which should be considered to ensure quality measurements are reported.
Introduction
Portable direct-reading organic vapor monitors (monitors) have found application as survey tools and as a means of assessing hazards. Some advantages of monitors include (1) short lag time between measurement and reporting of result, (2) rapid response and logging of changes in concentration, (3) allowing both peak and time-integrated concentration determinations, (4) providing real-time exposure information to the worker for modification of work habits during a shift, and (5) allowing more informed decisions regarding any environmental controls and respiratory protection needed.
One hazard assessment area where direct-reading monitors play an increasingly important role is emergency response. Direct-reading monitors are being used to provide information on the level of personal protective equipment needed by first responders entering a contaminated area, to differentiate between hot and cold zones, and to define evacuation radii. Unlike in industrial settings, first responders may not have direct knowledge of the contaminants and concentrations they may encounter. In order for first responders to make the most informed decision, they need to have detailed knowledge of the capabilities of the monitors they are using. The monitors in this study were selected based on a review of the equipment list contained in the Responders Knowledge Base (InterAgency Board, Citation2014).
Since most emergency response situations involve multiple gases and/or vapors, it is necessary for first responders to know how these interferents affect the performance of the monitors in detecting the gas/vapor of interest. Few studies have investigated the effect of interferents on performance. Of the studies available, the one most comparable to this study was conducted in 2000 by Longworth, Barnhouse, and Ong, who tested three MIRAN SapphIRe Portable Ambient Air Analyzers to characterize their ability to detect chemical warfare agent vapors (Longworth et al., Citation2000).
The authors tested three individual SapphIRes against tabun, sarin, and mustard. The study determined the minimum detectable concentration level of each agent, investigated the effects of humidity and temperature on detection response, established response curves for each agent, and determined the effects of potential interfering vapors on detector performance. They concluded that the SapphIRe did not provide sufficient warning to ensure the safety of first responders when exposed to chemical warfare agents: Interferents affected the monitor’s detection performance, and the monitor was affected by humidity, with high humidity decreasing the monitor’s response.
The next most comparable study used the TVA-1000B (Longworth et al., Citation1999). Those authors found that the PID detectors were easily contaminated and needed frequent cleaning, which is impractical in the field. The FID was strongly affected by interferents. Both the PID and FID detectors could not be relied upon for the detection of chemical warfare agents, and other contaminants adversely affected their performance. No studies were found that used agents other than chemical warfare agents. This is an important deficiency in the guidance provided to first responders.
Direct-reading monitors have been assessed for specific applications using a single monitor under generally limited environmental conditions (Barsky et al., Citation1985; Coffey et al., Citation2009; Coy et al., Citation2000; Drummond, Citation1997; Poirot et al., Citation2004). The National Institute for Occupational Safety and Health (NIOSH) recently published a technical report that provides guidance on evaluation of direct-reading monitors for gases and vapors (NIOSH, Citation2012). This report outlines the importance of assessing monitor performance in the presence of both environmental effects (i.e., temperature and relative humidity) and environmental interferences (e.g., gases present in the test atmosphere that would change monitor response). NIOSH researchers began investigating these topics as part of a large study to characterize the performance of organic monitors. The following two studies were conducted to evaluate the environmental effect as well as the effect of calibration environment on monitor performance. The two studies were also the initial research used to inform the current study.
LeBouf et al. determined the performance of three MIRAN SapphIRe portable ambient air analyzers (SAP) and three Century portable toxic vapor analyzers (TVAs) when calibrated at different environmental conditions (LeBouf et al., Citation2013). Prior to sampling, the monitors were calibrated per the manufacturer’s instructions using methane for the TVA flame ionization detector (FID) and isobutylene for the photoionization detector (PID), whereas the SapphIRe monitors were zeroed and the monitor’s manufacturer-supplied library was used. For the first series of tests (“same condition”), the monitors were calibrated under the same environmental conditions as those present during sampling. They were then challenged with four cyclohexane concentrations (30, 150, 300, and 475 ppm) under two extreme environmental conditions: 5°C and 30% RH (same/cold) and 38°C and 90% RH (same/hot). For the second series of tests (“different condition”), the monitors were calibrated at approximately normal indoor environmental conditions (21ºC and 50% RH) and sampled at extreme environmental conditions (different/cold and different/hot). The monitor readings from the two methods were compared with the actual cyclohexane concentration determined from charcoal tubes using ratios and root mean square errors. Monitor failures were identified as values greater than two times the challenge concentration or below the detection limit of the instrument. A number of monitor failures occurred in each part: same condition 20.7% (149 failures/720 trials) and different condition 42.4% (305/720), with a majority of the failures (>78%) during the hot and humid conditions. All monitors had the lowest bias and within-monitor variability at the same/cold condition, followed by the same/hot condition. The ranked choice of monitors for same/cold was PID > SAP > FID (i.e., best > less good > worst); for different/cold, FID > PID > SAP; for same/hot, SAP > PID > FID; and for different/hot, PID > SAP (FID not included due to 100% failure rate).
Using the same monitors, Coffey et al. investigated monitor relationships using two different calibration methods at four cyclohexane concentrations, three temperatures, and four relative humidities (Coffey et al., Citation2012). For the first method, the TVA monitors were calibrated with a single concentration of methane for the FID, and isobutylene for the PID. The SapphIRe monitors were zeroed and the monitor’s manufacturer-supplied library was used. For the second method, a five-point cyclohexane (100, 200, 300, 400, and 500 ppm) calibration curve was created for each monitor. Comparison of the monitor results of each calibration method indicated a significant difference between methods (t-test, p < 0.001) at a 95% confidence level. The SapphIRe group had results closer to the charcoal tubes with the second calibration method, while the PID and FID monitor groups performed better using the first calibration method. The PID monitor group’s performance was affected only at the 90% relative humidity condition. Using the first method, the monitor readings were compared with the charcoal tube average using analyses of variance (ANOVA) and regression. The ANOVA results showed there was a statistically significant difference among readings from all monitor types (p < 0.0001). The regression results demonstrated that the SapphIRe (r2 = 0.97) and FID (r2 = 0.92) monitor groups correlated well with the charcoal tubes. The PID monitor group had a similar correlation when 90% RH was excluded (r2 = 0.94) but had a much worse correlation when it was included (r2 = 0.58). The authors concluded that operators should take care when using these monitors at high concentrations and the PID monitors at high humidities, consider the variability between units of the same monitor, and conduct performance verification of the monitor being used.
The overall objective of this laboratory study was to determine the performance of direct-reading organic vapor monitors using simulated real-world conditions where chemical gas interferents may be present in the test atmosphere when attempting to measure a gas of interest. The specific aim was to evaluate the performance of monitors compared to National Institute for Occupational Safety and Health (NIOSH) Method 1500 (hydrocarbons) when challenged with a specific gas of interest at varying concentrations and with varying amounts of potential interferents in the challenge atmosphere.
Materials and Methods
Instrumentation
This study evaluated three units of two different analyzers: MIRAN SapphIRe Portable Ambient Air Analyzers with a single-beam infrared spectrophotometer (series 205B, model 100; Thermo Environmental Instruments, Inc., Franklin, MA) and Century Portable Toxic Vapor Analyzers (TVA, model TVA-1000B, Thermo Environmental Instruments, Inc.) equipped with photoionization (PID) with a 10.6-eV lamp and flame ionization detector (FID).
In addition to being in the Responders Knowledge Base (InterAgency Board, Citation2014), the SapphIRe was chosen because it is a single-beam infrared spectrophotometer that allows the first responders to make an identification of any unknown contaminants. The TVA-1000B was selected to provide a contrast to the SapphIRe since it has a PID detector, which is nonspecific. In addition, it contains a FID, making it one of the few dual detectors available.
Each monitor was calibrated by the manufacturer prior to the beginning of the study. The built-in library supplied with the SapphIRe monitors was used and has the following parameters: gas high range limit (HRL) of 500 ppm, a detection limit of 6 ppm, wavelength of 11.156 µm, and a path length of 12.5 m (Thermo Electron Corporation, Citation2004). Monitors were zeroed everyday using a zero particulate filter (part number TR101ZU, Thermo Fisher Scientific, Inc.) and a zero gas chemical filter (TR101PU, Thermo Fisher Scientific, Inc.). The TVAs were calibrated using the manufacturer-recommended calibration method, which included zeroing the monitor and challenging with a span gas (500 ppm methane for FID and 1000 ppm isobutylene for PID) (Coffey et al., Citation2012).
Instrumentation testing
The test setup has been described previously and only a summary is provided (Coffey et al., Citation2012; LeBouf et al., Citation2013). Testing was performed in a 22-m3 walk-in environmental chamber (Nor-Lake ENVIROLINE; Nor-Lake Scientific, Hudson, WI) providing control of temperature (21°C) and relative humidity (90% RH). Elevated relative humidity is a worst-case scenario chosen to reflect operating conditions in the field that would provide the most challenge to the instruments. The inlet of the monitors and charcoal tubes were placed in a 0.4-m3 Rochester-style (exposure) chamber inside the environmental chamber. Variability among inlets was 3.1% relative standard deviation, which was assessed using charcoal tubes at each location. The test vapor atmospheres were generated using an in-house vapor generation system connected to the exposure chamber. The test system was automated as described previously (Coffey et al., Citation2012).
Challenge agents
Cyclohexane was selected since it is a high-production-volume chemical (i.e., annual production and/or importation volumes above one million pounds) (Scorecard Citation2014). Therefore, first responders and others may come into contact with this chemical. It may also be used as a chemical warfare agent simulant (NIOSH, Citation2005; Chemical Security Analysis Center [CSAC] e-mail message to author, October 3, 2008). Cyclohexane (certified ACS grade, catalog number C556-1, Fisher Scientific, Pittsburgh, PA) has an ionization potential (IP) of 9.88 eV so it is readily detectable by the PID of the TVA.
A personal communication report from the Chemical Security Analysis Center (e-mail message to author, October 3, 2008) indicated that exhaust gases, gasoline, kerosene, chlorine bleach, insect repellant, and diesel fuel vapors could be used as interferents in detector evaluation for first responders. All of these except for chlorine bleach are complex mixtures of volatile and semivolatile organic compounds. Using these as interferents in the study would add confounding variables that may make the detection of differences in performance difficult. The main component of chlorine bleach (sodium hypochlorite) is not detectable by charcoal tubes. Due to these considerations, simulants for the interferents used were hexane (IP = 10.18 eV), methyl ethyl ketone (IP = 9.54 eV), trichloroethylene (IP = 9.45 eV), and toluene (IP = 8.82 eV) (NIOSH, Citation2010). These contaminants are common chemicals that are likely to be encountered during emergency response scenarios and represent different classes of organic compounds (straight-chain hydrocarbons, ketones, chlorinated hydrocarbons, and aromatics) that may elicit different monitor responses. Hexane was selected since it is the straight-chain version of cyclohexane. Toluene was chosen because it is a component of gasoline. In addition, it and methyl ethyl ketone are found in car and truck exhausts. Trichloroethylene was chosen as the substitute for chlorine bleach over methylene chloride. The TVA PID has a 10.6-eV lamp, which cannot detect methylene chloride since methylene chloride has an IP of 11.32 eV. Trichloroethylene (TCE), having an IP of 9.45 eV, can be ionized and detected by the TVA PID.
Four concentrations of cyclohexane were used (30, 150, 300, and 475 ppm by volume). These concentrations are based on the 0.1, 0.5, 1.0, and 2.0 times the recommended exposure limit (REL) for a NIOSH standard method fit for publication in the NIOSH Manual of Analytical Methods. Two times the cyclohexane REL (300 ppm) is 600 ppm. The upper limit of both the SapphIRe and the TVA PID is 500 ppm. Therefore, 475 ppm (95% of 500 ppm) was used to in order to ensure all concentrations would be within the monitors’ ability to detect. Each of the four vapor concentrations had multiple proportions of cyclohexane:interferent with volume ratios of 33%:67%, 50%:50%, 67%:33%, and 0%:100% for each of the four concentrations. The interferents were used singly and as a combination of all four (mixture of interferents) in equal proportions. Five replicates each lasting 30 min were conducted at each test condition. For each concentration condition, the five replicates were run consecutively due to time constraints. The monitor-measured concentrations were then compared to concentrations measured using NIOSH Method 1500 for cyclohexane and total hydrocarbons.
Charcoal tube analysis
The charcoal tubes were analyzed in-house using NIOSH Method 1500 for hydrocarbons, boiling points 36–216°C (NIOSH, Citation2003), with the following modified operating parameters: 0.2 μL injection volume; 100% dimethyl polysiloxane fused silica capillary column with dimensions of 60 m × 0.32 mm ID × 1.00 μm film (Rtx-1; Restek Corporation, Bellefonte, PA); and a 20:1 split flow. In addition, 167 ng of p-cymene 99+% (Acros Chemicals, Thermo Fisher Scientific, Geel, Belgium) was added to each sample as an internal standard. The analyzer was an Agilent 6890N gas chromatograph with an FID (Agilent Technologies, Santa Clara, CA). Two five-point response factor calibration curves (both r2 > 0.999) were developed (3.05 ng/sample to 61.1 ng/sample and 61.1 ng/sample to 763.7 ng/sample). The limit of detection for the modified method was considered to be 3.05 ng.
Data Analysis
An average of the 3600 data points from each monitor was used to calculate a 30-min time-weighted average (TWA) of each trial. The data from the five replicates at each condition were combined for a total of 420 trials per monitor. Pure cyclohexane accounted for 20 (5 replicates × 4 concentrations) of 420 trials. The rest of the trials consisted of cyclohexane plus interferents at different ratios (5 replicates × 4 concentrations × (4 interferents + 1 mixture of interferents) × 4 interferent ratios) (). The trials for different interferent volume ratios were pooled since no significant effect of interferent ratio was observed on monitor performance (p = 0.29). The mean, median, coefficient of variation (CV), and the minimum and maximum values were computed for each monitor, monitor group (i.e., devices of the same monitor), and tube. The detection limit values were: charcoal tubes, 2.9 ppm (Kennedy et al., Citation1995); SapphIRe, 6 ppm (Thermo Electron Corporation, Citation2004); PID, 0.5 ppm (Thermo Electron Corporation, Citation2003); and FID, 1 ppm (Thermo Electron Corporation, Citation2004). The data were reviewed for values below the detection limit (BDL) as an indicator of a false negative response. Since the monitors were exposed to a known concentration of chemical but measured BDL during some trials, these trials were considered monitor failures.
Table 1. Test matrix indicating sample numbers and independent variables
All data were subsequently analyzed with the BDL values removed to reflect a more representative comparison to the reference sorbent tube method. The monitor TWA concentrations were statistically compared to the appropriate charcoal tube concentration with and without response factors applied. The monitor comparisons in terms of reported concentration in ppm were examined using an analysis of variance (ANOVA) with Tukey’s multiple comparison test (α = 0.05) in JMP 10 (SAS Institute, Inc., Cary, NC). The following categorical variables were included as treatment variables in the model: target concentration, interferent condition, and replicate. Each monitor measurement was also compared to the reference method using a percent recovery referred to here as a percentage of the charcoal tube value (%CT = measurement/tube value × 100%). The cyclohexane and the interferent response factors were taken from the Thermo Environmental TVA Response Factor manual (Thermo Electron Corporation, Citation2003). Manufacturer response factors can be applied to this data set generated under an extreme 90% RH since they are applicable to the entire environmental operating range of the instrument. Three separate analyses were conducted on three data sets: raw monitor readings (no response factor applied), cyclo RF (cyclohexane response factor applied), and mixed RF (mixed response factor applied). Data were analyzed under these three scenarios to reflect the varying use of these monitors in laboratory and field settings.
A mixed response factor was the most appropriate data conversion method for this study since the proportion of test and chemical interference concentrations were well known. Since some monitor measurements were substantially overestimating the charcoal tube value, the mixed RF data was reviewed for twice the target concentration (200%CT) as an indicator of overestimation; none of these data were removed from the analyses. The 200% criterion was chosen to roughly approximate the 75th percentile of the distributions of measurements from the worst performing monitor group (i.e., PID). The mixed response factor was a weighted summation of the individual chemical response factors calculated using eq 1 adapted from the manufacturer’s response factor manual:
Results and Discussion
Information on individual and group monitor performance in terms of %CT values and associated summary statistics by target concentration are available in Supplementary Material (Tables S1–S4).
lists the monitor concentration values that were BDL indicating a false negative response. The SapphIRes had the lowest percentage of BDL values (0.2%) and were consistent across target concentrations. The PID group had the highest percentage (21.1%). Two of the PID monitors had the same percentage of BDL (18.1%), while the third had a slightly higher percentage (27.1%). When the BDL percentage was analyzed by target concentration, the number of BDL values for the PID increased as target concentration decreased, indicating less reliable measurements toward the lower measurement range of the monitor. This observed concentration effect may have been due to a reduction in monitor response resulting from the high humidity test condition combined with an increased measurement variability at the lowest target concentration (30 ppm). For the FID monitors, the percentages of BDL values were approximately the same (3.8–5.4%) regardless of target concentration. In terms of least amount of false negatives, the rank order of best performing monitors was SAP > FID > PID.
Table 2. Frequency (in percentage) of trials where monitor measurement was below the detection limit (false negatives) in the presence of a known concentration
contains the percentage of monitor concentration values equal to or greater than twice the corresponding tube concentration indicating a substantial overestimation of the reference concentration. Of the three monitor types, the FID group had the lowest percentage (7.9%) and the PID group the highest (26.0%). For the PID group at 30 ppm target concentration, the percentage overestimation (10.5%) was lower than at the other three concentrations (range 24.1–34.9%) and tended to increase as target concentration increased. For the FID group, the percentage overestimation was dominated by FID 2 (16%). For the SAP group at 30 ppm target concentration, 30.2% of the monitor values were greater than two times the tube concencentration and tended to decrease with increasing target concentration. These results indicate that monitor measurements can be significantly greater than actual airborne concentration levels measured by traditional sorbent-based methods. In addition, percentage overestimation varied among monitors of the same type. In terms of least amount of overestimation, the rank order of best performing monitors was FID > SAP > PID.
Table 3. Frequency (in percentage) of trials where monitor measurement (mixed response factor applied) was over twice the tube value
A Tukey’s multiple comparison test on reported instrument concentration for all test concentration levels combined for the three monitor groups with a mixed RF applied to the FID and PID measurements and BDL values removed showed that the FID group mean (mFID = 326.9 ppm) was not statistically different from the SAP group mean (SAP = 345.3 ppm) (). These group means were statistically different from both the tube mean (Tube = 247.6 ppm) and the PID group mean (mPID = 526.8 ppm), which were also statistically different from each other. Application of a mixed response factor was the most appropriate data conversion method for this study, since the proportions of test and chemical interference concentrations were well known. It was surprising that all monitor types still failed to match the reference method in a controlled atmosphere, but the FID group mean was the closest to the tube mean. When a cyclohexane RF was applied to the FID and PID measurements, the FID group mean (cFID = 276.6 ppm) was not statistically different from the tube mean (Tube = 247.6 ppm) but was statistically different from the SAP group mean (SAP = 345.3 ppm) and the PID group mean (cPID = 668.7 ppm). Application of a single response factor would be most appropriate when the test atmosphere was known to contain one dominant chemical, such as in a chemical plant or responding to a chemical spill. When no response factor was applied to the FID and PID measurements, the FID group mean (FID = 643.4 ppm) was statistically different from all monitor groups: the SAP group mean (SAP = 345.3 ppm), the tube mean (Tube = 247.6 ppm), and the PID group mean (PID = 284.8 ppm). Using raw monitor measurements (i.e., measurements remaining in methane equivalents for FID and in isobutylene equivalents for PID) is appropriate when the VOC composition of the test atmosphere is unknown, such as in most emergency response scenarios, and when relative readings are needed for monitoring process changes or assessing VOC stability of an atmosphere. An ANOVA of the five replicates for all monitors at each condition (target concentration and interferent mixtures) showed there were no statistical differences between any of the replicates (p = 0.55).
Figure 1. Mean monitor concentration in ppm with 95% confidence intervals. Tube = charcoal tube; mPID = PID with mixed response factor applied; mFID = FID with mixed response factor applied; cPID = PID with cyclohexane response factor applied; cFID = FID with cyclohexane response factor applied; PID = PID with no response factor applied; FID = FID with no response factor applied.
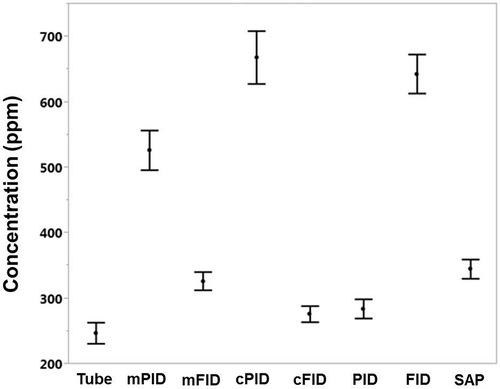
Another measure of monitor performance is the group mean %CT (displayed as a dot) and the 95% confidence interval (CI; displayed as an error bar) (). This figure was developed using data corrected by a mixed response factor using eq 1; it displays the monitor group mean %CT values segregated by target concentration, monitor type, and interferent condition. The smaller the width of the CI, the more closely the values agree among the three devices of the same type of monitor. The mean %CT minus 100% can be equated to percentage bias (i.e., relative bias). The CI can be equated to precision. The PIDs and SAPs had larger CIs at the 30 ppm target concentration except for PIDs measuring pure cyclohexane; CIs at this condition increased with increasing target concentration. When the CI encompasses the ratio of 100%, the monitor closely matched the tube concentration (i.e., the reference method). When the lower confidence limit is above 100%, there is a statistically significant positive bias associated with the monitor measurements. When the upper confidence limit is below 100%, there is a statistically significant negative bias associated with the monitor measurements.
Figure 2. Mean percentage charcoal tube values (%CT) with 95% confidence intervals. CYC = cyclohexane; HEX = hexane; TOL = toluene; MEK = methyl ethyl ketone; TCE = trichloroethylene; and MIX = mixture of all interferents.
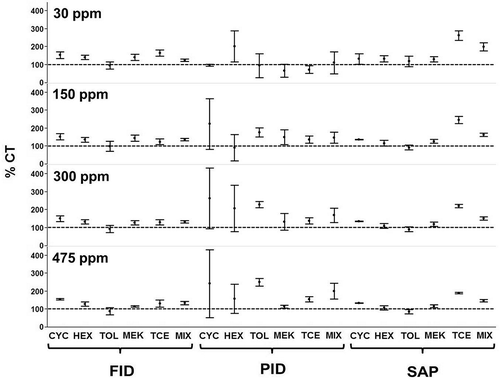
The FID group was the least affected by the presence of interferents, as can be seen from the consistency in %CT values across all interferent conditions from pure cyclohexane to a mixture of cyclohexane and all four interferents (). This is presumably due to the ability of the flame ionization technique to more efficiently ionize compounds (related to the combustion efficiency) than photoionization (related to the ionization potential), as well as its ability to handle the higher RH (90% RH) found in this study. FID sensitivity is greatest for hydrocarbons. PID has a lower sensitivity for low-molecular-weight hydrocarbons.
The PID group showed an effect of interferent on the %CT values; this is most notable in the 150-, 300-, and 475-ppm conditions (). In these target concentration conditions, the PID group had CIs that were widest (±100 to ±136%CT) when exposed to pure cyclohexane. The hexane exposure condition also showed considerable variability (±19 to ±77%CT). The toluene exposure condition generally had mean %CT values greater than methyl ethyl ketone (MEK) (30 ppm, 178 vs. 152%CT; 150 ppm, 230 vs. 135 %CT; and 475 ppm, 252 vs. 116%CT) and trichloroethylene (30 ppm, 178 vs. 141%CT; 150 ppm, 230 vs. 147 %CT; and 475 ppm, 252 vs. 158 %CT). This observed difference in performance of the PIDs for various interferents may have been due to the high RH condition of this study and/or the varying ionization potentials of the interferents. The order of ionization potentials is hexane > cyclohexane > methyl ethyl ketone ≈ trichloroethylene > toluene. Since toluene has the lowest ionization potential of the interferents, it requires the least amount of energy to break the molecule into positively charged ions. When cyclohexane and toluene are simultaneously measured by the PID, cyclohexane will preferentially absorb photons over toluene since the ionization potential for cyclohexane is higher than that of toluene. Thus, PID response in terms of isobutylene equivalents should be lower in this two-component system compared to toluene measured alone at the same total vapor concentration condition. This phenomenon was observed as the relative proportion of toluene to cyclohexane decreased from 100% to 0%, the PID response decreased (data not shown). This phenomenon is also reflected in the difference between the two chemicals’ manufacturer-recommended response factors. Cyclohexane response factor is greater than 1, meaning the monitor response is increased by applying the response factor; toluene response factor is less than 1, meaning the response is decreased when the factor is applied. The high RH of this study also affected the PID performance in terms of variability as well as direct comparison to the tube reference method. This high RH condition was chosen to reflect the worst case scenario in terms of monitor performance as seen by these researchers in a previous study (Coffey et al., Citation2012).
The SAP group showed an effect of interferent on performance in terms of %CT values. This is most apparent in the 150-, 300-, and 475-ppm graphs (). The monitor is tuned to a specific absorption wavelength for cyclohexane (11.156 µm) as recommended by the manufacturer; transmittance at this wavelength is approximately 69%. The interferents also absorb slightly at this wavelength (HEX 88%, TOL 84%, MEK 91%, and TCE 82%). The lower the percent transmittance, the more infrared (IR) energy is absorbed by the molecule at this wavelength. TCE had the lowest percent transmittance, meaning it should have the greatest positive interference with cyclohexane measurement, which was confirmed in this study with TCE mean %CT values being the largest (e.g., 192%CT at 475 ppm) compared to cyclohexane mean %CT (e.g., 136%CT at 475 ppm).
Conclusion
These results confirmed that PID monitors may not be reliable for accurately determining well-controlled concentrations of mixtures at high humidity conditions. Reliability was assessed by tracking monitor failures and comparing measurements to a standard reference method. The SAP monitors gave fairly consistent and reliable results, but responded to the presence of interferents when tuned to a cyclohexane absorption wavelength. Interferents should always be considered when looking for selective compound monitoring using this detector. This monitor, and the measurements from it, should be used by trained technicians to ensure the measurements are correctly interpreted. The FID gave the most consistent and reliable results when compared to the reference method. The FID is a suitable choice when measuring total hydrocarbons instead of specific chemicals in a humid environment containing interferents.
The PID and FID monitors are nonspecific detectors and should have measured all compounds in this study. The SAP monitors may be more suitable for identification and quantification of target compounds in mixtures, but positive bias in measurements was observed here due to interfering chemical absorption at the wavelength chosen. Variability and bias associated with all these monitors preclude supplanting traditional sorbent-based tube methods for measuring volatile organic compounds, especially for compliance monitoring.
Funding
This work was supported by the National Institute for Occupational Safety and Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Mention of any company or product does not constitute endorsement by the National Institute for Occupational Safety and Health (NIOSH). In addition, citations to websites external to NIOSH do not constitute NIOSH endorsement of the sponsoring organizations or their programs or products. Furthermore, NIOSH is not responsible for the content of these websites.
Supplemental Materials
Supplemental data for this article can be accessed at http://10.1080/10962247.2014.986308.
Supplementary_Material.docx
Download MS Word (63 KB)Acknowledgment
The authors acknowledge Michael Commodore and Judith Hudnall for conducting the environmental tests, Catherine Calvert for performing the charcoal tube analysis, and John Powers, Larry Lee, and Bradley Newbraugh for their assistance in the development of the LabView program and associated hardware.
Additional information
Notes on contributors
Ryan F. LeBouf
Ryan F. LeBouf is a senior service fellow with the Division of Respiratory Disease Studies at the National Institute for Occupational Safety and Health, Morgantown, WV.
Christopher C. Coffey
Christopher C. Coffey is the associate director for Science in the Office of the Director, National Personal Protective Technology Laboratory, National Institute for Occupational Safety and Health, Morgantown, WV.
References
- Barsky, J.B., S.S. Que Hue, and C.S. Clark. 1985. An evaluation of some portable, direct-reading 10.2 eV and 11.8 eV photoionization detectors, and a flame ionization gas chromatograph for organic vapors in high humidity atmospheres. AIHA J. 46: 9–14.doi:10.1080/15298668591394293
- Coffey, C., T. Pearce, R. Lawrence, J. Hudnall, J. Slaven, and S. Martin. 2009. Effect of calibration and environmental condition on the performance of direct-reading organic vapor monitors. J. Occup. Environ. Hyg. 6:1–8.
- Coffey, C., R.F. LeBouf, L. Lee, J. Slaven, and S. Martin. 2012. Effect of calibration and environmental condition on the performance of direct-reading organic vapor monitors. J. Occup. Environ. Hyg. 9:670–80. doi:10.1080/15459624.2012.725015
- Coy, J.D., P.L. Bigelow, R.M. Buchan, J.D. Tessari and J.O. Parnell. 2000. Field evaluation of a portable photoionization detector for assessing exposure to solvent mixtures. AIHA J. 61:268–74. doi:10.1202/0002-8894(2000)061<0268:FEOAPP>2.0.CO;2
- Drummond, I. 1997. On-the-fly calibration of direct reading photoionization detectors. AIHA J. 58:820–22. doi:10.1080/15428119791012333
- Kennedy, E.R., T.J. Fischbach, R. Song, P.M. Eller, and S.A. Shulman. 1995. Guidelines for air sampling and analytical method development and evaluation. NIOSH Publication No. 95–117. http://www.cdc.gov/niosh/docs/95-117/ (accessed December 23, 2014).
- LeBouf, R.F., J.E. Slaven, and C.C. Coffey. 2013. Effect of calibration environment on the performance of direct-reading organic vapor monitors. J. Air Waste Manage. Assoc. 63:528–533. doi:10.1080/10962247.2013.772926
- Longworth, T.L., J.I. Barnhouse, and K.Y. Ong. 2000. Domestic preparedness program—Testing of MIRAN SapphIRe portable ambient air analyzers against chemical warfare agents. Summary report. Aberdeen Proving Ground, MD: Soldier and Biological Chemical Command. http://www.ecbc.army.mil/downloads/reports/ECBC_miran_sapphire_analyzers.pdf (accessed August 30, 2014).
- Longworth, T.L., J.C. Cajigas, J.I. Barnhouse, K.Y. Ong, and S.A. Procell. 1999. Testing of commercially available detectors against chemical warfare agents. Summary report. Aberdeen Proving Ground, MD: Soldier and Biological Chemical Command. http://www.ecbc.army.mil/downloads/reports/ECBC_detectors_summary.pdf (accessed August 30, 2014).
- National Institute for Occupational Safety and Health. 2003. Method 1500. http://www.cdc.gov/niosh/docs/2003-154/pdfs/1500.pdf (accessed August 30, 2014).
- National Institute for Occupational Safety and Health. 2005. Determination of CBRN organic vapor (cyclohexane) service life test, air-purifying respirators standard test procedure. Pittsburgh, PA: NIOSH.
- National Institute for Occupational Safety and Health. 2010. Pocket Guide to Chemical Hazards. Cincinnati, OH: NIOSH.
- National Institute for Occupational Safety and Health. 2012. Components for evaluation of direct-reading monitors for gases and vapors, pp. 1–99. http://www.cdc.gov/niosh/docs/2012-162/ (accessed December 23, 2014).
- Poirot, P., I. Subra, F. Gerardin, V. Baudin, S. Grossman, and M. Hery. 2004. Determination of short-term exposure with a direct-reading photoionization detector. Ann. Occup. Hyg. 48:75–84. doi:10.1093/annhyg/meg079
- Scorecard. 2014. Chemical profile for cyclohexane. http://scorecard.goodguide.com/chemical-profiles/summary.tcl?edf_substance_id=110%2d82%2d7 (accessed August 30, 2014).
- InterAgency Board. 2014. Standardized equipment list. http://iab.gov/SELint.aspx (accessed August 30, 2014)
- Thermo Electron Corporation, 2003. TVA-1000B Toxic Vapor Analyzer Instruction Manual (part number BK3500). Franklin, MA.
- Thermo Electron Corporation, 2004. Miran® 205B Series SapphIRe Portable Ambient Air Analyzers Instruction Manual (part number BK3538). Franklin, MA.
