Abstract
Using laboratory experiments, the authors investigated the impact of dry-heat and moist-heat treatment processes on hazardous trace elements (As, Hg, Cd, Cr, and Pb) in food waste and explored their distribution patterns for three waste components: oil, aqueous, and solid components. The results indicated that an insignificant reduction of hazardous trace elements in heat-treated waste—0.61–14.29% after moist-heat treatment and 4.53–12.25% after dry-heat treatment—and a significant reduction in hazardous trace elements (except for Hg without external addition) after centrifugal dehydration (P < 0.5). Moreover, after heat treatment, over 90% of the hazardous trace elements in the waste were detected in the aqueous and solid components, whereas only a trace amount of hazardous trace elements was detected in the oil component (<0.01%). In addition, results indicated that heat treatment process did not significantly reduce the concentration of hazardous trace elements in food waste, but the separation process for solid and aqueous components, such as centrifugal dehydration, could reduce the risk considerably. Finally, combined with the separation technology for solid and liquid components, dry-heat treatment is superior to moist-heat treatment on the removal of external water-soluble ionic hazardous trace elements.
Implications: An insignificant reduction of hazardous trace elements in heat-treated waste showed that heat treatment does not reduce trace elements contamination in food waste considerably, whereas the separation process for solid and aqueous components, such as centrifugal dehydration, could reduce the risk significantly. Moreover, combined with the separation technology for solid and liquid components, dry-heat treatment is superior to moist-heat treatment for the removal of external water-soluble ionic hazardous trace elements, by exploring distribution patterns of trace elements in three waste components: oil, aqueous, and solid components.
Introduction
With the accelerated urbanization and rapid economic development, a substantial amount of food resources are lost in the form of food waste (Soare et al., Citation2011; Mason et al., Citation2004). Therefore, the resource utilization of food waste, such as anaerobic biogas production (Cekmecelioglu and Uncu, Citation2013; Chen et al., Citation2014; Forster-Carneiro et al., Citation2008; Tawfik and El-Qelish, Citation2012), feedstuff production (Kwak and Kang, Citation2006; Jin et al., Citation2012; Westendorf et al., Citation1998; Yang et al., Citation2006), and composting (Hwang et al., Citation2002; Seng et al., Citation2013; Kim et al., Citation2008), draws particular attention of researchers as well as administrators. For instance, from 2010 to 2014, the Chinese government launched a program for the resource utilization and safe treatment of food waste in 67 pilot cities, with the total treatment capacity as 17,770 tons/day.
In this paper, food waste refers to the food residues from public eating places, such as restaurants, hotels, and cafeterias, and it usually comprises a complex mixture of oil, aqueous, and solid components (Chen et al., Citation2012). In addition, regarding sources, food waste primarily derives from the residues of human food consumption, with a low background concentration of hazardous trace elements (García et al., Citation2005; Viana and Schulz, Citation2003). However, hazardous trace elements might be introduced during the process of the collection, transportation, or treatment of food waste for various reasons. For example, García et al. (Citation2005) found that the high amount of trace elements in food waste from household was higher than the values permitted by European legislation, due to the presence of inorganic substances such as cans, sand, metals, or other particles. Moustatsou et al. (Citation2003) reported the metal concentrations of Fe up to 157.5, Cu up to 82.5, Zn up to 31, and Ni up to 8.5 mg/L in the waste from an alcoholic anis-type beverage, mainly derived from the bronze pot stills. Onay et al. (Citation2010) observed that up to 80% heavy metals existed in the synthetic solid waste containing 76% food waste by sorption. In China, the contamination of hazardous trace elements seems more serious, because of the low-level source separation and inappropriate collection. According to the results of our investigation of cafeterias, waste treatment stations in residential communities, and restaurant food waste treatment facilities in Suzhou, China, the average concentrations of As, Hg, Cd, Cr, and Pb (trace elements) were relatively low and were in accordance with the data reported by García et al. (Citation2005) and Chen et al. (Citation2011). However, the concentrations of trace elements in food waste gradually increase or exceed the related standards due to an increase in the range of waste collection and the complexity of waste sources, and occasionally, they were found with high amount of heavy metals in food waste (Chen et al., Citation2011). Thus, the utilization of food waste is limited. For instance, a concentration of Hg that slightly exceeded the health standards of feedstuffs was observed sometimes in the food waste from waste treatment facilities, which rendered it unsuitable for feedstuff production (Chen et al., Citation2011). In addition, a high concentration of hazardous trace elements can affect microbial activity and render them poisonous to microbes (Breton et al., Citation2013; Lyubun et al., Citation2013) and less functional in biological processing, such as anaerobic digestion or high-temperature composting. Therefore, the investigation of the regularity of hazardous trace elements migration and transformation is significantly important for resource utilization involving food waste.
Furthermore, there are some research about the influence of trace elements on the recycling of food waste; for example, Zhang et al. (Citation2011b) suggested that food waste was deficient with some trace elements required for anaerobic digestion; however, the separation studies of trace elements from food wastes are rare. Additionally, Bernstad et al. (Citation2013) found that ground food waste collected from tank-connected systems had a low content of trace elements. However, due to the unique dietary characteristics in some countries such as China, South Korea, etc., food waste contains a relatively high concentration of fat (Wang et al., Citation2013; Zheng et al., Citation2012), which has not only a considerably high economic recycling value but also would significantly affect the stability of follow-up processing and the effectiveness of treatment. Hence, the heating pretreatment is required to increase the dissolution of solid fats and to recover a substantial amount of oil (Canakci, Citation2007; Zheng et al., Citation2012). In general, for the heating pretreatment process for food waste, the main approaches in the actual practice include dry-heat and moist-heat treatments (Wang, Citation2013), both of which are the earliest forms of sterilization practiced. In addition, as the name indicates, dry-heat utilizes hot air that is either free from water vapor, or has very little of it, whereas moist-heat utilizes hot air that is heavily laden with water vapor. In other words, whether the moisture plays the most important role or not is the significant difference between dry-heat and moist-heat treatments. Moreover, moist-heat treatment involves the application of heat under pressure an aqueous medium in a sealing apparatus (Chen et al., Citation2012). Generally for food waste, dry-heat treatment is considered as a physical modification that changes the physicochemical properties of food (such as starch, etc.) without destroying its granule structure (Sun et al., Citation2014). However, its application is restricted to organic materials that can’t tolerate high temperatures. Moist-heat treatment refers to splitting (breaking) up of the organic particles into smaller organic fragments in water through a variety of mechanisms, including hydrolysis, oxidation, and gasification (Jomma et al., Citation2003), which also acts on the large organic molecules reducing them into smaller fragments, some of which dissolve in water. Furthermore, proteins in food waste are easier to be destroyed during dry-heat treatment process compared with moist-heat treatment as Maillard reactions (Henle, Citation2001).
Currently, numerous studies have focused on these two heating approaches. And consequently, studies on dry-heat treatment have mainly focused on the treatment of sludge, fly ash, and other materials (Liang et al., Citation2013; Ohm et al., Citation2009; Ramaroson et al., Citation2008; Zhang et al., Citation2011a), whereas studies on moist-heat treatment have primarily focused on material synthesis and carbonization treatment (Gao et al., Citation2012; Lin et al., Citation2010; Reza et al., Citation2013; Xiang et al., Citation2002; Xu and Lancaster, Citation2008; Yang et al., Citation2013). But only a few studies have been conducted on the application of these two heat treatments on food waste (Chen et al., Citation2012; Jin et al., Citation2012; Kelley and Walker, Citation2000), and in particular, studies comparing the effect of trace elements removal on these two heating treatment processes are rare.
By simulating dry-heat and moist-heat treatments for food waste samples in the laboratory, we analyzed the removal of hazardous trace elements (As, Hg, Cd, Cr, and Pb) after these two processes and explored the posttreatment distribution pattern of trace elements in solid, aqueous, and oil components of food waste. And as a result, this study would provide a strong theoretical support for the development of the subsequent treatment and recycling technologies of food waste.
Material and Methods
Study description
Firstly, we collected 10 kg of fresh food waste from a college cafeteria (with a water content of 76.8 ± 0.8%). And after the manual sorting to remove unrelated wastes, such as plastic spoons and napkins, the remaining sample was ground, mixed in a food grinder, and divided into two portions for the subsequent dry-heat and moist-heat experiments, respectively.
The measurement results for the trace elements in the raw samples of fresh food waste collected from the cafeterias are shown in . And similar to the results of García et al., the background value for most of trace elements in the food waste was relatively low (García et al., Citation2005) and less than the most stringent upper limit value recommended by the health standards for feedstuffs (Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China [AQSIQ], Citation2001; GB/T 13078-2001). This result was primarily attributed to the direct sampling locations, which would simplify the transportation process of food waste, reduce the potential risk of external pollution, and ensure that the hazardous trace elements in the waste samples primarily derived from food for human consumption as well. The high amount of Hg observed in raw food waste may be due to the contact with disinfectant and the presence of rice, vegetables residues, or inedible parts of food such as fish, etc. (Kao, Citation2004; Qiu et al., Citation2008, Citation2009; Zhang et al., Citation2010).
Table 1. Results for trace elements in the fresh raw food waste (dry basis)
Experiment description
Regarding sources, the concentration of trace elements in food waste was relatively low (Chen et al., Citation2011; García et al., Citation2005; Viana and Schulz, Citation2003). Therefore, the concentration of trace elements in the original waste, shown in , was used as a background value in this study. Moreover, in order to investigate the impact of external contamination (e.g., through intentional addition if dissatisfied to some policies from the standpoint of economic, etc., factors), we added standard trace element samples containing 1 mg/mL of Pb, As, Cr, or Cd into every kg of the waste sample (National Test Center of Steel Material of China), with 20 mL for each type of trace elements, to reduce the experimental error. And the concentration of Hg without external addition was used as the reference, mainly because it exceeded the most stringent maximum quantity allowed by the legislation (). In addition, for the heat treatment, all samples were subsequently placed in a homemade reactor as described by Chen et al. (Citation2012).
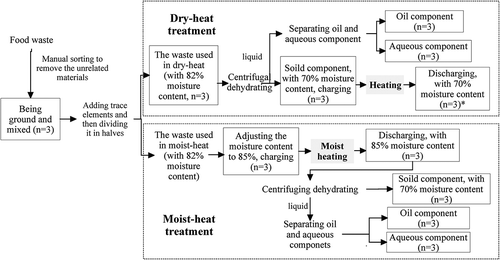
Dry-heat and moist-heat treatments are distinctively different. Compared with moist-heat treatment, the waste of dry-heat treatment is operated with lower moisture content and nonpressure circumstance. In this experiment, the water, especially free water of food waste, should be removed as much as possible by centrifuging before dry-heat treatment, whereas some water would be added to waste before moist-heat treatment. Furthermore, during the dry-heat treatment, the samples with trace elements were centrifugally dehydrated at a centrifugation speed of 15,000 rpm for 20 min at room temperature, prior to heating and drying, until a water content of approximately 70% was attained. Then, these samples were placed in a preheated airtight reactor with stirrer at 140 ± 5 °C, nonpressure for 40 min. In particular, during the moist-heat treatment, the water content of the samples with trace elements was adjusted to 85%, and the samples were subject to heat treatment in the humid and airtight environment at 120 ± 2 ° C, 0.5 MPa for 40 min, followed by a centrifugal dehydration for 20 min at a centrifugation speed of 15,000 rpm at room temperature. The procedures are shown in . In addition, in order to keep the material properties as much as possible, the technical parameters for the heat treatment in this experiment were established based on the processing procedures and the sterilization requirements used in the actual practice, as follows: 120 °C at 0.5 MPa for 40 min for the moist-heat treatment and 140 °C at normal pressure for 40 min for the dry-heat treatment (Chen et al., Citation2012). Moreover, because of the airtight reactor used in the experiment, there was little change with the moisture of the food waste after the heating process (the gray filled box in ), if the centrifugal dewatering wasn’t considered.
Figure 1. Procedures for dry-heat and moist-heat treatments (the rectangular boxes indicate the sampling points, and the number within the parentheses indicates the number of samples; * indicates the discharging in dry-heat treatment also means the solid component).
The specific sampling points and sample number for the experiment are shown in . In the experiments, a total of 11 sampling points were determined. For each sampling point, we collected three parallel samples in the different positions distributed in the upper, middle, and lower parts of the reactor and then mixed and homogenized them to form an analytic sample. Each analytic sample was measured three times in parallel. Then, the mean value multiplied by material mass could be gained as the mass of trace elements.
Measurement method
The samples were subject to microwave-assisted digestion (CEM MARS; Mathews, NC, USA) in the Chemical Analysis Center of Tsinghua University. The concentration of the hazardous trace elements was measured by inductively couple plasma atomic emission spectroscopy (ICP-AES; Vista-MAX; Varian Inc.), after a microwave digestion (WX-8000; PreeKem Scientific Instruments Co., Shanghai, China) (U.S. Environmental Protection Agency [EPA], Citation1994).
Statistical analysis
The results were expressed as the mean values and standard deviations of the raw data. The data were analyzed using Excel (Microsoft, 2007) and OriginPro7.5 (OriginLab Corporation; Northampton, MA, USA). Significant differences were determined by the Fisher least significant difference (LSD) test at the P < 0.05 level.
Results and Discussion
The performance of heat treatment in the removal of hazardous trace elements from food waste
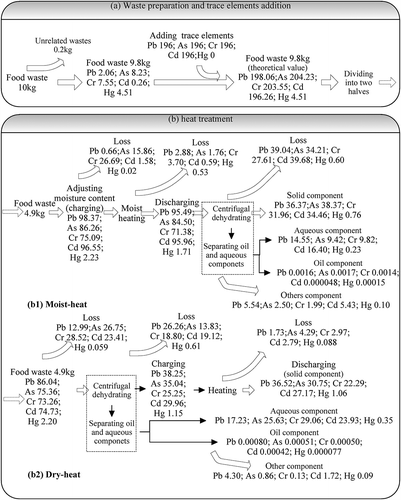
showed the mass balance of trace elements during the heat treatment. During the treat process, lots of trace elements (13.5–40.4%) were lost in the centrifugal dehydrating and oil recovery steps, which may be due to the residues in vessels and test loss. And most trace elements still remained in food waste, mainly distributed in solid and aqueous component. Moreover, the loss amount of trace elements was slight both at dry-heat and moist-heat steps.
Figure 2. The mass balance of trace elements (mg) during the heat treatment process, consisting of two parts: (a) material preparation and elements addition; (b) heat treatment, including dry-heat and moist-heat treatments separately.
In order to indicate the effect of the removal of hazardous trace elements from food waste during heat treatment further, the contents of hazardous trace elements in food waste (dry basis) were detected, as shown in , by comparing the values of charging and discharging; Hg was used as the reference without external addition. And according to , the legend “after centrifugate” of moist-heat treatment in is equal to the trace element contents of solid component, whereas “before centrifugate” in dry-heat treatment is the trace element contents of food waste after adding trace elements. After the moist-heat treatment, the concentration of trace elements in the waste decreased by 0.61–23.68%. And the largest reduction in concentration was obtained for Hg, which decreased from 0.38 ± 0.12 to 0.29 ± 0.09 mg/kg, and the smallest reduction in concentration was obtained for Cd, which decreased from 16.42 ± 3.0 to 16.32 ± 3.4 mg/kg. In addition, the Hg concentration decreased after the moist-heat treatment, which was most likely due to the volatility of Hg in food waste under hot and humid conditions. Furthermore, the differences among the reductions in concentration for Pb, As, and Cd were not significant—2.93% (decreased from 16.73 ± 2.0 to 16.24 ± 1.8 mg/kg), 2.04% (decreased from 14.67 ± 1.9 to 14.37 ± 1.5 mg/kg), and 4.93% (decreased from 12.77 ± 2.5 to 12.14 ± 2.7 mg/kg), respectively. After the dry-heat treatment, the concentration of trace elements decreased from 4.53% to 12.25%. The largest reduction in concentration was obtained for As, which decreased from 11.92 ± 1.4 to 10.46 ± 0.8 mg/kg, and the smallest reduction in concentration was obtained for Pb, which decreased from 13.01 ± 2.1 to 12.42 ± 2.7 mg/kg. The concentration of Cr, Cd, and Hg decreased by 11.76% (decreased from 8.59 ± 0.3 to 7.58 ± 0.2 mg/kg), 9.31% (decreased from 10.19 ± 1.6 to 9.24 ± 1.3 mg/kg), and 7.69% (decreased from 0.39 ± 0.14 to 0.36 ± 0.08 mg/kg), respectively.
Figure 3. Impact of heat treatment on the concentration of hazardous trace elements in food waste (calculated on a dry basis; the symbols # and & indicate a significant difference, P < 0.5).
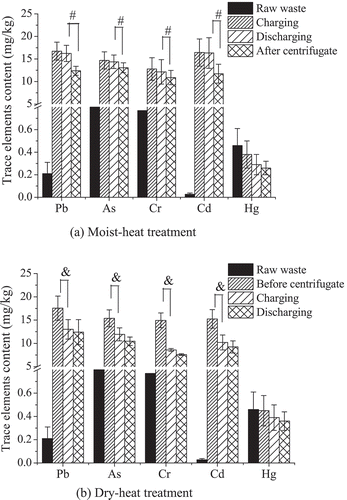
Results showed that the concentration and mass of the trace elements in the food waste discharged from the heat treatment were slightly lower than those in the feed-in waste, with no significant difference being observed. Therefore, the heat treatment did not have a remarkable impact on the concentration and removal of hazardous trace elements in the food waste. This may be because the temperatures of the heat treatments (120 °C for moist heating and 140 °C for dry heating) were not sufficiently high and remained below the volatilization temperature of trace elements.
Moreover, after the centrifugal dehydration, the concentration of trace elements in the waste was considerably decreased (P < 0.5), with the exception of Hg (). This finding may be attributed to the notion that the addition of standard samples of trace elements involves water-soluble ions, which can be partially removed with the separation of solid and aqueous components by centrifugal dehydration due to their dissolution in the aqueous component (). In addition, for Hg without external addition, the main form of Hg in food is methylmercury (Kao, Citation2004; Qiu et al., Citation2008, Citation2009; Zhang et al., Citation2010). And according to the description of Kao (Citation2004), mercury vapor that evaporates from soil and water converts to a soluble form (Hg2+) and returns to the earth surface with rainwater. This soluble form may attach itself to aquatic sediments and be microbially converted into methylmercury (MeHg). Methylmercury then enters into the aquatic food chain and becomes concentrated at the top of the aquatic food chain, with the highest concentrations found in long-lived predatory fish such as tuna, swordfish, shark, and bass. Moreover, the methylmercury concentrated in food is difficult to be eliminated by conventional treatments, even chelators are not effective (Clarkson and Magos, Citation2003). Thereby, the separation process for solid and aqueous components, such as centrifugal dehydration, could reduce the risk of water-soluble ionic trace element contamination in waste.
Distribution of hazardous trace elements in the oil, aqueous, and solid components of food waste
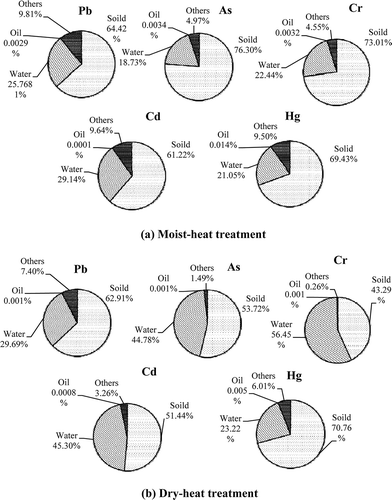
Based on the mass balance of trace elements, the distribution pattern of trace elements in the oil, aqueous, and solid components of food waste after heat treatment is shown in . The trace elements predominantly remained in the solid and aqueous components (>90%) and were negligible in the oil component (<0.01%). In , the other sinks of trace elements in food waste might be caused by the volatilization of some volatile trace element compounds or the experimental loss during the heat treatment or drying processes (Ramaroson et al., Citation2008). In the study, the slight volatilization of trace elements could be thought to be relevant to the high organics as well as Cl contents of food waste due to the probable formation of volatile chlorides (Folgueras et al., Citation2003), except for the emission features of some volatile elements, such as Hg, which is very volatile, Pb and Cd, which are partially volatile. Furthermore, Cr has properties of both very volatile and hardly volatile with different chemical speciation (Huang et al., Citation2004). Moreover, that the residual ratio of trace elements in other component after moist-heat treatment was more than dry-heat treatment may due to the effect of pressure (Liu et al., Citation2006). High pressure leads to the formation and enhancement of the reduced species and increases the condensation temperature of the volatile elements.
For the trace element Hg without external addition, the results indicated that a greater percentage of Hg (70.76%) remained in the solid waste after the dry-heat treatment compared with after the moist-heat treatment (69.43%). In addition, regarding Pb, As, Cr, and Cd with external addition, only a small percentage remained in the solid waste component after dry-heat treatment because the trace elements added during the experiment consisted of aqueous-soluble ions, which can be removed during the separation process for solid and aqueous components in the dry-heat treatment (as shown in ). As a result, the proportion of trace elements in the water component discharged during the dry-heat treatment was relatively high, even close to 60%. However, during the process of moist-heat treatment, a portion of trace elements may experience morphological changes (Gao et al., Citation2012; Reza et al., Citation2013; Xiang et al., Citation2002) or may be intercepted and absorbed (Lin et al., Citation2010) by the solid waste component due to a series of complicated thermal chemical reactions in the humid heat tank (Ren, Citation2006), which results in a smaller proportion of trace elements in the water after the separation of aqueous and solid components (18.73–29.14%) compared with the proportion of trace elements after the dry-heat treatment (23.22–56.45%). Moreover, in the treatment of food waste, the moist-heat reaction is widely used to disintegrate the organic components, converting the complex organic matter into simpler by-products or harmless end products for discharging into the environment. In detail, the main chemical components of food waste typically are protein, cellulose, fat, and minerals. For proteinaceous wastes, amino acids are produced from degradation of proteins. Amino acids further degrade to organic acids. In the case of cellulosic wastes, cellulose is mainly converted into glucose by hydrolysis. Glucose further decomposes to other products, including aldehydes and ketones, from which organic acids are produced. Organic acids may also be produced from monomers obtained from hydrolysis of plastic wastes. Finally, the moist-heat treatment process results in the production of some dissolved organic matter (Jomaa et al., Citation2003). The dissolved organic matter, especially dissolved organic carbon (DOC), can remarkably enhance the complexation and sorption of trace elements (Onay et al., Citation2010).
Generally, according to the emission features, various factors could affect the distribution of trace elements, including temperature, pressure, the characteristics of the trace elements, etc. Furthermore, based on the performance of heat treatment in the removal of hazardous trace elements, heat treatment is insufficient for significantly reducing the concentration of trace elements in food waste; however, the separation process of aqueous and solid components, such as centrifugal dehydration, can considerably reduce the risk of trace element contamination in solid food waste. Consequently, combined with the separation technology for aqueous and solid components, dry-heat treatment shows a relatively superior removal effect on the external contamination of ionic trace elements, whereas moist-heat treatment plays a specific role in the removal of background trace element contamination in food waste.
Conclusion
In order to evaluate the effect of heat treatment on the trace element reduction in food waste, two widely used processes of dry-heat and moist-heat treatment were simulated in this study respectively. And according to the results, there was no significant difference by comparing the concentration and mass of the trace elements in the food waste before and after heat treatment, which means neither dry-heat treatment nor moist-heat treatment is significantly effective in the removal of hazardous trace elements from food waste. Therefore, the control of the external pollution of trace elements in raw materials is critical for the effective eliminating the impact of hazardous trace elements on the resource utilization and the subsequent treatment of food waste. Moreover, a significant reduction in hazardous trace elements (except for Hg without external addition) after centrifugal dehydration (P < 0.5) indicated that the separation process for solid and aqueous components, such as centrifugal dehydration, could reduce the risk of water-soluble ionic elements remarkably. However, the centrifugal dehydration is simply a physical separation process and can hardly remove some insoluble pollutants. Thus, the effect of other processes (such as absorption, chemical reaction, etc.) as well as the properties of food waste will be our subsequent study focus. Anyhow, different heat treatment processes should be selected for pretreatment of contaminated food waste after the identification of the various forms of hazardous trace element pollutants; for instance, dry-heat treatment is a preferable option for the removal of water-soluble ionic contaminants compared with moist-heat treatment method.
Funding
This work was supported by the project funded by China Postdoctoral Science Foundation (no. 2014M550746), the collaborative project funded by Japan International Cooperation Agency and National Development and Reform Commission of China, and the Public Science and Technology Research Funds Projects of Environmental Protection, Ministry of Environmental Protection of the People’s Republic of China (no. 201109035).
Additional information
Funding
Notes on contributors
Ting Chen
Ting Chen is a postdoctoral scientist, Yiying Jin is an associate research professor, and Xin Chen is a research assistant at the School of Environment, Tsinghua University, Beijing, P.R. China.
Yiying Jin
Xiaopeng Qiu is a technical engineer at China Urban Construction Design & Research Institute, Beijing, P.R. China.
References
- Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (AQSIQ). 2001. Hygienical Standard for Feeds. GB/T 13078-2001. Beijing: AQSIQ.
- Bernstad, A., A. Davidsson, J. Tsai, E. Persson, M. Bissmont, and J.L. Jansen. 2013. Tank-connected food waste disposer systems-current status and potential improvement. Waste Manage. 33:193–203. doi:10.1016/j.wasman.2012.09.022
- Breton, J., C. Daniel, J. Dewulf, S. Pothion, N. Froux, M. Sauty, P. Thomas, B. Pot, and B. Foligne. 2013. Gut microbiota limits heavy metals burden caused by chronic oral exposure. Toxicol. Lett. 222:132–138. doi:10.1016/j.toxlet.2013.07.021
- Canakci, M. 2007. The potential of restaurant waste lipids as biodiesel feedstocks. Bioresour. Technol. 98:183–190. doi:10.1016/j.biortech.2005.11.022
- Cekmecelioglu, D., and O. Uncu. 2013. Kinetic modeling of enzymatic hydrolysis of pretreated kitchen wastes for enhancing bioethanol production. Waste Manage. 33:735–739. doi:10.1016/j.wasman.2012.08.003
- Chen, T., Y.Y. F. Liu, X. Meng, H. Li, and Y.F. Nie. 2012. Effect of hydrothermal treatment on the levels of selected indigenous bacteria in food waste. J. Environ. Manage. 106:17–21. doi:10.1016/j.jenvman.2012.03.045
- Chen, T., Y.Y. Jin, and X. Meng. 2011. Food waste originating from three different sources: Characterization and potential use as animal feedstuff. Presented at the 1st International Conference WASTES: Solutions, Treatments and Opportunities. Guimarães, Portugal, September 12–14, 2011.
- Chen, X., W. Yan, K.C. Sheng, and M. Sanati. 2014. Comparison of high-solids to liquid anaerobic co-digestion of food waste and green waste. Bioresour. Technol. 154:215–221. doi:10.1016/j.biortech.2013.12.054
- Clarkson, T.W., and L. Magos. 2003. The toxicology of mercury. Current exposures and clinical manifestations. N. Engl. J. Med. 349:1731–1737. doi:10.1056/NEJMra022471
- Folgueras, A.B., R.A. Diaz, J. Xiberta, and I. Prieto. 2003. Volatilization of trace elements for coal-sewage sludge blends during their combustion. Fuel 82:1939–1948. doi:10.1016/S0016-2361(03)00152-2
- Forster-Carneiro, T., M. Perez, and L.I. Romero. 2008. Influence of total solid and inoculum contents on performance of anaerobic reactors treating food waste. Bioresour. Technol. 99:6994–7002. doi:10.1016/j.biortech.2008.01.018
- Gao, Y., X.H. Wang, H.P. Yang, and H.P. Chen. 2012. Characterization of products from hydrothermal treatments of cellulose. Energy 42:457–465. doi:10.1016/j.energy.2012.03.023
- García, A.J., M.B. Esteban, M.C. Márquez, and P. Ramos. 2005. Biodegradable municipal solid waste: Characterization and potential use as animal feedstuffs. Waste Manage. 25:780–787. doi:10.1016/j.wasman.2005.01.006
- Henle, T. 2001. Maillard reactions of food proteins: Chemical, nutritional and functional aspects. Nahrung 45:149–149.
- Huang, Y.J., B.S. Jin, Z.P. Zhong, X. Rui, Z.Y. Tang, and H.F. Ren. 2004. Occurrence and volatillity of several trace elements in pulverized coal boiler. J. Environ. Sci. China 16:242–246.
- Hwang, E.J., H.S. Shin, and J.H. Tay. 2002. Continuous feed, on-site composting of kitchen garbage. Waste Manage. Res. 20:119–126. doi:10.1177/0734242X0202000203
- Jin, Y.Y., T. Chen, and H. Li. 2012. Hydrothermal treatment for inactivating some hygienic bacteria from food waste amended animal feed. J. Air Waste Manage. Assoc. 62:810–816. doi:10.1080/10962247.2012.676999
- Jomma, S., A. Shanableh, W. Khali, and B. Trebilco. 2003. Hydrothermal decomposition and oxidation of the organic component of municipal and industrial waste products. Adv. Environ. Res. 7:647–653. doi:10.1016/S1093-0191(02)00042-4
- Kao, R. 2004. Understanding the mercury reduction issue: The impact of mercury on the environment and human health. J. Calif. Dent. Assoc. 32:574–579.
- Kelley, T.R., and P.M. Walker. 2000. Bacterial concentration reduction in swine waste amended livestock feed using a single-screw dry-extrusion process. Bioresour. Technol. 75:189–195. doi:10.1016/S0960-8524(00)00063-8
- Kim, J.K., G.H. Han, B.R. Oh, Y.N. Chun, C.Y. Eom, and S.W. Kim. 2008. Volumetric scale-up of a three stage fermentation system for food waste treatment. Bioresour. Technol. 99:4394–4399. doi:10.1016/j.biortech.2007.08.031
- Kwak, W.S., and J.S. Kang. 2006. Effect of feeding food waste-broiler litter and bakery by-product mixture to pigs. Bioresour. Technol. 97:243–249. doi:10.1016/j.biortech.2005.02.008
- Liang, X., X.A. Ning, G.X. Chen, M.Q. Lin, J.Y. Liu, and Y.J. Wang. 2013. Concentrations and speciation of heavy metals in sludge from nine textile dyeing plants. Ecotoxicol. Environ. Saf. 98:128–134. doi:10.1016/j.ecoenv.2013.09.012
- Lin, L.C., M. Thirumavalavan, Y.T. Wang, and J.F. Lee. 2010. Surface area and pore size tailoring of mesoporous silica materials by different hydrothermal treatments and adsorption of heavy metal ions. Colloids Surf. A 369: 223–231. doi:10.1016/j.colsurfa.2010.08.032
- Liu, S.Q., Y.T. Wang, L. Yu, and J. Oakey. 2006. Volatilization of mercury, arsenic and selenium during underground coal gasification. Fuel 85:1550–1558. doi:10.1016/j.fuel.2005.12.010
- Lyubun, Y.V., E.V. Pleshakoya, M. Mkandawire, O.V. Turkoyskay. 2013. Diverse effects of arsenic on selected enzyme activities in soil-plant-microbe interactions. J. Hazard. Mater. 262:685–690. doi:10.1016/j.jhazmat.2013.09.045
- Mason, I.G., A. Oberender, and A.K. Brooking. 2004. Source separation and potential re-use of resource residuals at a university campus. Resour. Conserv. Recy. 40:155–172. doi:10.1016/S0921-3449(03)00068-5
- Moustastsou, A., E. Chalarakis, and G. Zarangas. 2003. Influence of raw materials and distillation equipment on the heavy metal content of waste from an alcoholic anis-type beverage. J. Hazard. Mater. 96:53–64.
- Ohm, T.L., J.S. Chae, J.E. Kim, H.K. Kim, and S.H. Moon. 2009. A study on the dewatering of industrial waste sludge by fry-drying technology. J. Hazard. Mater. 168:445–450. doi:10.1016/j.jhazmat.2009.02.053
- Onay, T.T., N.K. Copty, B. Demirel, and A. Bacioglu. 2010. Impact of food waste fraction in municipal solid waste on sorption of heavy metals. Waste Manage. Res. 28:936–943. doi:10.1177/0734242X09349556
- Qiu, G., X. Feng, P. Li, S. Wang, G. Li, L. Shang, and X. Fu. 2008. Methylmercury accumulation in rice (Oryza stativa L.) grown at abandoned mercury mines in Guizhou, China. J. Agric. Food Chem. 56:2465–2468. doi:10.1021/jf073391a
- Qiu, G., X. Feng, S. Wang, X. Fu, and L. Shang. 2009. Mercury distribution and speciation in water and fish from abandoned Hg mines in Wanshan, Guizhou Province, China. Sci. Total Environ. 407:5162–5168. doi:10.1016/j.scitotenv.2009.06.007
- Ramaroson, J., S. Kribi, J.-L. Dirion, A. Nzihou, P. Sharrock, and G. Depelsenaire. 2008. Chemical and thermal treatment of dredged sediments (sludges): Modification of heavy metal mobilities. In Global Symposium on Recycling, Waste Treatment and Clean Technology, REWAS 2008. Cancun, Mexico, October 12–15, 2008, 769–774. Warrendale, PA: Metals & Materials Society (TMS).
- Ren, L.H. 2006. Chapter 2: Feasibility study of restaurant garbage used as animal feed by hydrothermal treatment. In Research and Application of Thermal Hydrolysis Process for Restaurant Garbage Treatment, 17–20. Beijing: Tsinghua University.
- Reza, M.T., J.G. Lynam, M.H. Uddin, and C.J. Coronella. 2013. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 49:86–94. doi:10.1016/j.biombioe.2012.12.004
- Seng, B., K. Hirayama, K. Katayama-Hirayama, S. Ochiai, and H. Kaneko. 2013. Scenario analysis of the benefit of municipal organic-waste composting over landfill, Cambodia. J. Environ. Manage. 114:216–224. doi:10.1016/j.jenvman.2012.10.002
- Soare, I.C.C., E.R. da Silva, S.E. Priore, R.D.L. Ribeiro, N.M.L.D. Pereira, H.M. Pinheiro-Sant’Ana. 2011. Quantification and analysis of the cost of food wastage in the cafeterias of a large company. Rev. Nutr. 24(4):593–604.
- Sun, Q.J., M. Gong, Y. Li, L. Xiong. 2014. Effect of dry heat treatment on the physicochemical properties and structure of proso millet flour and starch. Carbohydr. Polym. 110:128–134. doi:10.1016/j.carbpol.2014.03.090
- Tawfik, A., and M. El-Qelish. 2012. Continuous hydrogen production from co-digestion of municipal food waste and kitchen wastewater in mesophilic anaerobic baffled reactor. Bioresour. Technol. 114:270–274. doi:10.1016/j.biortech.2012.02.016
- U.S. Environmental Protection Agency. 1994. Determination of metals and trace elements in water and wastes by inductively coupled plasma-atomic emission spectrometry. In Methods for the Determination of Metals in Environmental Samples, Supplement I. EPA/600/R-94-111. Washington, DC: U.S. Environmental Protection Agency; May 1994.
- Viana, E., and H.E. Schulz. 2003. Collection, processing and characterization of food residues from residential waste for use in broiler chicken feed. J. Solid Waste Technol. Manage. 29:24–30.
- Wang, L.J. 2013. Production of bioenergy and bioproducts from food processing wastes: A review. Trans. ASABE 56:217–229.
- Wang, P., L.H. Ren, and M. Zhao. 2013. The investigation and analysis of current output of restaurant garbage in Qingdao. Environ. Pollut. Control 35:99–103.
- Westendorf, M.L., Z.C. Dong, and P.A. Schoknecht. 1998. Recycled cafeteria food waste as a feed for swine: Nutrient content, digestibility, growth, and meat quality. J. Anim. Sci. 76:2976–2983
- Xiang, L., Y.P. Yin, and Y. Jin. 2002. Hydrothermal formation of Ni-Zn ferrite from heavy metal co-precipitates. J. Mater. Sci. 37:349–352. doi:10.1023/A:1013608530417
- Xu, C.B., and J. Lancaster. 2008. Conversion of secondary pulp/paper sludge powder to liquid oil products for energy recovery by direct liquefaction in hot-compressed water. Water Res. 42:1571–1582. doi:10.1016/j.watres.2007.11.007
- Yang, J.K., Y.F. Shi, X. Yang, M. Liang, Y. Li, and N. Ye. 2013. Durability of autoclaved construction materials of sewage sludge-cement-fly ash-furnace slag. Constr. Build. Mater. 48:398–405. doi:10.1016/j.conbuildmat.2013.07.018
- Yang, S.Y., K.S. Ji, Y.H. Baik, W.S. Kwak, and T.A. McCaskey. 2006. Lactic acid fermentation of food waste for swine feed. Bioresour. Technol. 97:1858–1864. doi:10.1016/j.biortech.2005.08.020
- Zhang, D.Q., H. Zhang, C.L. Wu, L.M. Shao, and P.J. He. 2011a. Evolution of heavy metals in municipal solid waste during bio-drying and implications of their subsequent transfer during combustion. Waste Manage. 31: 1790–1796. doi:10.1016/j.wasman.2011.04.006
- Zhang, H., X. Feng, T. Larssen, G. Qiu, and R.D. Vogt. 2010. In inland china, rice, rather than fish is the major pathway for methylmercury exposure. Environ. Health Perspect. 118:1183–1188. doi:10.1289/ehp.1001915
- Zhang, L., Y.W. Lee, and D. Jahng. 2011b. Anaerobic co-digestion of food waste and piggery wastewater: Focusing on the role of trace elements. Bioresour. Technol. 102:5048–5059. doi:10.1016/j.biortech.2011.01.082
- Zheng, L.Y., Q. Li, J.B. Zhang, and Z. Yu. 2012. Double the biodiesel yield: Rearing black soldier fly larvae, Hermetia illucens, on solid residual fraction of restaurant waste after grease extraction for biodiesel production. Renew. Energy 41:75–79. doi:10.1016/j.renene.2011.10.004