Abstract
Aircraft turbine engines are a significant source of particulate matter (PM) and gaseous emissions in the vicinity of airports and military installations. Hazardous air pollutants (HAPs) (e.g., formaldehyde, benzene, naphthalene and other compounds) associated with aircraft emissions are an environmental concern both in flight and at ground level. Therefore, effective sampling, identification, and accurate measurement of these trace species are important to assess their environmental impact. This effort evaluates two established ambient air sampling and analysis methods, U.S. Environmental Protection Agency (EPA) Method TO-11A and National Institute for Occupational Safety and Health (NIOSH) Method 1501, for potential use to quantify HAPs from aircraft turbine engines. The techniques were used to perform analysis of the exhaust from a T63 turboshaft engine, and were examined using certified gas standards transferred through the heated sampling systems used for engine exhaust gaseous emissions measurements. Test results show that the EPA Method TO-11A (for aldehydes) and NIOSH Method 1501 (for semivolatile hydrocarbons) were effective techniques for the sampling and analysis of most HAPs of interest. Both methods showed reasonable extraction efficiencies of HAP species from the sorbent tubes, with the exception of acrolein, styrene, and phenol, which were not well quantified. Formaldehyde measurements using dinitrophenylhydrazine (DNPH) tubes (EPA method TO-11A) were accurate for gas-phase standards, and compared favorably to measurements using gas-phase Fourier-transform infrared (FTIR) spectroscopy. In general, these two standard methodologies proved to be suitable techniques for field measurement of turbine engine HAPs within a reasonable (5–10 minutes) sampling period. Details of the tests, the analysis methods, calibration procedures, and results from the gas standards and T63 engine tested using a conventional JP-8 jet fuel are provided.
Implications: HAPs from aviation-related sources are important because of their adverse health and environmental impacts in and around airports and flight lines. Simpler, more convenient techniques to measure the important HAPs, especially aldehydes and volatile organic HAPs, are needed to provide information about their occurrence and assist in the development of engines that emit fewer harmful emissions.
Introduction
As worldwide air travel continues to increase, so does the consumption of jet fuel. This increase in overall fuel usage further intensifies the need for reliable and quantitative methods for characterizing gaseous emissions from aviation sources. However, implementation of viable techniques can be difficult due to the complex sampling environment for turbine engine exhaust. Although the overall mass emissions from aircraft may be significantly lower in magnitude than other mobile sources (e.g., gasoline and diesel engine sources), they can be locally concentrated at high altitude, at flight lines, and near airports (Federal Aviation Administration, Citation2005). These concentrated emissions can have adverse local health and environmental impacts.
Gaseous emissions of general interest from turbine engines include carbon dioxide (CO2), carbon monoxide (CO), nitrogen oxides (NO and NO2), sulfur oxides (SOx), and total unburned hydrocarbon (UHC) emissions. The UHC emissions, which are primarily released at low power conditions, include unburned fuel constituents and oxidized or pyrolyzed combustion products. Some of these compounds may have higher potential environmental and health impacts and are considered hazardous air pollutants (HAPs). HAPs are chemical species which are regulated emissions under the Clean Air Act (CAA) Amendment of 1990 (U.S. Environmental Protection Agency [EPA], Citation2007); however, they are only regulated for stationary sources and not for aircraft. The International Civil Aviation Organization (ICAO) has established limits for emissions of total NOx, CO, UHC, and particulates (smoke number) from commercial jet engines, but these guidelines do not include speciated emissions of individual UHCs, but rather, total response for all UHCs (Anderson et al., Citation2006; Kurniawan and Khardi, Citation2011). Consequently, there are presently no federal regulatory guidelines that address aircraft engine-related HAP emissions. The CAA Amendment of 1990 defines a major (stationary) emission source as one that emits 10 tons per year of any single HAP or 25 tons per year of any combination of HAPs. While airports are not technically regulated by the CAA, some may have HAPs emissions comparable to stationary sources (Federal Aviation Administration, Citation2005). Therefore, it is important to identify the levels of HAPs emitted from aircraft since they may represent a significant local emission source that may be regulated in the future.
The original list of HAPs from the CAA of 1990 includes many compounds not typically present in aircraft emissions (EPA, Citation1990). Spicer and others have examined HAPs from this original list, and produced shorter lists of HAPs from aircraft emissions (see ) (URS Corp., Citation2003; Federal Aviation Administration, 2009; Spicer et al., Citation2009; EPA, Citation1999a; Kinsey, Citation2009). The aldehydes in this list constitute a significant fraction of the total engine emissions; formaldehyde levels alone have been reported to comprise approximately 12% of the organic emissions from military engines tested (Federal Aviation Administration, 2009). Spicer estimates the average formaldehyde levels to be more than 1,300 mg/kg fuel for specific aircraft (Spicer et al., Citation2009). Kinsey examined six commercial engines and found formaldehyde levels between 130 and 357 mg/kg fuel using a DNPH sampling technique (Kinsey, Citation2009). This study also showed the aldehydes to represent more than half of the total organic emissions. Since the Air Force used more than 2 billion gallons of JP-8 jet fuel in 2010 (Defense Logistics Agency, Citation2010), a significant amount of formaldehyde and other pollutants were likely emitted near airports or bases. Overall, the amount of emitted HAPs by aircraft may be both significant and similar in concentration to many stationary source emissions.
Table 1. Table of hazardous air pollutants commonly emitted from jet engines and their chemical properties (EPA, Citation2009)
Development and implementation of simple methodologies that can be used to accurately and reliably sample and quantify selected HAPs from aviation sources would be useful to characterize the potential environmental burden of current and future systems. Specific compounds of interest pertinent to turbine engine exhaust include carbonyls (primarily aldehydes), C1–C6 hydrocarbons, unburned fuel components, and polycyclic aromatic hydrocarbons (PAHs). In addition to the general need for quantifying HAPs, both the Federal Aviation Administration (FAA) and the Department of Defense (DoD) have been actively pursuing the approval and certification of alternative (non-petroleum-derived) aviation fuels for use as blending feedstocks with petroleum-derived aviation fuel (Corporan et al., Citation2011). This may lead to substantial changes in the base fuel chemical composition and resulting emissions characteristics. Improved understanding of the impact of fuel formulation on HAPs emissions will assist in assessing the potential environmental impact of these fuels.
Measurement Methodologies for HAPs
Previous efforts have been performed to sample and quantify HAPs emissions from aircraft engines (Spicer et al., Citation2009; Spicer et al., Citation1994; Knighton et al., Citation2009; Corporan et al., Citation2005; Kinsey, Citation2009). The most complete of these methods for measuring a wide range of hydrocarbon species, including low-molecular-weight hydrocarbons (C1–C5), aromatics, and unburned fuel constituents, was completed by Spicer (Spicer et al., Citation2009). Exhaust sampling was performed in the field directly into passivated canisters, followed by subsequent laboratory analysis. Spicer also quantified speciated aldehydes by collection on dinitrophenylhydrazine (DNPH)-coated tubes and analysis using proton transfer reaction–mass spectrometry (PTR-MS) analysis. Aldehydes are important species for quantification due to their high relative concentrations in the exhaust and their classification as HAPs. Alternative methodologies for sampling and quantitation of aldehydes have been implemented.
Kinsey (Citation2009) also presented a complete methodology of sampling HAPs including DNPH cartridges and gas sample canister work for nonmethane volatile organic compounds (NMVOCs) in six aircraft turbine engines. This work showed emission indexes for both the aldehydes and NMVOCs in which the aldehydes represented between 31% and 53% of the emissions measured. NMVOCs measured included C2 through C4 components, in addition to alkenes and alkanes. While the great majority of these were not HAPs, most of the HAPs were measured and quantified using these methods.
For example, EPA compendium Method TO-11A (EPA, Citation1999b) measures aldehydes by sampling in DNPH coated silica tubes followed by off-line solvent extraction and quantification using high-pressure liquid chromatography (HPLC). TO-11A was created to quantify 14 different aldehyde compounds for ambient air monitoring, but could be appropriate for sampling combustion or engine emissions if the required total sample volume remains low (<10 L) and the adsorptive capacity of the sampling tubes is not exceeded. This sampling approach has been used in combination with either HPLC–ultraviolet (UV) or gas chromatography–mass spectrometry (GC-MS) detection for quantitation. Real-time measurement techniques, such as tunable diode laser absorption spectroscopy (TDLAS) and gas-phase Fourier-transform infrared (FTIR) spectroscopy, can be used for characterizing engine emissions, but quantitation can be challenging and must be developed for each individual aldehyde due to measurement interferences.
Several techniques are available for the sampling of speciated (nonaldehyde) hydrocarbons from engine exhaust streams. The aforementioned studies by Spicer and coworkers used passivated sampling containers for collection, followed by detailed GC and GC-MS analysis. Although these containers can be convenient for field work due to their ease of implementation during sampling (e.g., pumps and electricity are not required), storage/transport of the large containers and posttest cleaning present several logistical challenges. An alternate methodology is to use charcoal tubes to sample and extract gas-phase hydrocarbons in exhaust streams. Charcoal tubes have been effectively used for ambient sampling of organic species (C6–C20 and speciated aromatics) and occupational safety applications (National Institute for Occupational Safety and Health [NIOSH] Method 1501) (NIOSH, Citation2003). The potential exists to incorporate charcoal tubes for adsorbing hydrocarbons in jet engine exhaust due to their simplicity and suitability for the species of interest. Charcoal tubes are relatively inexpensive, can be used for relatively short-term sampling (5–10 minutes), and are readily transportable and stored. Following sampling, the adsorbed hydrocarbons can be extracted using a solvent (e.g., carbon disulfide) and analyzed via gas chromatography–mass spectrometry (GC-MS) or GC–flame ionization detection (GC-FID). The methods for these tubes were developed for occupational safety applications and have reduced application for some compounds and quantitation levels, especially higher polarity compounds. However, they are appropriate for many occupational exposure compounds that are also found in jet engine exhaust.
In this effort, the potential use of DNPH-coated tubes and charcoal tubes for sampling and quantitation of speciated aldehydes and hydrocarbons in jet engine exhaust was investigated. This included development of a viable methodology for sampling a known volume of engine exhaust under relevant conditions, evaluation of the collection and extraction efficiencies of the two types of tubes, identification of appropriate analytical techniques following extraction, and demonstration on a turbine engine platform. While other researchers have conducted successful HAPs analysis using canister sampling and other methods, the simplicity of sorbent tubes such as these is suitable in terms of HAP characterization. Charcoal tubes are much less expensive and easier to transport in the field than canisters.
Experimental Methods
Exhaust gas sampling
Turbine engine gaseous emissions measurements are typically performed via extractive probe sampling at the engine exit plane. Samples are transported to analytical instrumentation using heated lines to prevent condensation of moisture and condensable hydrocarbons (Corporan, Citation2010). Major and minor gaseous emissions (e.g., CO2, CO, NOx, UHCs) are typically quantified on-line using nondispersive infrared (NDIR), chemiluminescence, FTIR, and FID instrumentation. FTIR can provide real-time on-line simultaneous measurement of several major and minor gaseous emissions, which is also extremely beneficial for determining steady-state engine operation and stabilization following engine transients. It is possible to use FTIR to attempt to monitor and quantify speciated HAPs emissions, but this can be difficult due to spectral interferences during measurement (e.g., multiple species absorbing at the same wavenumber range), making it difficult to fully resolve and quantify individual species.
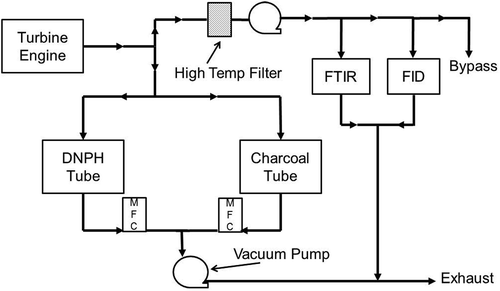
For practical purposes, it is desirable to implement techniques for HAPs sampling using existing gas analysis systems. A basic flow schematic for implementing charcoal and DNPH tube sampling within a typical sampling system is shown in . In this configuration, the heated transport line is split to provide a parallel sampling path for transport of raw sample to the DNPH and charcoal tubes. Total volume of sample gas through each sample tube can be independently controlled and quantified using high-precision mass flow controllers and sampling pumps, while specifying the total sampling duration with constant flow. The total sample duration can be varied to insure quantifiable total mass of the target HAPs are collected without exceeding the total adsorption capacity. While not used in this study, backup DNPH tubes can be used to monitor for breakthrough; in this study, short sampling times were used to reduce breakthrough through a single DNPH tube. Charcoal tubes have a separate breakthrough region in each tube. Following sample collection, the tubes can be removed and stored for subsequent laboratory analysis, and new sampling tubes can be inserted into the flow path. A parallel bypass pathway can be implemented to allow for continuous flow of exhaust through the sample pathway during inactive sampling. The following subsections describe how this sampling approach has been used with both DNPH and charcoal tubes.
Aldehyde collection, extraction, and quantitation
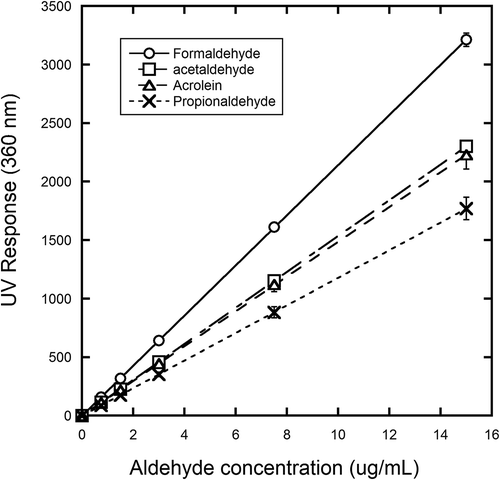
Measurement of aldehydes was performed using DNPH tubes (Supelco LpDNPH H30) via a modified EPA Compendium Method TO-11A. The method is based on a specific reaction between the organic carbonyl compounds and DNPH-coated silica gel in the presence of a strong acid to form a stable hydrazone derivative, and provides a time-weighted average (TWA) measurement. DNPH tubes can adsorb a wide range of aldehyde/ketone compounds pertinent to turbine engine exhaust, with representative compounds shown in . Of the 14 components typically measured using the DNPH-coated tubes, only four are classified as HAPs: formaldehyde, acetaldehyde, acrolein, and propanal (propionaldehyde).
Table 2. Extraction efficiencies (EE) of an aldehyde standard solution, evaluated by EPA Method TO-11A
During exhaust sampling through the DNPH tube, a constant flow rate (between 1 and 2 SLPM) was maintained for a predetermined amount of time (typically 5–10 minutes). These parameters were determined to be appropriate for typical aldehyde levels in turbine engine exhaust. While it was difficult to know beforehand how long to collect sample through tubes, short sampling times were used so that breakthrough was avoided. No backup tubes were used due to the shorter sampling times. An ozone scrubber (Supelco, 505285) was placed immediately upstream of the DNPH tube to prevent undesired reaction of the DNPH and aldehyde derivatives with ozone (Knighton et al., Citation2009). Following sample collection, the cartridges were removed and capped, placed into aluminum foil lined bags, and stored at 4ºC prior to posttest laboratory analysis. Derivatized aldehydes were extracted from the DNPH tubes using 5 mL acetonitrile (total collected volume). Analysis of the extracted compounds was performed using an HPLC-UV technique using the conditions listed in Supplemental .
A commercial standard mixture (Supelco 47285) of derivatized aldehydes (0–15 µg/mL) was used to develop a calibration curve for the HPLC-UV analysis and to determine the extraction and recovery efficiency for each compound of interest. In the event that a measured species concentration is greater than 15 µg/mL, the sample is diluted with additional acetonitrile to be within the calibration range. The corresponding gas-phase concentration of each species (Cx in µg/L) is determined as
where mx is the mass of component x quantified from the DNPH tube (µg), and VT is the total standard volume of exhaust gas sampled (L). The mass of each extracted compound is determined by
where Ci is the concentration of compound x from the HPLC-UV analysis (µg/mL), VACN is the total volume of acetonitrile recovered during extraction (mL), and EE is the extraction efficiency (%) for each compound from the DNPH tubes.
Aromatics and C6–C20 hydrocarbons sampling, extraction, and quantitation
Sampling of aromatic compounds and C6–C20 hydrocarbons was performed via adsorption with charcoal tubes (Supelco ORBO 32). The charcoal tubes comply with all NIOSH and OSHA specifications for tube dimensions, adsorbent quality and particles size, divider composition, and pore size. Prior to sampling, the tube ends are removed using a tube cutter and the tube is installed in the sampling system (). A constant sample flow rate (typically 1.0 SLPM) was drawn through the tube for a predetermined amount of time (typically 5 minutes). Following sample collection, the tubes are removed and capped, and stored at 4ºC prior to analysis. Each charcoal sample tube is comprised of two adsorbent beds separated by a section of urethane foam: The front bed (100 mg charcoal with glass wool) is used for primary sample collection while the back bed (50 mg charcoal and two foam plugs) is used to identify the occurrence of sample breakthrough. If sample analytes break through the first charcoal bed and get adsorbed on the foam, they will be counted with the smaller 50-mg bed. During laboratory analysis, the charcoal tube is scored and broken, separating each bed, with its glass wool or foam plug, into a separate GC vial. Extraction is performed directly in the vial via addition of 1.0 mL carbon disulfide (CS2) and manual agitation. An internal standard (n-C20) is added to each vial prior to GC-MS or GC-FID analysis. Aromatic species are measured via GC-MS using a characteristic ion for each specific compound while GC-FID response and retention time is used to quantify other hydrocarbons. The conditions used during GC analysis are shown in Supplemental . The corresponding gas phase concentrations of speciated and total hydrocarbon emissions is performed using the same methodology described in eqs 1 and 2.
Results and Discussion
Evaluation of aldehydes in turbine engine exhaust
HPLC-UV analysis
Prior to implementation of DNPH tubes for sampling of turbine engine exhaust, evaluation of the analytical accuracy and adsorption/extraction efficiencies was performed. A standard solution of 14 derivatized carbonyl compounds (15 µg/mL of each), Supelco 47285, was analyzed using the HPLC-UV method and conditions shown in Supplemental . A representative HPLC chromatogram for analysis under these conditions is shown in Supplemental . As shown, there was good separation and resolution for most compounds of interest, with the exception of acrolein and acetone, which coelute. However, the impact of coelution of these two specific compounds during actual implementation is minimized since acrolein is typically emitted in turbine engine exhaust at more than an order of magnitude higher level than acetone (Federal Aviation Administration, 2009) (acetone and acrolein were at the same concentration for this standard sample). displays the linearity of the calibration curves for the HPLC response versus concentrations for the aldehydes of interest in this study.
Extraction efficiency of derivatized DNPH compounds
The extraction efficiency (EE) of derivatized compounds from the DNPH tubes is required to accurately quantify the mass of each species adsorbed during exhaust sampling. The extraction efficiencies were determined by injecting known volumes of the derivatized standard (15 µg/mL) onto DNPH tubes and performing acetonitrile extraction and quantification. Four measurements were conducted; the results with relative standard deviations (RSD) are shown in . Three important HAPs (formaldehyde, acetaldehyde, and propionaldehyde) showed extraction efficiencies greater than 93% with low standard deviations. Two specific aldehydes, acrolein and crotonaldehyde, had extraction efficiencies of 59% and 51%, respectively, with relative standard deviation (standard deviation/mean [or RSD]) of 25 and 39%. Attempts to perform additional extraction with acetonitrile showed negligible improved recovery. The poor results for crotonaldehyde and acrolein are most likely due to decomposition of these compounds on the DNPH tubes (Ho et al., Citation2011; Goelen et al., Citation1997) and the inability to separate acrolein from acetone. This known decomposition of crotonaldehyde and acrolein on DNPH tubes precludes the use of this method for accurate quantitation of these specific compounds in turbine engine exhaust. Overall, the magnitude and consistency of the EE for the majority of compounds of interest were good, providing confidence in the application of the technique for this purpose.
Recovery efficiency from sampling of gas-phase aldehydes
The potential use of a modified EPA Method TO-11A for turbine engine exhaust applications was assessed by measuring the overall efficiency of transport of HAP species through the sampling system, adsorption on the DNPH tubes under representative conditions, and solvent extraction for off-line quantitation. The combined transport, collection, and recovery efficiencies were evaluated using a standard gas mixture containing formaldehyde, acetaldehyde, and acrolein with concentrations of 6.56, 2.20, and 1.10 ppmv (parts per million volume), respectively, in nitrogen. Tests were performed using a flow system configured as represented in , with the standard gas mixture introduced at the inlet of the sampling system. The standard mixture was drawn via a vacuum pump through heated lines maintained at 150ºC. The sample was split to provide separate streams for DNPH tube sampling and on-line measurement using an MKS Multi Gas 2030 FTIR based analyzer. This instrument was operated at a scanning rate of 1 scan/8 seconds at a spectral resolution of 0.5 cm−1 and a 5.11-m gas cell. The cell was operated at ambient pressure with a temperature of 150°C. The FTIR measurement provided a concurrent formaldehyde measurement and verified a steady sample flow and exhaust stream concentration. Tube sampling was performed using a constant sample flow rate of 1.0 SLPM with durations of 5, 10, 15, and 20 minutes. The total sampling time was varied to investigate the effect of tube sample loading on the measurement accuracy. Concentrations of each component quantified using HPLC-UV were corrected to equivalent gas phase concentrations using eqs 1 and 2 and the corresponding EE shown in .
Experimental results are shown in . The horizontal lines correspond to the concentration of each species in the gas standard. For the sample transport system and conditions employed, the DNPH tube method (EPA Method TO-11A) provided excellent accuracy for recovery and quantitation of both formaldehyde and acetaldehyde, with a larger uncertainty for acrolein. The average corrected gas-phase concentrations for the four different sample durations were 6.36 ± 0.12 ppmv for formaldehyde, 2.22 ± 0.05 ppmv for acetaldehyde, and 1.37 ± 0.46 ppmv for acrolein. Consistent, accurate values were obtained for sampling with higher total gas volumes, indicating sufficient adsorption capacity for the quantity of compounds extracted.
Figure 3. Comparison of measurement accuracy for DNPH tube sampling of selected gas-phase compounds with varying total sample gas volume. The horizontal lines represent the standard gas concentration.
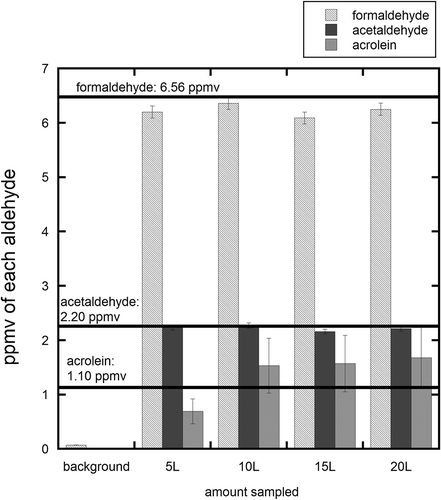
These experiments were conducted to understand the absolute accuracy of the measurement for a known mixture sampled over a wide range of total volume sampled and mass of analyte. Sampling up to 20 L of gas with excellent accuracy and precision for three solutes indicated that breakthrough of components was not readily occurring. If breakthrough were occurring, this experiment would have yielded poor or inconsistent results, especially in the higher gas volumes sampled. The 20 L of sample used in this study was 2–4 times more sample than typically used in this technique, indicating the high capacity of these sampling tubes.
The formaldehyde measurement from the FTIR was also consistent over the test duration, but was approximately 20% higher than the DNPH measurement, with an average concentration of 7.45 ± 0.14 ppmv. The cause of the differences is not readily known, but could be due to the FTIR calibration or more likely, interferences with the other compounds in the gas mixture. On-line FTIR analysis of formaldehyde is an important measurement in the field, as it can indicate when background levels are high or if memory effects are present.
In the case of acrolein, the corrected concentrations were overestimated for the higher sample volumes and significantly underestimated for the lowest sample volume. The inaccuracies are believed to be primarily due to the poor extraction consistency and efficiency for this compound, and also possibly influenced by degradation on the DNPH tube. In general, poor extraction efficiency, poor stability, and (generally) low concentrations of this compound in turbine engine exhaust make acrolein quantitation inappropriate using this method.
Uncertainty analysis for aldehyde quantitation
Propagation of error analysis was performed to estimate the measurement uncertainty for the quantitation of aldehydes using the modified method TO-11A. The relative errors, shown in , account for the respective uncertainties in the total volume of gas sampled, solvent extraction from the DNPH tube and HPLC quantitation. In general, the RSD associated with aldehyde measurement is less than 10%. Limits of detection (LOD) were estimated using the minimum respective concentration for which there is a quantifiable HPLC response. This HPLC response was used for each component to calculate the amount possible to detect, given a 10 L sample of exhaust emissions.
Table 3. Relative errors of aldehyde compounds evaluated by EPA Method TO-11A and their limits of detection
Overall, the calculated relative errors are consistent with the aforementioned experiment results from testing using the gas standard mixture, shown in . The low estimated relative errors associated with formaldehyde and acetaldehyde are also consistent with the good experimental agreement between the measured and known concentrations.
Evaluation of modified NIOSH Method 1501 for aromatics and C6–C20 hydrocarbon analysis
An initial motivation for using the NIOSH Method 1501 was to quantify individual aromatic compounds in turbine engine exhaust. In addition to being health hazards and harmful for the environment (designated as HAPs, such as benzene), these compounds are of interest due to the potential role as intermediates in the formation of polycyclic aromatic hydrocarbons (PAHs), and subsequently soot. Charcoal has a high affinity for hydrocarbon adsorption, making it a viable adsorbent for this application. Following sample collection and extraction with carbon disulfide, quantitation of specific aromatics can be performed via GC-MS using extracted ion analysis. Extracted ion response is necessary due to the presence of partially combusted and unburned fuel components, which coelute with the aromatic compounds of interest. For example, shows a gas chromatogram of hydrocarbons extracted from a charcoal tube following sampling from a T63 turboshaft engine operated with JP-8 fuel at idle power. The chromatogram in represents a standard solution of several mono- and diaromatic compounds of interest.
Figure 4. Gas chromatographic analysis of a charcoal tube sampled from a previous test on a T63-A-700 turboshaft engine operating at idle power (a) and a standard solution of several aromatic compounds (b).
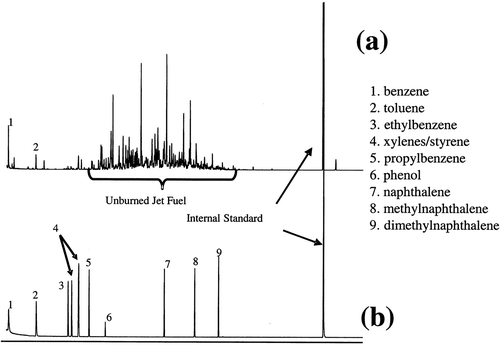
An internal standard (n-C20) was added to both solutions for quantitation. Individual calibration curves are developed for each species of interest. As shown, there are numerous hydrocarbon species that are present in the engine exhaust, with many being residual unburned fuel species. Due to coelution, it is not feasible to fully speciate and quantify individual components using a universal detection method (e.g., FID); however, extracted ion analysis allows for measurement of species with unique ions and is used for quantitation of aromatics, which are the primary hydrocarbon HAPs of interest for this application. No breakthrough of organics into the second charcoal bed occurred during these analyses due to the short sampling duration. The short sampling times may have resulted in less sensitivity for these compounds than longer times would have allowed.
The presence of unburned fuel constituents in turbine engine exhaust is noteworthy. It is typically assumed that UHC emissions are partially oxidized or pyrolyzed products, and not unreacted fuel constituents, especially for high-efficiency turbine engines. Identification and quantification of unreacted fuel in turbine engine exhaust demonstrates that fuel-rich pockets can survive the high pressure and temperature combustion zone without reaction. Unreacted fuel constituents have been observed in the exhaust of the aforementioned T63 engine, and during recent testing from higher-efficiency turbine engines (CFM56 and F117) (Cain et al., Citation2013). The measurement and quantitation of the unreacted fuel make up a relatively simple analysis that can be performed directly using a flame ionization detector (FID).
Extraction efficiency from sampling of aromatic HAP compounds
Similar to the DNPH tube methodology, estimation of the extraction efficiency of aromatic compounds from the charcoal tubes is required to determine the mass of each species adsorbed during sample collection. Efficiencies were determined by injecting small volumes of a standard solution of aromatic compounds onto the front bed of the charcoal tubes using a microliter syringe. Following injection, the front bed was removed and extracted using the solvent extraction method described previously. The corresponding extraction efficiencies were calculated using the ratio of the extracted concentrations to those obtained for the standard solutions injected directly into 1.0 mL of carbon disulfide.
The extraction efficiencies, shown in , were evaluated over a wide concentration of aromatic compounds (1–500 µg). Averages of lower concentrations (1–20 µg) and higher concentrations (21–500 µg) show that for most compounds, there is a reasonable agreement in extraction efficiency over this wide concentration range. However, styrene and phenol showed significantly different and inconsistent extraction efficiencies depending on the concentration range. At low concentrations, it is more difficult to recover these two polar compounds. Diaromatics, such as naphthalene and alkylnaphthalenes, also have lower extraction efficiencies than alkylbenzenes, likely due to their increased polarity. In general, compounds present at higher concentrations were extracted with a slightly better efficiency than when present at lower concentrations. Highly polar compounds, such as phenol, have very low extraction efficiencies, indicating that this methodology is not appropriate for accurate quantitation of such compounds, even though it may occasionally be detected.
Table 4. Extraction efficiencies of select aromatic compounds from charcoal tubes
The RSD for the extraction efficiencies were calculated over 4–6 different concentrations between 1 and 500 µg/mL by comparing spiked tube samples to spiked solvent samples; these show excellent consistency for mono-aromatic compounds. Averages for EE are shown in for two ranges (1–20 and 20–500 µg). Diaromatics have relatively low extraction efficiencies (29–45% for the 1–20 µg range) with extraction efficiency RSD of 25–34%. These allow reasonable estimation of the emission levels of these types of compounds, but with a high uncertainty interval.
Based on previously reported emission inventories (Spicer et al., Citation2009), it is expected that exhaust gas concentrations for several engines of interest should be on the order of 0–2 µg/L gas sampled for individual alkyl aromatics and 0–0.5 µg /L gas sampled for naphthalene. For expected sampling durations (5–10 minutes) and flow rates (1 liter per minute) used with this technique, it is anticipated that the mass range of approximately 1–20 µg of each compound will be adsorbed during sampling.
Relative errors for aromatic HAP analysis
Propagation of error analysis was performed for the measurement of aromatic species via the NIOSH 1501 method, assuming a total adsorbed mass of 0–20 µg. lists the calculated relative error of each compound under sample conditions of 1 SLPM for 10 minutes. In this propagation of error analysis, the replicate (n = 3) analyses of a 5 µg spike of a standard solution on a charcoal tube were used to estimate the standard deviation of the extraction and analysis.
Table 5. Relative errors of selected HAPs from charcoal tubes and their limits of detection
Limits of detection were calculated using the lowest concentration of the standard solution. A 1-µL spike of this mixture provided some minimally detectable responses, from which were calculated concentrations, adjusted using blank correction and EE for each compound. There were two compounds that were not well quantified using this method, styrene and phenol. Low extraction efficiencies (shown in ) are the primary reason for not including limits of detection.
Demonstration of DNPH and charcoal tube sampling methodologies during sampling from a turboshaft engine
Demonstration of the two modified methods was performed during measurement of turbine engine exhaust from a T63-A-700 turboshaft engine housed in the Engine Environment Research Facility at Wright-Patterson Air Force Base. This engine has been used to investigate the effect of fuel chemical and physical properties, additives, and engine power setting on gaseous and PM emissions; details on the engine are provided elsewhere (Corporan et al., Citation2005, 2009; Wilson et al., Citation2013). During this effort, the engine was operated at several power settings, 3% (idle) and 30% and 85% (cruise) of maximum rated power, with a specification-quality, conventional JP-8 fuel. Results reported here are for the engine operated at idle and 30% power.
Exhaust measurements were performed using a sampling system consistent with the flow schematic shown in . Raw engine exhaust was extracted at the engine exit plane using an open bore probe (1.19 mm orifice diameter) installed parallel to the exhaust flow. The sample was transported through 25 m of 7.75-mm internal diameter stainless-steel heated lines maintained at 150ºC to prevent condensation of moisture and hydrocarbons. For HAPs sampling, the gas sample was split and connected to a manifold assembly. In addition to tube sampling, real-time FTIR (formaldehyde response) measurements were performed.
Comparisons of speciated aldehyde and aromatic measurements for several separate test cycles for the idle and 30% power settings are shown in and , respectively. As expected, the absolute emissions of these compounds are highest at the lower power setting, and decrease significantly with increasing power setting due to higher combustion efficiencies. The levels of compounds emitted at the two higher power conditions were below the lower detection limit of the respective methods. In , the average aldehyde concentrations for the three test cycles, conducted over a 3-day period, are shown for the engine operated at idle and intermediate (30%) power. The relative standard deviation (RSD) based on n = 6 samples taken at idle and n = 4 samples at 30% power over the 3-day test period are below 18% for formaldehyde and acetaldehyde. The data variation is believed to be mostly due to variation in engine operation over three separate days. All other aldehyde compounds of interest were below the lower detection limit of the method. It is possible to quantify these other aldehyde species by increasing the total volume of exhaust sampled.
Figure 5. Aldehydes from T63 engine at two conditions, idle and 30% power. The sampling was conducted over three consecutive days at each condition; error bars represent 2 standard deviations, calculated using mean extraction efficiencies from .
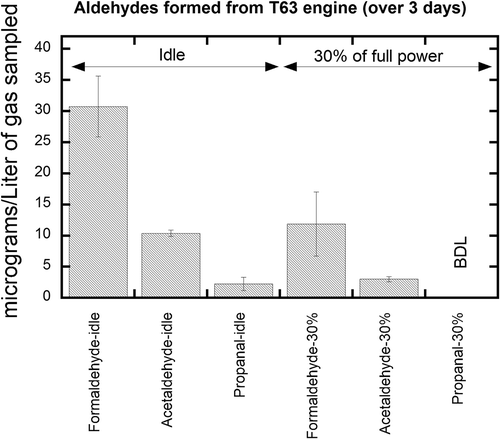
Figure 6. T63 HAP emissions captured by charcoal tubes and measured by GC-MS at idle and 30% conditions. The sampling was conducted over three consecutive days at each condition; error bars represent 2 standard deviations, calculated using mean extraction efficiencies from .
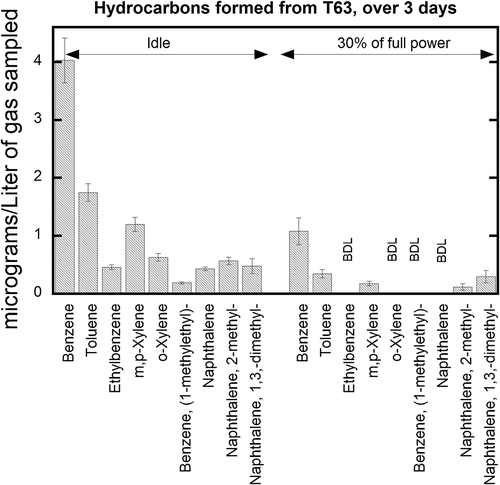
Because acrolein degrades on the tube and extraction efficiencies are poor, quantitation levels for these engine tests are not reported. However, acrolein was detected at idle power conditions and was below detectable levels at 30% power. Poor extraction efficiencies did not allow acrolein to be accurately and confidently quantified.
The HAPs measured using charcoal tubes are shown in . Benzene levels over the three days of test cycles show an RSD of 16% for the idle condition; in contrast, the RSD for the method using standard solutions () was less than 10%, indicating that the engine variability contributes to the measurement uncertainty. As observed in , aromatic compounds were present in the exhaust in significant concentrations at low engine power. These compounds can be emitted directly as unburned fuel constituents, in addition to being produced during combustion. Monoaromatic compounds were emitted at the highest concentrations, while diaromatics such as naphthalene and alkyl naphthalenes were observed at lower, but measurable, levels. These results agree in general with those of Spicer (Spicer et al., Citation1994), which are reported in an emission index format (mg/kg fuel). Like acrolein, styrene and phenol are both detected under idle conditions, but because of poor extraction efficiencies, their concentrations could not be reported accurately. Their detection implies that their actual concentration in the engine exhaust is likely higher than the amount detected (about 4 µg/L gas sampled).
The error bars shown in indicate one standard deviation from the mean for the measurements of hydrocarbon at idle (n = 6) and 30% (n = 4) engine power for the T63 over 3 consecutive days. The major components of those measured using charcoal tubes (benzene and toluene) have approximately half the RSD for idle measurements as for the 30% power setting. Like the aldehyde measurements, it is suspected that much of the deviation present in the measurements was likely due to the operation of the engine over a period of 3 days. Like acrolein, styrene and phenol are not well quantified using this method, as explained earlier. However, styrene was formed at detectable levels during idle combustion.
It was difficult to compare emissions levels with literature data (Spicer, Citation2009; Kinsey, Citation2009) because the aircraft engines and operating conditions were different. However, at low power conditions (idle) the benzene concentration determined during this study was lower than that reported by the other two studies: emissions index of 0.4 mg/kg fuel as compared to 9.25 mg/kg fuel (Spicer, Citation2009) or 32.4 mg/kg fuel (Kinsey, Citation2009). The T63, a helicopter engine, is very different than the engines from the F-15 or C-130 used in the two studies as it is older, and T63 testing was conducted on the engine in a laboratory, not while the engine was on the wing of an operational aircraft.
This technique could have been improved by sampling a higher volume of gaseous emission, either by increasing the flow rate through each tube, or sampling for longer times. However, the pressures of keeping short sampling times to reduce engine time-at-condition (especially in the field) were significant and larger samples from higher flow rates increased the amount of water coating the sorbents, as well as concerns about breakthrough. The conditions and methods chosen represented a reasonable, simple, and inexpensive way to obtain the aldehyde and hydrocarbon data. While methods of canister sampling have advantages in measuring polar hydrocarbons (which are not well measured in sorbent tubes), the simplicity and economy of sorbent tubes provide a reasonable alternative for many labs.
Conclusion and Recommendations
Two established methods, EPA Method TO-11A and NIOSH Method 1501, typically used for the sampling and analyses of hydrocarbon pollutants in ambient air monitoring, were evaluated for the potential to quantify HAP emissions in turbine engine aircraft exhaust. Results show that the EPA Method TO-11A (DNPH tubes) is suitable for measuring speciated aldehydes for the expected concentration ranges, except for acrolein and crotonaldehyde, which decompose following adsorption. Among the aldehydes, formaldehyde and acetaldehyde are well measured by this technique, as validated using gas-phase standards. This result is promising, as these two species have been reported to be present at high concentrations in turbine engine exhaust. Real-time monitoring of selected aldehydes, which can be performed using FTIR in conjunction with DNPH sampling, can provide an effective approach to verify the stability of engine operation and transfer line background before conducting composite DNPH tube sampling.
NIOSH Method 1501 (charcoal tubes) offers an effective technique for sampling and measuring organic volatiles in engine exhaust. A wide range of unburned hydrocarbons, including unreacted jet fuel and single-ring aromatics, can be characterized with a reasonable degree of accuracy using this methodology. Higher molecular weight hydrocarbon compounds (e.g., naphthalene) have lower extraction efficiencies than monoaromatics; however, the consistent extraction efficiencies provide the potential for accurately quantifying these types of species in engine exhaust. This method has limitations with regard to polar species such as phenol and styrene due to poor extraction from the charcoal matrix. Efforts are underway to characterize the sampling and solvent extraction efficiency of unburned fuel onto charcoal tubes, which will allow correlation to total UHC concentrations using a standard on-line FID.
Acknowledgment
The authors acknowledge the efforts of Joe Mantz (UDRI) for his support of experimental setup and testing, and Matt Wagner and Dean Brigalli of (AFRL/RQTM) for their technical support and operation of the T63 engine.
Funding
The efforts of UDRI were sponsored by the Air Force Research Laboratory under Cooperative Research Agreement FA8650-10-2-2934. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the Air Force Research Laboratory or the U.S. government.
Supplemental Materials
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/10962247.2014.991855.
Supplemental_Material.docx
Download MS Word (88 KB)Additional information
Funding
Notes on contributors
David Anneken
David Anneken and Christopher Klingshirn are research engineers, Richard Striebich is a senior research engineer, and Matthew J. DeWitt is a distinguished senior research engineer at the University of Dayton Research Institute (UDRI), Dayton, OH, USA.
Richard Striebich
Edwin Corporan is a senior research engineer at the Air Force Research Laboratory at Wright-Patterson Air Force Base, Dayton, OH, USA.
References
- Anderson, B.E., G. Chen, and D.R. Blake. 2006. Hydrocarbon emissions from a modern commercial airliner. Atmos. Environ. 40:3601–12. doi:10.1016/j.atmosenv.2005.09.072
- Cain, J., M.J. DeWitt, D. Blunck, D, Corporan, E., R.C. Striebich, D. Anneken, C. Klingshirn, W.M. Roquemore, and R. Vander Wal. 2013. Characterization of gaseous and particulate emissions from a turboshaft engine burning conventional, alternative, and surrogate fuels. Energy Fuels 27: 2290–302. doi:10.1021/ef400009c
- Corporan, E., R. Reich, O. Monroig, M. J. DeWitt, V. Larson, T. Aulich, M. Mann, and W. Seames. 2005. Pollutant emissions of a JP-8-fueled turbine engine. J. Air Waste Manage. Assoc. 55(7): 940−49. doi:10.1080/10473289.2005.10464680
- Corporan, E., M.J. DeWitt, C.D. Klingshirn, R. Striebich, and M.-D. Cheng. 2010. Emissions characteristics of military helicopter engines with JP-8 and Fischer–Tropsch fuels, J. Propulsion Power 26:317–24. doi:10.2514/1.43928
- Corporan, E., T. Edwards, L. Shafer, M.J. DeWitt, C. Klingshirn, S. Zabarnick, Z. West, R. Striebich, J. Graham, and J. Klein. 2011. Chemical, thermal stability, seal swell, and emissions studies of alternative jet fuels. Energy Fuels, 25:955–66. doi:10.1021/ef101520v
- Defense Logistics Agency. 2010. Petroleum Quality Information System (PQIS) annual report (2010). Fort Belvoir, VA. http://www.energy.dla.mil
- Federal Aviation Administration, 2005. Primer on emissions from aircraft engines. http://www.faa.gov/regulations_policies/policy_guidance/envir_policy/media/aeprimer.pdf
- Goelen, E., M. Lambrechts, and F. Geyskens. 1997. Sampling intercomparisons for aldehydes in simulated workplace air. Analyst 122:411–19. doi:10.1039/a607047g
- Ho, S.S.H., K.F. Ho, W.D Liu, S.C. Lee, W.T. Dai, J.J. Cao, and H.S.S Ip. 2011. Unsuitability of using the DNPH-coated solid sorbent cartridge for determination of airborne unsaturated carbonyls. Atmos. Environ. 45: 261–65. doi:10.1016/j.atmosenv.2010.09.042
- Kinsey, J.S. 2009. Characterization of emissions from commercial aircraft engines during the Aircraft Particle Emissions eXperiment (APEX) 1 to 3. EPA-600/R-09/130. http://nepis.epa.gov/Adobe/PDF/P1005KRK.pdf
- Kline, S.J., and F.A. McClintock. 1953. Describing uncertainties in single-sample experiments. Mech. Eng. 75:3–8.
- Knighton, W.B., S.C. Herndon, and R.C Miake-Lye. 2009. Aircraft engine speciated organic gases: speciation of unburned organic gases in aircraft exhaust. EPA-420-R-09-902. http://www.epa.gov/nonroad/aviation/420r09901
- Kurniawan, J.S., and S. Khard. 2011. Comparison of methodologies estimating emissions of aircraft pollutants, environmental impact assessment around airports. Environ. Impact Assess. Rev. 31:240–52. doi:10.1016/j.eiar.2010.09.001
- National Institute for Occupational Safety and Health. 2003. Method 1501. NIOSH Manual of Analytical Methods, 4th ed. http://www.cdc.gov/niosh/docs/2003-154/pdfs/1501.pdf
- Spicer, C.W., M.W Holdren, R.M. Riggin, and T.F. Lyon. 1994. Chemical composition and photochemical reactivity of exhaust from aircraft turbine engines. Ann. Geophys. 12(10/11): 944−55. doi:10.1007/s005850050116
- Spicer, C.W., M.W. Holdren, K.A. Cowen, J.D. Joseph, J. Satola, B. Goodwin, H. Mayfield, A. Laskin, L. Alexander, J.V. Ortega, M. Newburn, R. Kagann, and R. Hashmonay. 2009. Rapid measurement of emissions from military aircraft turbine engines by downstream extractive sampling of aircraft on the ground: Results for C-130 and F-15 aircraft. Atmos. Environ. 43:2612–22. doi:10.1016/j.atmosenv.2009.09.034
- URS Corporation. 2003. Select resources materials and annotated bibliography on the topic of hazardous air pollutants (HAPs) associated with aircraft, airports and aviation. DTFA 01-00-Y-01002. http://www.epa.gov/nscep/index.html.
- U.S. Environmental Protection Agency. 1990. Hazardous air pollutants list. http://www.epa.gov/ttnatw01/orig189.html
- U.S. Environmental Protection Agency. 1999a. Evaluation of air pollutant emissions from subsonic aircraft. EPA 420-R-99-013. http://www.epa.gov/oms/regs/nonroad/aviation /r99013.pdf
- U.S. Environmental Protection Agency. 1999b. Compendium Method TO-11a (1999). EPA/625/R-96/010b. http://www.epa.gov/ttnamti1/files/ambient/airtox/to-11ar.pdf
- U.S. Environmental Protection Agency. 2007. Plain English guide to the Clean Air Act. Publication EPA-456/K-07-001, Office of Air Quality. http://www.epa.gov/airquality/peg_caa/pdfs/peg.pdf
- U.S. Environmental Protection Agency. 2009. Recommended best practice for quantifying speciated organic gas emissions from aircraft equipped with turbofan, turbojet and turboprop engines. Federal Aviation Administration, U.S. EPA. www.epa.gov/nonroad/aviation/420r09901.pdf
- Wilson, G.R., J.T. Edwards, E. Corporan, and R.L. Freerks. 2013. Certification of alternative aviation fuels and blend components Energy Fuels 27: 962–66. doi:10.1021/ef301888b