Abstract
This work presents an overview over heterogeneous photocatalysis performed in gas phase towards the degradation of o-xylene, n-hexane, n-octane, n-decane, methylcyclohexane and 2,2,4-trimethylpentane. The experimental set-up composed by a titanium plug flow reactor vessel contained a quartz tube with a 100 W UV lamp placed at center position from 1.7 cm to the quartz wall. A titanium dioxide film was immobilized on the internal walls of the reactor and used as catalyst. All measurements were taken after reaching steady state condition and evaluated at the inlet and outlet of the system. Conversion rates were studied in a wide range of residence times yielding to a 90% or above conversion as from 20 seconds of residence time. During experiments the temperature of reactor’s wall was monitored and remained between 52 and 62 °C. Temperature influence over degradation rates was negligible once a control experiment performed at 15 °C did not modify outgoing results. Humidity effect was also evaluated showing an ideal working range of 10 - 80% with abrupt conversion decay outside the range. By varying inlet concentration between 60 and 110 ppmv the VOC degradation curves remained unchanged. Loss over catalytic activity was only observed for o-xylene after 30 minutes of reaction, the catalyst was reactivated with a solution of hydrogen peroxide and UV light followed by additional deposition of the catalytic layer. The kinetic study suggests a first order reaction rate.
Implications: The study of effective and economically viable techniques on the treatment of volatile organic compounds (VOCs) has being highlighted as an important parameter on the environmental research. The heterogeneous photocatalysis in gas phase was proved to be an effective process for the degradation of the nonaromatic VOCs tested, yielding high conversion values for the optimized systems.
Introduction
Volatile organic compounds (VOCs) are of particular concern in the issue of air pollution, mainly because in the presence of light they undergo photochemical reactions that form tropospheric ozone and photochemical smog. This term is used to describe the haze of contaminants resulting from the combination of smoke and fog (Albuquerque, Citation2007). Besides this indirect environmental damage, VOCs can also affect human health directly, since most of these compounds are toxic and many are carcinogenic, mutagenic, or teratogenic (International Agency for Research on Cancer [IARC], Citation2012; Tunsaringkarn et al., Citation2014).
The heterogeneous photocatalysis is an advanced oxidation process that has been extensively studied in cases of degradation of VOCs. This method employs a semiconductor material that receives photon energy coming from light irradiation. This energy, which must be higher than the energy band gap of the material, will cause a jump of electrons from the valence band to the conduction band. When an electron jumps, it leaves a positive vacancy (l+) in its place and many pairs (e−/l+) are formed. These species may recombine internally or migrate to the catalyst surface where they react with the adsorbed species and initiate the reactions of reduction and oxidation (Alberici, Citation1996).
Titanium dioxide (TiO2) is a white particulate dust widely used as pigment in paper, cosmetic, and paint industries. In this work, it was used as the catalyzer for heterogeneous photocatalysis because of its several advantages, such as confirmed photoactivity and stability, atoxicity, high surface area, and relatively low cost when compared with other possible semiconductor materials. TiO2 is found in nature in three allotropic forms: anatase, rutile, and brookita, anatase being the most photoactive phase and therefore more recommendable in catalytic application (Alberici, Citation1996).
Some advantages of the heterogeneous photocatalysis in relation to other VOC treatment techniques are low cost, possibility of application on a large variety of compounds, possibility of working at low temperatures requiring little energy, and achieving high rates of degradation. Besides, no addition of chemical oxidants is required; also, being a destructive technique, there is no need to have a posterior stage for waste disposal, since the process generates mainly CO2 and water (Alberici and Jardim, Citation1997).
An overview of heterogeneous photocatalysis phenomenon should include the study of some process variables that affect in different ways the occurrence and the efficiency of reactions. For example, as heterogeneous photocatalysis is activated by photons, it does not require additional energy and can occur at relatively low temperature. Therefore, researchers have suggested that temperature is not a major factor in the rate of degradation reactions for this kind of processes, since low inlet concentrations of organic compounds are used (Peral and Ollis, Citation1992; Kutsuna, et al., Citation1993; Alberici and Jardim, Citation1997). According to Hewer (Citation2006), at temperatures between 20 and 80 °C, the rate of degradation reactions does not depend on temperature due to the low adsorption energy for this range.
The humidity in the inlet is a very important factor for the reactions of heterogeneous photocatalysis in gas phase, since this is the main source of hydroxyl radicals. As these radicals are the primary oxidants of reactions and therefore are consumed during the process, it is necessary to maintain a minimum level of water vapor concentration so as to facilitate the photodegradation reactions. Higher humidity in the system could inhibit the reactions. This fact may be connected to a possible consumption of superoxide radicals (O2•−) by the water molecules in excess, to the competitive adsorption on the catalyst surface between the water vapor and other reagents or intermediate compounds, or even to an increase in the rate of recombination of the pair electron (−)/gap (+) caused by the reduction of light penetration due to the higher number of water molecules adsorbed (Alberici, Citation1996). Thus, the humidity range should be optimized for each specific case of heterogeneous photocatalysis.
For the influence of VOC inlet concentration, from the earliest studies in this field (Al-Ekabi and Serpone, Citation1988; Raupp and Dibble, Citation1990), it has been observed that for compounds with high inlet concentrations, there is a first-order dependence to the rate of degradation. However, for low inlet concentrations (less than 10,000 ppmv), the kinetics observed was zero order, which means there is no direct influence between these variables (Raupp and Junio, Citation1993; Alberici, Citation1996).
Regarding the catalytic deactivation of TiO2, this has been reported in some gas-phase studies, particularly when working with compounds having aromatic rings and nitrogen (Alberici, Citation1996; Sitkiewitz and Heller, Citation1996; Einaga and Futamura Ibusuki, Citation2004). These authors determined that the main factor that contributes to catalytic activity loss of TiO2 should be the formation of intermediate compounds in the surface that, when adsorbed by the catalyst, may affect the access to active sites by compounds of interest. This phenomenon is also called catalyst poisoning.
This work studied the heterogeneous photocatalysis of some VOCs with 6–10 carbons (n-hexane, n-octane, and n-decane), especially because there is a significant amount of these kinds of compounds in atmospheric emissions arising from the manipulation of organic volatiles such as solvents and fuels. Two closed-chain compounds were also tested (2,2,4-trimethylpentane methylcyclopentane) to observe some possible differences from the open-chain compounds. Besides these, an aromatic compound (o-xylene) was also qualitatively tested. n-Octane was chosen as the starting compound in order to determine the optimal conditions of the system such as humidity, inlet concentration, and temperature using a jacket in the reactor. This choice was due to n-octane being a straight-chain and low-toxicity compound.
The main objective of this work is to study the efficiency of heterogeneous photocatalysis in the degradation of the selected VOCs. The catalytic coating process with TiO2 and the kinetic behavior of the reaction will also be analyzed.
Experimental Methods
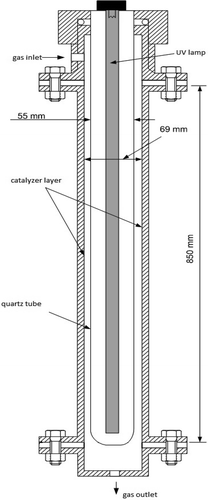
The tubular photoreactor used was constructed from a titanium cylinder with 6.9 cm internal diameter and 85 cm length. Inside this structure, a quartz cylinder of 5.5 outer diameter and 85 cm length was positioned so that the effective internal volume of the annular reactor was 1160 mL. In the center of the quartz cylinder, a 100-W ultraviolet (UV) lamp was installed 0.5 cm from the walls. In this setup, the flow of gases takes place in the annular region between the cylinders. The input is through the top of the photoreactor and the exit through the bottom (). The experimental system also contains rotameters and pressure-regulating valves. The inlet gas is composed of atmospheric air enriched with VOC gas and moisture. The VOC enrichment is obtained from the bubbling of the liquid phase. The moisture is to ensure the presence of hydroxyl radicals.
The concentration values in both input and output of the reactor are obtained from a continuous monitor of hydrocarbons using a flame ionization detector (Environnement SA, HC 51M, no. 159; Poissy, France). This equipment provides the concentration values of total hydrocarbons present in the sample, therefore measuring the complete mineralization of VOCs not allowing the chemical speciation. The minimum detection limit of the total hydrocarbon analyzer used is 0.05 and the maximum limit is 1000 ppmv; however, compounds with concentrations above 900 ppmv are not used. The calibration was performed with standard gas composed of 300 ppmv propane (891,045 Cylinder; White Martins Praxair Specialty Gases Inc., certificate no. 16749391; Campinas, SP, Brazil) before the beginning of the experiments and checked at each change of the VOC used. The supply gases for operation of the analyzer are synthetic air and hydrogen.
The temperature on the outside wall of the reactor was monitored with a thermocouple sensor adequately insulated from thermal exchange with the environment. Thus, possible heat losses due to conductivity through the walls of the reactor and convection through contact with room air were negligible. Measurements remained constant between 52 and 62 °C and these values were considered as the operating temperature of the system in each VOC tested. As the operating temperatures were relatively high (between 52 and 62 °C), a jacketing system was assembled using a heat bath for evaluation of the effects of temperature reduction on the photodegradation curves. Water was used as cooling fluid and only n-octane and n-decane were tested, as these compounds yielded conclusive results that may be extended to others. With the system jacketed, reactor temperatures were maintained stably at 15 °C during experiments.
The presence of humidity in input stream was obtained from measurements of the water mass passing the sample through a glass tube containing silica gel, the adequate water flow was achieved with a constant flow pump (model Ametek, BSS 100, serial number SN 0026900105; Largo, Florida, USA). The mass in grams of dry air present in the initial stream was determined by the ideal gas equation considering atmospheric pressure of 708.85 mm Hg for the city of Campinas (Centro de Previsão de Tempo e Estudos Climáticos [CPTEC], Citation2012) and the average temperature during the experiments, 26.5 °C or 300 K. The value of the ideal gas constant R was considered to be 62.364 L·mm Hg/K·mol, and the molar mass of each compound was in g/mol. This way, the relative humidity value was determined from the psychometric chart for the city of Campinas, with the dry bulb temperature (considered as the average temperature measured for each experiment) and the ratio between mass of steam water and mass of dry air being used as input data.
An immobilized catalyzer was used on the inner walls of the reactor, since studies have shown that this method offers greater degradation efficiency and less by-product generation when compared, for example, with reactors with the solid catalyst in suspension (Alberici, Citation1996). To impregnate the surface a solution containing 10 g of commercial TiO2 (Degussa P25 lot no. 86/2009; Tokyo, Japan) in 250 mL of water and 250 mL of ethanol (Chemco Anhydrous 99.3° INPM, lot no. 22363/2010; Hortolândia, SP, Brazil) was used. According to the manufacturer, the catalyzer has a surface area of 50 m2/g, average particle diameter of 20 nm, and structural composition of 80% rutile and 20% anatase. The solution was applied on the walls of the reactor in several layers, each application being followed by hot air drying. The surface formed was visually uniform, well distributed, and adhered to the inner surface of the photoreactor ().
To examine the kinetics of the VOC catalytic photodegradation reaction, three different kinetic models were investigated: first-order, second-order, and Langmuir-Hinshelwood adsorption model. The experimental data collected were applied to these models, and the quality of their matching was analyzed.
The system was considered as a plug flow reactor (PFR), to which the fluid composition varies from point to point along the flow. The mass balance of this reactor can be made for a volume differential element (dV) as a function of a time differential element (dt), according to eq 1 below, where Q is the flow rate of the gaseous stream, C is the substance concentration, and dC is a concentration differential element:
For an irreversible monomolecular reaction of first order, considering that it occurs at constant volume, the rate of consumption of a substance (rA) is given by eq 2 bellow, where K′ is the first-order constant:
It is possible to use the mass-balance equation for this first-order rate and integrate the input (C0) and output (C) concentration limits and the volume from initial (zero) at the end (the total volume of the reactor), reaching eq 3 below. This equation considers the variable V/Q, which is called residence time in the reactor (τ) and is measured in seconds.
In this work, experiments were performed varying the flow rate and consequently residence time, allowing the study of the kinetic reaction behavior. A first-order kinetic model was fitted to experimental data through a curve ln(C/C0) versus τ, and the first-order constant K′ was obtained from the angular coefficient of the line.
For an irreversible monomolecular reaction of second order, considering that it occurs at constant volume, the rate of consumption of a substance (rA) is given by eq 4 below, where K″ is the second-order constant:
Similarly to what was done in the first-order model, it is possible to use the mass-balance equation for this second-order rate and integrate the concentration and volume limits, reaching eq 5 below.
In this work, the second-order kinetic model was fitted to experimental data collected through a curve (1/C − 1/C0) versus τ, and the value of second-order constant K″ was obtained from the slope.
Besides the analysis of kinetic models, an application of Arrhenius equation model was also performed; this equation suggests that an increase in temperature of the reaction should cause a proportional increase in the reaction rate constant. It can be seen in eqs 6 and 7, where A represents a constant preexponential, Ea the activation energy for the reaction to occur, R the ideal gas constant, and T the ideal operating temperature.
Results and Discussion
Influence of humidity
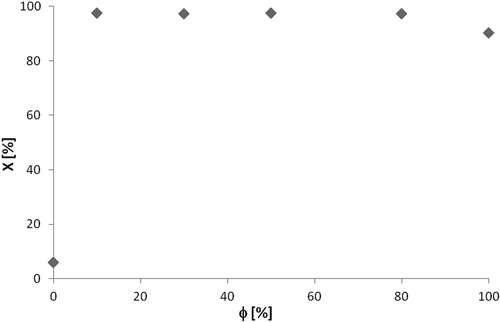
The presence of hydroxyl radicals is essential for the photocatalytic reaction; they are regarded as the primary oxidant for degradation. For n-octane, the photodegradation was measured over a wide range of relative humidity; these results for average inlet concentration of 108 ppmv, residence time of 23 sec, and an average temperature of 56 °C can be observed in .
For the experiment under dry conditions, that is, in the absence of humidity, there was a low VOC conversion, about 6%, most probably due to the absence of hydroxyl radicals. For the situation of greater humidity close to 100%, there was a slight reduction in conversion value, reaching approximately 90%. This result is in agreement with the literature, which shows that the rate of degradation is considerably reduced for flows with high values of humidity (Dibble and Raupp, Citation1992; Alberici, Citation1996). From these results, it was considered that for the photodegradation of n-octane, conversion values are not affected by the variation of relative humidity of the system, since it is kept within a range of safety values between 10% and 80%.
Thus, for all other compounds tested, humidity supplied in the input stream was maintained between 25% and 40% saturation by the techniques of molecular diffusion or bubbling. This humidity range is close to that typically found in indoor atmosphere, which is between 20% and 60% relative humidity.
Inlet concentration influence
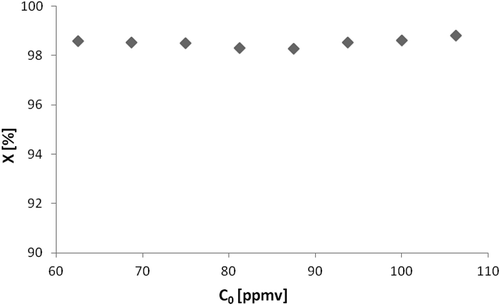
The influence of the inlet concentration of VOCs was studied in the degradation of n-octane, using between 60 and 110 ppmv in the input stream, with fixed residence time of 24 sec, 40% of relative humidity, and temperature of 54 °C. shows the conversion values obtained, demonstrating that, for the range studied, the inlet concentration does not affect the conversion. This result is consistent with the literature, in the case of low values of inlet concentration, it is expected that there is little or no influence of this parameter on the conversion of photodegradation (Dibble and Raupp, Citation1990; Alberici and Jardim, Citation1997; Jo, Citation2013).
Catalytic deactivation
In the photodegradation of o-xylene, a very low conversion for the first three points collected was observed. From the fourth point, the values of concentration collected at the inlet and outlet were about the same, indicating that the degradation was not occurring anymore. This test confirmed the occurrence of the deactivation for o-xylene, as already reported by other authors (Peral and Ollis, Citation1992; Alberici, Citation1996; Zhonga et al., Citation2009). The results of photodegradation of o-xylene to average inlet concentration of 101 ppmv, relative humidity 40%, and temperature of 56 °C can be seen in .
In this case, a catalytic deactivation of titanium dioxide occurred and interrupted the continuity of the degradation reaction, as foreseen in the literature for aromatic compounds. Small, yellowish-brown crystals were observed in the inner walls of the reactor after the test with o-xylene. These crystals have already been reported in the literature (Fu et al., Citation1995; Alberici, Citation1996; Zhonga et al., Citation2009). It is assumed that possible intermediate species are formed and are adsorbed by catalyst surface competing with hydroxyl radicals, blocking the active sites and interrupting the continuity of the reaction. Some of the researchers who reported such phenomenon attributed this effect to the possible formation of toluene, generated as a probable intermediate compound that may be responsible for poisoning the catalyst.
In this work, catalytic deactivation was observed only for o-xylene, not for any other nonaromatic compounds studied. Some of the techniques suggested by the literature to regenerate the TiO2 photocatalytic activity were tested, including UV illumination of the catalyst with pure air flow (decontaminated and without humidity) and humid air flow in the absence of illumination; however, none of them resulted in the desired effects even having been applied for a period of more than 15 consecutive hours. The catalyst was reactivated only upon insertion of a hydrogen peroxide liquid solution in the presence of UV light for a few hours. The disappearance of the yellowish crystals was observed, and the reactivation of catalyzer was accomplished. Even so, it was decided to reapply the TiO2 layer with subsequent drying with compressed air to ensure that the resulting film catalyst was the same used in the experiments performed before and after the test with o-xylene.
Kinetic models analysis
As mentioned, the kinetics of the VOC catalytic photodegradation reaction was examined by applying the experimental data collected to first-order kinetic model, second-order kinetic model, and Langmuir-Hinshelwood adsorption model.
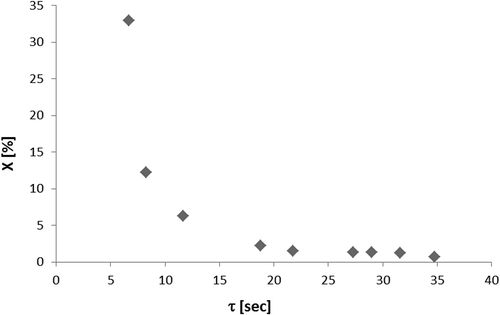
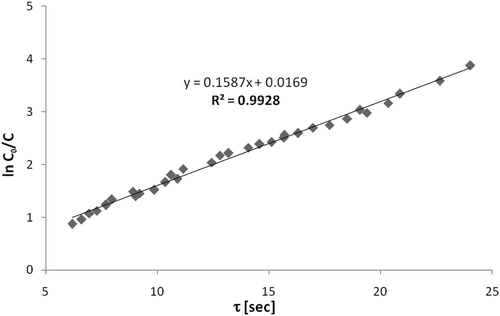
First-order model. To investigate the first-order kinetic model, the experiments were performed varying residence time, and the model was fitted to experimental data through a curve ln(C/C0) versus τ. The first-order constant K′ was obtained by the angular coefficient of the line. shows this curve obtained for the photodegradation of n-octane with inlet concentration of 103 ppmv average, relative humidity of 27%, and an average temperature of 56 °C. Similarly, the first-order kinetic model was also tested for another studied VOC, except for o-xylene, which suffered catalytic deactivation.
shows the minimum and maximum values of inlet concentration for each compound (C0min, C0max; ppmv), relative humidity (ϕ; %), and temperature (T; °C) used for the experiments of VOC photodegradation and the data obtained for the linear adjustments of the first-order kinetic model, which are the coefficients of determination (R2), the first-order kinetic constants (K′; sec−1) obtained from the slopes of the adjustments, and the linear coefficients (b). The high values of R2 obtained (0.9908–0.9991) show that the first-order kinetic model represents well the system of photodegradation reactions for the studied VOCs. The first-order kinetic constants are also in agreement with the values found in the literature for similar hydrocarbons (Alberici, Citation1996; Vorontsov et al., Citation1997).
Table 1. Data for linear adjustments of first-order kinetic model
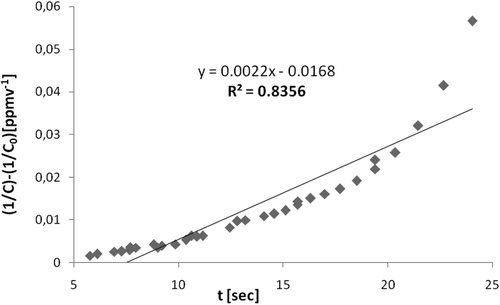
Second-order model. To investigate second-order kinetic model, experimental data collected were fitted to the model through a curve (1/C − 1/C0) versus τ. The value of second-order constant K″ was obtained from the slope. shows this curve obtained in the photodegradation of n-octane with inlet concentration of 103 ppmv average, relative humidity of 27%, and an average temperature of 56 °C.
The other tested VOCs presented the same curve behavior compared with n-octane, and the adjustments of the curves obtained with a linear trend were low (R2 of 0.9249 for n-decane, 0.4679 for n-hexane, 0.9271 for methylcyclohexane, and 0.9526 for 2,2,4-trimethypentane), especially when compared with those obtained for the first-order kinetic models. This demonstrates that the second-order kinetic model is not adequate to represent the system with the experimental data in any of VOC tested; thus, the values of the second-order constant K″ (which could be obtained from the angular coefficient) were not calculated.
Influence of temperature
As previously mentioned, the literature reports no significant effects of temperature on degradation rate of VOCs for experiments performed at elevated temperatures. In this study, the effect of temperature on degradation rate of n-octane and n-decane was analyzed using the jacketed reactor maintained at 15 °C with heat bath. The results obtained at 15 °C were compared with those reported earlier (not jacketed reactor) with an average temperature of 56 °C for n-octane and 52 °C for n-decane. The first-order kinetic model, which proved to be the best suited for the system, was also fitted to data obtained at 15 °C, and shows a comparison of the compounds studied at different temperatures.
Table 2. First-order data at different temperatures: Comparison for photodegradation of n-octane and n-decane
For both VOCs tested, it is possible to observe that the first-order kinetic model represented well the experimental data, R2 values presented good linear adjustments to the curves of the experiments without temperature control (56 °C for n-octane and 52 °C for n-decane) or the jacketed reactor (temperature maintained at 15 °C), and calculation of first-order constants resulted in quite similar values regardless temperatures. These results suggest that temperature variation does not significantly influence the photodegradation of the VOCs tested under the experimental conditions studied. However, it is known that for reactions that conform to a purely kinetic model, characterized, for example, by first-order kinetic constant K′, the rate of reaction is dependent on the temperature variation. According to the Arrhenius equation, an increase in temperature of the reaction should cause a proportional increase in the reaction rate constant.
allows viewing that for both studied VOCs, the rise in temperature results in a slight increase in the first-order constant, although this effect is minimal. For n-octane with a 41 °C increase in temperature (T from 15 to 56 °C), an increase of only 8% in the first-order constant (K′ from 0.1465 to 0.1594 sec−1) was found; and for n-decane with a 37 °C increase in temperature (T from 15 to 52 °C), the constant was increased by only 11.5% (K′ from 0.0193 to 0.0218 sec−1).
This minimum effect of temperature in relation to the kinetics may be due to its low energy activation. It is known that reactions involving highly reactive species such as hydroxyl radicals in this case have such low activation energy that temperature causes no significant influence in the reaction rate (Levenspiel, Citation1966). Using the values of the first-order constants for the two temperatures used () and the ideal gas constant R = 8.314472 J/K·mol, it was possible to estimate activation energy and preexponential constant from eq 6; values obtained for n-octane were Ea = 1.62 kJ/mol and A = 0.288, and for n-decane were Ea = 2.56 kJ/mol and A = 0.056. Although no experiments have been carried out at a third temperature, which would allow the obtaining of a curve for analyzing the behavior of Ea due to the variation of T, it is possible to observe through the magnitude range (Ea = 1–3 kJ/mol) that in the studied reactions the activation energy found is relatively low.
Another possibility to explain the weak dependence of kinetic reaction on temperature is that the mass transfer can considerably influence the system, affecting the calculation of reaction rate constant. One way to evaluate the effects of mass transfer would be increase turbulence in the system, for example, by doubling the reactor volume and the flow of current, thus maintaining the same residence time and allowing a comparison between results obtained in both situations. However, this research was not the focus of this paper.
Conclusions
Heterogeneous photocatalysis in gas phase with TiO2/UV proved to be a viable and highly efficient way to photodegradate the VOCs tested (n-hexane, n-octane, n-decane, methylcyclohexane, and 2,2,4-trimethylpentane), obtaining high conversion rates for the system with optimized operational conditions considering the minimum detection limit of the analyzer hydrocarbons (0.1 ppmv).
For dry conditions and atmospheres with more than 90% relative humidity, the degradation of compounds is compromised. Therefore, the relative humidity should be maintained within a safe range, which does not affect the process, this being between 10% and 80%. Relative humidity range is close to the range typically found in indoor atmospheres, which is about 20–60%. Influence of the temperature reduction was evaluated for n-octane and n-decane, not yielding, however, significant impact on the results of rates of photodegradation. It is concluded that the temperature influence is irrelevant within the studied range, that is, between 15 and 62 °C. Influence of VOC inlet concentration was studied using a range between 60 and 110 ppmv concentration of VOC in inlet; for this range, inlet concentration did not affect conversion rates. Working with low inlet concentrations, these results are consistent with those published in the literature.
In the experiments performed, catalytic deactivation was observed only for o-xylene, possibly due to an aromatic compound already reported in the literature. TiO2 reactivation was achieved by the insertion of a hydrogen peroxide liquid solution in the presence of UV light remaining in the system for some hours, and reapplication of the catalyst layer with subsequent drying with compressed air. For the other compounds, there was no deactivation problem.
The kinetic study has shown good fits to the mathematical first-order kinetic model; for all tested VOCs, it was considered that this model adequately represents the kinetics of degradation and complete oxidation of organic compounds studied. Second-order kinetic model did not fit well with any of the VOCs studied in this work.
For all tested VOCs, with 20 sec of average residence time the photodegradation presented conversion of around 90%. This result puts this alternative treatment of gaseous pollutants as feasible for application. It is assumed that the combined use of TiO2 with other metal catalysts should improve the process, making it especially useful in controlling emissions of VOC currents at temperatures around ambient and low concentrations. These widely found conditions in industries, such as vents in atmospheric tanks and vessels, or even releases from process through forced-flow ducts, may utilize this technology, which does not require high temperatures as in the case of thermal or catalytic oxidizer. On the other hand, the phase-transfer processes as in the case of adsorption, which could be implemented effectively to the same applications, require the need for further treatment of solid adsorbents.
Further studies like this and the transition from laboratory scale to industrial scale will enable the development of devices based on heterogeneous photocatalysis that may have immediate application in the world. One of these applications is in the degradation of VOCs arising from vents of storage tanks containing solvents, fuel, and all kinds of VOCs in general. This may be extended to any sorts of vents in industrial process equipment such as reactors, columns, vases, and others. These vents are very common and account for most part of industrial VOC emissions. Another important application may occur in the treatment and control of odors with the advantage of not generating nitrogen compounds (NOx) as it happens in existing thermal treatments for decontamination of such malodorous atmospheres. Moreover, this oxidative process works at relatively low temperatures, close to room temperature; in this way, devices based on heterogeneous photocatalysis do not need flame for VOC oxidation, this will allow it to be installed safely even in areas vulnerable to fire and explosion.
Additional information
Notes on contributors
Ursula Luana Rochetto
Ursula Luana Rochetto has a BS and MS in chemical engineering with specialization in environmental engineering from the University of Campinas, Campinas SP, Brazil.
Edson Tomaz
Edson Tomaz is a researcher at the Department of Process Engineering, head of the LPDTA, at the University of Campinas, Campinas SP, Brazil.
References
- Alberici, R.M. 1996. Destruction of volatile organic compounds in the gas phase by heterogeneous photocatalysis. Doctorate thesis, Universidade Estadual de Campinas, Campinas, Brazil.
- Alberici, R.M., and W.F. Jardim. 1997. Photocatalytic destruction of VOCs in the gas-phase using titanium dioxide. Appl. Catal. B Environ. 14:55–68. doi:10.1016/S0926-3373(97)00012-X
- Al-Ekabi, J., and N. Serpone. 1988. Kinect studies in heterogeneous photocatalysis—Photocatalytic degradation of chlorinated phenols in aerated aqueous solutions over TiO2 supported on glass matrix. J. Phys. Chem. 92:5726–5731. doi:10.1021/j100331a036
- Albuquerque, E.L. 2007. Volatile organic compounds in the urban atmosphere of the metropolitan region of São Paulo. Thesis for Doctoral Degree in Chemistry, Universidade Estadual de Campinas, Campinas, Brazil.
- Centro de Previsão de Tempo e Estudos Climáticos (CPTEC), Brazil. 2012. INPE—Instituto Nacional de Pesquisas Espaciais. http://www.cptec.inpe.br/ (accessed November 2012).
- Dibble, L.A., and G.B. Raupp. 1990. Kinetics of gas-solid heterogeneous photocatalytic oxidation of trichloroethylene by near UV illuminated titanium dioxide. Catal. Lett. 4:345–354. doi:10.1021/j100331a036
- Dibble, L.A., and G.B. Raupp. 1992. Fluidized-bed photocatalytic oxidation of trichloroethylene in contaminated airstreams. Environ. Sci. Technol. 26:492–495. doi:10.1021/es00027a006
- Einaga, H.,T. Ibusuki, and S. Futamura. 2004. Improvement of catalyst durability by deposition of Rh on TiO2 in photooxidation of aromatic compounds. Environ. Sci. Technol. 38:285–289. doi:10.1021/es034336v
- Fu, X., W.A. Zeltner, and M.A. Anderson. 1995. The gas-phase photocatalytic mineralization of benzene on porous titania-based catalysts. Appl. Catal. 6:209–224. doi:10.1016/0926-3373(95)00017-8
- Hewer, T.L.R. 2006. Synthesis and surface modification of TiO2 to increase efficiency of the heterogeneous photocatalysis process in the treatment of phenolic compounds. Dissertation for Masters Degree in Chemistry, Universidade de São Paulo, São Paulo, Brazil.
- International Agency for Research on Cancer (IARC). 2012. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 100F, Chemical Agents and Related Occupations. Lyon, France: IARC.
- Jo, W. 2013. Coupling of titania with multiwall carbon nanotubes for decomposition of gas-phase pollutants under simulated indoor conditions. J. Air Waste Manage. Assoc. 63:963–970. doi:10.1080/10962247.2013.801931
- Kutsuna, S., Y. Ebihara, K. Nakamura and T. Ibusuki. 1993. Heterogeneous photochemical reactions between volatile chlorinated hydrocarbons and titanium dioxide. Atmos. Environ. 27A:599–604. doi:10.1016/0960-1686(93)90217-M
- Levenspiel, O. 1966. Chemical Reaction Engineering—An Introduction to the Design of Chemical Reactors, 2nd ed. New York: John Wiley & Sons.
- Peral, J., and D.F. Ollis. 1992. Heterogeneous photocatalytic oxidation of gas-phase organics for air purification. J. Catal. 136:554–565. doi:10.1016/0021-9517(92)90085-V
- Raupp, G.B., and C.T. Junio. 1993. Photocatalytic oxidation of oxygenated air toxics. Appl. Surf. Sci. 72:321–327. doi:10.1016/0169-4332(93)90369-M
- Sitkiewitz, S., and A. Heller. 1996. Photocatalytic oxidation of benzene and stearic acid on sol-gel derived TiO2 thin films attached to glass. New J. Chem. 20:233–241.
- Tunsaringkarn, T., T. Prueksasit, S. Sematong, W. Siriwong, N. Kanjanasiranont, K. Zapuang, D. Morknoy, and A. Rungsiyothin, A. 2014. Volatile organic compounds exposure and health risks among street venders in urban area, Bangkok. J. Environ. Occup. Sci. 3:31–38. doi:10.5455/jeos.
- Vorontsov, A.V., E.N. Savinov, G.B. Barannik, V.N. Troitsky, and V.N. Parmon. 1997. Quantitative studies on the heterogeneous gas-phase photooxidation of CO and simple VOCs by air over TiO2. Catal. Today 39:207–218. doi:10.1016/S0920-5861(97)00102-8
- Zhonga, J.B., Y. Lua, W.D. Jianga, Q.M. Menga, X.Y. Hea, J.Z. Li, and Y.Q. Chen. 2009. Characterization and photocatalytic property of Pd/TiO2 with the oxidation of gaseous benzene. J. Hazard. Mater. 168:1632–1635. doi:10.1016/j.jhazmat.2009.02.158