Abstract
During the months of October to November, many important festivals are celebrated in India. Celebration of these festivals are marked by extensive use of fireworks or pyrotechnics, bonfire, incense burning, open air community cooking, and temporary eateries using crude fuel such as coal, wood, kerosene, cow dung, burning of raw/semiwood, and coconut shells. The present study deals with the influence of these unregulated anthropogenic activities on ambient mixing level of volatile organic compounds (VOCs), especially some carbonyl compounds. The study was undertaken in the metropolitan city of Kolkata, India, with very high population density, which is even higher during festival period. The average total carbonyl level at different sites in Kolkata varied from 134.8 to 516.5 μg m−3 in pre-festival season, whereas in post-festival season the same varied from 252.2 to 589.3 μg m−3. Formaldehyde to acetaldehyde ratio altered from 0.62 in pre-festival season to 1.78 in post-festival season. Diurnal variation also altered, indicating variation in source composition of carbonyls. The total ozone forming potential calculated for all 14 carbonyls in pre-festival season increased by 35% in post-festival season. The effect of anthropogenic activities typical to the event of Diwali night characterized by intense execution of pyrotechnics resulted in significantly high level of carbonyl VOCs. Principal component analysis study for the event of Diwali shows clear contribution of the event on certain carbonyl VOCs. The results indicate elevated primary emissions of these pollutants and also their effect on formation of secondary pollutants. The study emphasizes the need of generating awareness among the communities in society as well as need for regulations to minimize the emissions and related hazards to the extent possible.
Implications: Altered anthropogenic activities typical of festival season including extensive use of pyrotechnics affect ambient level of volatile organic compounds, especially some carbonyls. Such activities have considerable effect on interspecies ratio and diurnal variation. They also affect formation of secondary pollutants such as tropospheric ozone. Principal component analysis (PCA) study shows clear contribution of the pyrotechnics execution on certain carbonyl VOCs. The findings emphasize the need of generating awareness in society and need for regulations to minimize the emissions.
Introduction
An almost month-long festival season is celebrated every year during October to November in India. The festival season in Kolkata is unique in many respects. More than 2000 temporary structures made of bamboo and cloth were set up within the Kolkata metropolitan city alone in 2009. The footfall in Kolkata in this season is increased noticeably due to people coming from suburbs and outskirts of Kolkata. A very common sight of any Kolkata street in this period is temporary eateries, serving different kinds of foods, mostly fried, to thousands of people, in open air. In one hand, some activities are increased, such as temporary stalls are established for food and other merchandises. Many of them use crude biomass fuel such as coal, wood, twigs, dry leaves and branches, cardboards, and cow dung for open burning. Food is prepared and eaten together at community level in temporary structures that involve burning of coal, wood, and straw in the open much more than usual. Activities in some small-scale industries related to the festival events are increased, such as firework production, garment, and leather industries. Above all in this period, various kinds of fireworks are played during the night. Especially on the day of “Diwali,” large quantities of fireworks or pyrotechnics are displayed in one night. On the other hand, some other activities are decreased, e.g., the vehicular movement is markedly reduced in this period mainly due to long holidays in schools, colleges, and offices. Also, the small-scale industries operating within the city are closed for a considerable time within this period, and even when the units are operating, they are not working in the full capacity due to many workers visiting home. All these activities are likely to alter the anthropogenic emissions, resulting in mixing ratios of air pollutants different than usual. Studies indicated that burning of crackers and sparkles is a very strong source of air pollution (Mandal et al., Citation1997). Releases of common air pollutants, such as sulfur dioxide (SO2), carbon dioxide (CO2), carbon monoxide (CO), and suspended particles including PM10 (particulate matter with an aerodynamic diameter <10 μm) from use of pyrotechnics are well reported. Concentration of SO2 was observed to be increased approximately 10-fold, whereas the concentrations of nitrogen dioxide (NO2) and PM10 increased 2–3 times, compared with a typical winter day in December 1999 during Diwali festival in Hisar City, India (Ravindra et al., Citation2003). High level of metals in air was reported by Kulshrestha et al. (Citation2004) on the occasion of Diwali fireworks display, with concentrations of Ba, K, Al, and Sr went up to 1091, 25, 18, and 15 times higher than the previous day of Diwali. Pyrotechnic displays also affect atmospheric PM2.5 mass concentration. The majority of this mass was composed of K and S, which originated from the combustion of black powder (Perry, Citation1999). Fleischer et al. (Citation1999) reported persistence organic pollutants emission in fireworks. Recently Yerramsetti et al. (Citation2013) studied the influence of Diwali fireworks episodes on urban air quality of Hyderabad City, India. They reported high level of nighttime ozone in the festival period, as well as 2–3-fold increase in the level of nitrogen oxides (NOx) and black carbon (BC) on Diwali day compared with control days. Similar observation with elevated concentrations of ozone (O3), NO2, nitric oxide (NO), and PM10 was reported by Nishanth et al. (Citation2012) during the intense usage of fireworks for Vishu Festival at Kannur, a semiurban costal region in India. One of their observations was nighttime production of O3 by the photo dissociation of NO2 from the flash of firecrackers, and the concentrations of NO2 and PM10 increased by 100%. Organic air pollutants, especially carbonyl compounds, are known emission products of fireworks burning. Drewnick et al. (Citation2006) reported high level of oxygenated organic aerosol associated with fireworks burning during New Year celebration in Germany. Croteau et al. (Citation2010) reported the emission factor of formaldehyde (<7.0–82 mg kg−1), acetaldehyde (43–210 mg kg−1), and acrolein (1.9–12 mg kg−1). However, effects of pyrotechnics or fireworks on volatile organic air pollutants are comparatively less studied.
Other activities, especially of this period, such as incense burning, open air cooking, and bonfires, are also significant sources of organic pollutants (Lee and Wang, Citation2004; Lemieux et al., Citation2004; Wang et al., Citation2007; Estrellan and Iino, Citation2010). Mixing ratios of volatile organic compounds (VOCs) including carbonyls are important factors in formation of ozone and other photochemical oxidants leading to urban smog. VOCs have been identified as toxic, carcinogenic, or mutagenic at concentrations levels found in urban environment since long ago (Edgerton et al., Citation1989; Kerns et al., Citation1983). It is worthwhile to study the effect of various activities during festival season on the mixing ratios of organic air pollutants.
In the present study, we intended to observe the immediate and short-term effects of various activities performed during the festival season on the ambient mixing ratios of a few volatile organic pollutants, especially some oxygenated compounds (carbonyls). The study was conducted during the festival season of 2009, 25 September to 20 October, and immediately before the season. We also monitored the event of “Diwali,” characterized by extensive fireworks display, in the year 2009.
Materials and Methods
Study area
Kolkata (22°32′N, 88°22′E) is the second largest metropolis in south Asia. The city is bounded to the west and northwest by the Hugli River spread along 80 km, which divides it from Howrah on the western bank. The city has a tropical climate with a distinct monsoon season. Average relative humidity (RH) is 66% and 69% in winter and summer, respectively. Mean monthly temperature ranges from 20 to 31 °C, with average wind speed of 7 km/hr blowing throughout the year.
Study design
The study was done in two parts.
Extensive study. Monitoring program was conducted for 2 weeks pre-festival season and for 2 weeks post-festival season for understanding the short-term effects of unregulated activities characteristics to the festival season. Variations in ambient level of oxygenated VOCs, i.e., carbonyls were monitored from 7 to 20 September 2009 (pre-festival season). The same monitoring was repeated from 19 October to 31 October 2009 (post-festival season). This extensive study was done in nine different sites in Kolkata, once a week at each site. Samples were collected for 24 hr; starting from 12:00 noon in the first day continued to next day till 12:00 noon (divided into three shifts: “Afternoon shift”: 12:00 noon to 6:00 p.m.; “Night shift”: 6:00 p.m. to 6:00 a.m.; “Morning shift”: 6:00 a.m. to 12:00 noon) at each location every week. The shifts were selected based on absence (night) or presence (morning and afternoon) of solar influx influencing photochemical reactions of the reactive volatile target species (n = 6 for each of nine sites in both pre-season and post-season; altogether n = 108 for the extensive study).
Intensive study. Monitoring program was taken up on 16–17 October 2009 at night. This study covered the major fireworks event of Diwali on 17 October in nine locations in Kolkata Metropolitan Corporation (KMC) area for observing the immediate effect of fireworks. Four hourly grab samples were taken (two parallel samples in each location) on the night of Diwali and on the previous night (n = 36) between 6:00 p.m. to 12:00 a.m. simultaneously at all the nine locations.
Sampling sites
The sites for both extensive and intensive studies are shown in .
For Kolkata, being a cosmopolitan metro city, no clear demarcation is possible based on activities of any area within the city. However, some activities are normally predominant in a particular area, whereas other activities are predominant in other location. For the extensive study, three types of locations were chosen based on predominant activity, viz., residential sites, industrial sites, and site in petrol pump vicinity. One location for each type of sites was chosen in North, Central, and South Kolkata to represent the overall city atmosphere. The descriptions of predominant activities in all nine locations around the sampling sites for extensive study are given in . The sites for extensive monitoring were chosen considering the various activities in the neighborhood, as this study aimed to monitor the effect of festival season activities on target pollutants.
Table 1. Description of the sites: Extensive study
On the other hand, the intensive study sites were selected considering the fireworks events, as the aim was to study the immediate effect of the fireworks. Nine sites distributed over the city of Kolkata were selected for grab sampling. For this purpose, sites with predominant residential activity were selected. Also, all the sites were located within 200 m radius of a major fireworks event. It is important to note that during the day of “Diwali,” celebration the usual activities of the sites such as traffic, industrial, or commercial activities were reduced significantly.
Estimation of carbonyl and noncarbonyl VOCs
A low-volume air sampler (Polltech model PEM-LVAS, Mumbai, India) was used to sample air at 60 mL/min. For noncarbonyl VOCs, the analysis was done by thermal desorption followed by detection on gas chromatography–mass spectrometry (GC-MS) in accordance with U.S. Environmental Protection Agency (EPA) compendium method TO-17 (EPA, Citation1997). The adopted method for determination of target VOCs is described in details in the authors’ previous publications (Central Pollution Control Board [CPCB], Citation2010; Majumdar and Srivastava, Citation2012; Majumdar et al., Citation2014). The target compounds were quantified using an external five-point calibration curve with VOCMIX 15 (Dr. Ehrenstrofer, Germany).
For carbonyl VOC compounds, air was sampled using Supelco model LpDNPH cartridge using similar pump at a flow rate of 80 mL min−1. Sampling and analysis have been carried out following EPA compendium method TO 11 (EPA, Citation1999) using reverse-phase high-performance liquid chromatography (HPLC) with ultraviolet (UV) detector (Dionex ICS3000; California) and details of the analytical and quality assurance/quality control (QA/QC) procedures followed is described in authors’ previous publication (Majumdar et al., Citation2014). The compounds were quantified against a five-point external calibration curve prepared using TO11/IP6A Carbonyl-DNPH Mix (Supelco). Altogether 14 carbonyl compounds have been identified and quantified in this study.
Result and Discussion
Extensive study
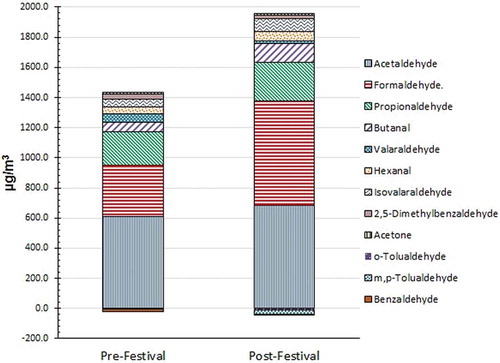
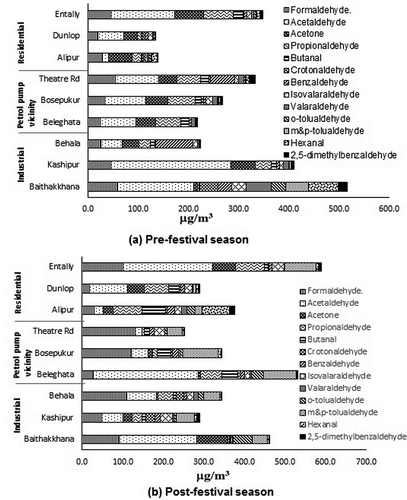
All 14 carbonyls, namely, formaldehyde, acetaldehyde, acetone, propionaldehyde, butanal, crotonaldehyde, benzaldehyde, isovalaraldehyde, valaraldehyde, o-, m&p-tolualdehyde, hexanal, and 2,5-dimethylbenzaldehyde were identified in most of the samples in both pre- and post-festival season. depict the Tcarb level (sum of 14 carbonyl VOCs quantified) as daily average of three shifts as observed in all nine sites. Contribution of each species towards total carbonyl level in both pre- and post-festival season can be also comprehended from . The levels of carbonyl obtained in pre-festival period were comparable to a previous study done in Kolkata City in three different sites during summer season by Dutta et al. (Citation2009). Site-wise mean formaldehyde level observed in the present study in pre-festival season was in the range of 20.0–59.0 μg m−3 in comparison with 14.07–26.12 μg m−3 reported by Dutta et al. (Citation2009). Acetaldehyde level in this study (site-wise mean ranging from 12.2 to 151.1 μg m−3), however, was somewhat higher than reported in the previous study (7.60–18.67 μg m−3). High mean acetaldehyde level of 151.1 μg m−3 was in fact observed at an Industrial site, Baithakkhana, indicating industrial usage.
Figure 2. The variation in contribution of different carbonyl VOC species towards total ambient concentration in (a) pre-festival season and (b) post-festival season.
The site-wise average Tcarb level varied from 134.8 μg m−3 in Dunlop, residential site, to 516.5 μg m−3 in Baithakkhana, industrial site in pre-festival season. In post-festival season, the site-wise average varied from 252.2 μg m−3 in Theatre Road, petrol pump vicinity, to 589.3 μg m−3 in Entally, residential site. The Tcarb level was increased in post-festival season for most cases except in three sites: petrol pump vicinity (Theatre Road) and industrial (Baithakkhana and Kashipur) categories. Analysis of variance (ANOVA) test (F = 2.89, P = 1.08) shows that the levels of Tcarb are not significantly different. The increase in Tcarb level varied from 27% in Bosepukur to 170% in Alipur from pre- to post-festival season. Among the individual components, tolualdehyde level was most affected. From pre- to post-festival season, m- and p-tolualdehyde levels increased 62 and 36 times, respectively, on an average across all sites. The other components increased around 1–5 times.
During the festival season, primary emission of carbonyls is reduced due to reduced number of cars in the road, decreased refueling activity, and reduced activity in industries. Again, open burning of crude fuel and increased activity in some small-scale industries increase the primary emission of the carbonyls. So Tcarb levels observed in these sites represent the mixed effect of change in regular activity and unregulated activities of this season; as a result, no clear trend is observed. However, Tcarb level has increased in all instances from pre- to post-festival season in the sites under residential category. There is no specific source (other than city background) of carbonyls in these areas, and it can be inferred that the activities characteristic of festival season has increased the Tcarb level in these area.
Acetaldehyde was found to be the highest contributing species, which accounted for 33% (95.8 μg m−3) and 28% (107.5 μg m−3) of Tcarb considering its average concentrations across all sites in pre- and post-festival season, respectively. It was followed by formaldehyde, contributing 13% (37.2 μg m−3) and 19% (75.0 μg m−3) in pre- and post-festival season, respectively. Other prominent contributing species in pre- and post-festival season were propionaldehyde (32.5 μg m−3, 11% and 37.7 μg m−3, 10%) and acetone (37.9 μg m−3, 13% and 27.3 μg m−3, 7%), respectively. The average formaldehyde/acetaldehyde (C1/C2) ratio in pre-festival season was found to be 0.62. A higher acetaldehyde level indicates influence of combustion from diesel-driven vehicle (Rodrigues et al., Citation2012). The entire fleet of heavy vehicle and more than 50% of the medium-duty vehicles are diesel-driven in Kolkata City. Low C1/C2 ratio also indicates contribution from industrial activities (Seo and Baek, Citation2011). The C1/C2 ratio was observed to be 1.78 in post-festival season measurement. High value of C1/C2 is conventionally associated with biogenic contribution, indicating that photolysis of natural hydrocarbons such as isoprene give rise to higher formaldehyde level (Duane et al., Citation2002; Pang and Mu, Citation2006). However, the urban environment of Kolkata metro city is expected to have little biogenic emission, which remains same in both the seasons. High formaldehyde level is also characteristic of residential wood burning (Cerqueira et al., Citation2013). Uncontrolled burning of biomass fuel characteristic of festival season has given rise to higher acetaldehyde level, which altered the C1/C2 ratio during post-festival season.
Diurnal variation
represents the diurnal variation of Tcarb level during both in pre- and post-festival season. During pre-festival season, the diurnal trend is similar for all sites except two: Theatre Road (petrol pump vicinity) and Baithakkhana (industrial) as depicted in . The general observed trend was low in the morning (6:00 a.m. to 12:00 noon), higher in the afternoon (12:00 noon to 6:00 p.m.), and again low at night (6:00 p.m. to 6:00 a.m.). The afternoon to night ratio ranges from 1.1 to 3.6 except at Baithakkhana (0.5). This trend is somewhat similar to the observation made in other studies in urban setup with similar meteorology, having long sunshine hour and high solar radiation (Moussa et al., Citation2006; Pang and Mu, Citation2006; Cheng et al., Citation2014). This indicates that secondary photochemical formation is also an important contributor at these sites along with primary emissions. As the sunlight became more intense at noon, oxides of nitrogen (NOx) break down and as a result tropospheric ozone is formed during afternoon (12:00 noon to 6:00 p.m.). At the same time, some of the oxides of nitrogen reacts with organic pollutants available in air and oxidizes them to carbonyls. After the sunset, the incoming solar radiation ceases and hence the ozone production stops; as a result, the secondary production of carbonyl also stops (Atkinson, Citation2000). This explains the lower level of carbonyls at night, which is the residual from the primary and secondary carbonyls formed during the previous day, in presence of solar radiation. The high levels of carbonyls during morning and nighttime at industrial site (Baithakkhana) indicate dominant effect of primary emission of carbonyls in the vicinity due to industrial usage in pre-festival season. High level of carbonyl observed at Theatre Road site (petrol pump vicinity) in the morning indicates the dominant primary emission associated with refueling of vehicle during morning rush hour.
Figure 3. Diurnal variation of ambient total carbonyl levels in pre- and post-festival season in different sites of Kolkata City.
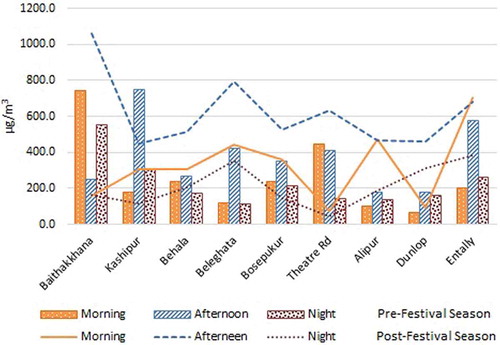
The general diurnal trend, as observed from , in the post-festival season remains the same as pre-festival season i.e., low in the morning (6:00 a.m. to 12:00 noon) increases in the afternoon (12:00 noon to 6:00 p.m.), and decreases at night (6:00 p.m. to 6:00 a.m.) except at two residential sites, Alipur and Entally. High morning carbonyl level in these sites may have resulted from primary emission from uncontrolled open burning of biomass and coal associated with cooking activity. It is interesting to note that the increase of afternoon carbonyl level is even prominent in this period than the pre-festival season in rest of the sites. As discussed earlier, the regular anthropogenic activity, e.g., the number of cars in the road associated with primary emission decreases in post-festival season. Lower level of carbonyls, in post-festival season, during morning in comparison with pre-festival season near petrol pump (Theatre Road) may have resulted from decrease in refueling associated with reduced number of car on road. Industrial site such as Baithakkhana does not show high morning level in post-festival season like pre-festival season due to decreased industrial activity. The afternoon to night ratio of Tcarb ranges from 1.5 to as high as 14.4, indicating increased contribution of secondary emission in total carbonyl level. The morning to afternoon ratio of Tcarb is increased in post-festival season in most cases, indicating higher level of nonoxygenated volatile organics in this season that are precursors of carbonyls. In two residential sites, Alipur and Entally, the morning to afternoon ratio has been altered in post-festival season. The high level of carbonyl during morning hours indicates impact of characteristic anthropogenic emission associated with festival season.
Effect of festival season on ozone forming potential of target carbonyls
Tropospheric ozone is a matter of concern globally due to its association with human health endpoint, including mortality (Cakmak et al., Citation2007). In India also, ozone is included as one of the criteria pollutants in National Ambient Air Quality Standards, 2009. Tropospheric ozone, being a secondary pollutant, is dependent on the formation from its precursors, i.e., VOCs and oxides of nitrogen (NOx) in presence of sunlight. The geographical location of city of Kolkata (22.57°N, 88.37°E) is such that it receives high solar influx with mostly clear sky and occasional rain during the study period (September to October, post-monsoon). The average NOx level varied from 11.3 μg m−3 in Entally (residential area) to 24.3 μg m−3 in Bosepukur (petrol pump vicinity). Such condition is favorable for formation of photochemical ozone in lower atmosphere (Silman, 1999). The Maximal Incremental Reactivity (MIR) scale developed by Carter (Citation1994, Citation2009) characterizes the incremental reactivity of individual VOC species for conditions in which the species has its maximum absolute MIR value. This method is widely practiced for comparing the ozone-forming potential (OFP) of various VOC species. Although the dimensionless MIR values may vary depending upon spatial and temporal variations, they can give a good general estimation of the importance of the different VOCs on tropospheric ozone formation.
Contribution of individual species in ozone formation during both the seasons is depicted in . The total ozone forming potential (OFP) calculated for all 14 carbonyls were 1416 μg m−3 of ozone in pre-festival season, which was increased by 35% in post-festival season to 1912 μg m−3 of ozone. It is interesting to note that among the target carbonyls, isomers of tolualdehyde and benzaldehyde have negative impact to ozone formation owing to their negative MIR values. In both seasons, more than 80% of total OFP is contributed by three species, i.e., formaldehyde, acetaldehyde, and propionaldehyde. Acetaldehyde was the highest contributor in pre-festival season, contributing 43%, followed by formaldehyde (24%). In the post-festival season, they both contribute equally (36% each). Hence, the unregulated anthropogenic activities characteristic of the festival season not only have a direct effect on emission of carbonyl VOCs in ambient air but also impose a significant influence on secondary pollutants such as ozone.
Intensive study
Immediate effect of fireworks on VOC and carbonyl levels
All 14 carbonyl compounds were studied in ambient air of Kolkata in the intensive study to understand the immediate effect of fireworks during the special event of “Diwali.” compares the average levels of individual and sum of the carbonyls in air on the night of Diwali with the same on the night before. The Tcarb level was found to be significantly high upon ANOVA test (F = 17.59, P = 0.001) on Diwali night than the previous night. The Tcarb level varied from 122.5 to 669.7 μg m−3 across different sites on the night before the event. The level was elevated in the range of 492.3–1116.6 μg m−3 on the night of the event ().
Figure 5. Comparison of ambient concentrations of (a) carbonyls and (b) noncarbonyl VOCs obtained during the intensive study depicting the effect of event of Diwali.
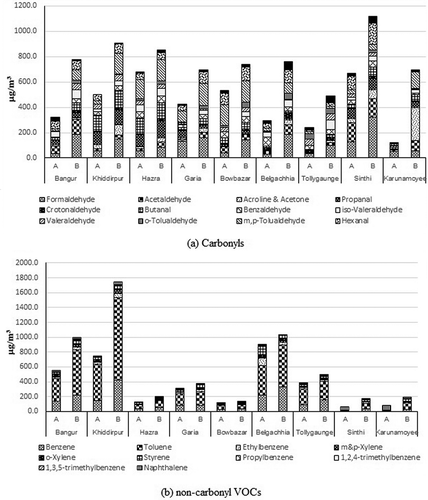
Alongside the carbonyls, 10 noncarbonyl VOC species were also identified and quantified, namely, benzene, toluene, ethylbenzene, m&p-xylene, o-xylene, styrene, propylbenzene, 1,2,4-trimethyl benzene, 1,3,5-trimethylbenzene, and naphthalene. depicts the average levels of individual and sum of the noncarbonyl VOCs (Tn-carb) in air on the night of Diwali and the same on the night before. Tn-carb varied from 76.2 to 748.9 μg m−3 on the night before the event across different sites in the city. The same varied in the range of 171.5–1743.9 μg m−3 on the Diwali night at different sites (), which was higher than the night before but not significantly (F = 1.14, P = 0.3). Benzene and toluene together contributed about 75% and 60% among Tn-carb on the night of the event and the night before, respectively. Chang et al. (Citation2011) reported that monoaromatic VOCs, i.e., BTEX, concentration went up to about 5–10 times its normal value during the fireworks display in Taiwan. In present study, average level for all the noncarbonyl VOCs and carbonyl compounds elevated except naphthalene, which was decreased by 32%. Carbonyls were more affected by the event than noncarbonyl VOCs. The increase in average level of carbonyls varied from 10% for benzaldehyde to 407% for acetone, whereas among noncarbonyl VOCs the average level increased from 5% for styrene to 81% for toluene while propylbenzene level remained almost same.
Principal component analysis for source characterization for the event of Diwali
Multivariate factor analysis using PCA was applied to the data set obtained in intensive study to identify potential sources of atmospheric VOCs. PCA was applied separately to the sets of data obtained from different sites before the night of Diwali (16 October 2009) and on the night of Diwali (17 October 2009). Factors were extracted using PCA, which involves varimax orthogonal rotation with Kaiser normalization to determine the eigenvalues of variance matrix of original variables. Only components with eigenvalues greater than unity were retained because they can account for a meaningful amount of variance. Factor loadings indicate the correlation of each species with each component, and a species was said to load on a given component if the factor loading was 0.5 or greater for that component.
The result of principal component analysis (PCA) is shown in for both the data sets for the night before Diwali (16 October 2009) and the night of Diwali (17 October 2009). In case of 16 October, VOCs were discriminated by the first six PCs, which together explained 92.6% variance of the data set. Most noncarbonyl VOCs such as toluene, ethylbenzene, styrene, and propylbenzene, had high relevance on the first principal component (PCv), as shown in . This PC (PCv) may be largely assigned to vehicle-related emission including evaporative emission from gasoline as well as combustion emission from vehicular exhaust (Yu et al., Citation2013; Joumard et al., Citation2004). PCv explains 38.2% of the variance. Characteristic compounds of fossil fuel, including coal combustion emission, such as propanal, butanal, and acetone, are separated in the second principal component (PCf). This source group explains 17.7% of variance in the whole data set. The third PC (PCc) has crotonaldehyde as characteristic compound. Crotonaldehyde has been associated with cooking and heated edible oil (Hecht et al., Citation2010; Ziping et al., Citation1994); hence, this source may be accredited to cooking activity, which corresponds to 14.9% variance. The fourth PC (PCph) could be attributed to photochemical formation with 9.9% variance, as the components separated in this group are formaldehyde, acetaldehyde, and acetone. Formaldehyde and acetaldehyde could be mainly produced by photochemical reactions of some VOCs and also removed by photolysis and reaction with OH radical at the same time during daytime (Atkinson, Citation2000; Silman, 1999). This intensive study was conducted during night; therefore, the contribution of photochemically associated carbonyls was less significant. The other two PCs (PCoth1 and PCoth2) may be attributed to other sources such as biomass burning (C5 carbonyls), consumer product (benzaldehyde, tolualdehyde), and smoking. However, it is difficult to comment on the exact nature of these two sources.
Table 2. Principal component analysis for comparison of source contribution towards the ambient level of oxygenated and nonoxygenated VOCs during intensive study
In case of 17 October, also VOCs were discriminated by the first six PCs, which together explained 95.6% variance of the data set. The first PC may be compared to PCv obtained in the data set from 16 October, i.e., vehicular emission including evaporative and incomplete combustion. PCv explains 34.4% of the variance. The indicative species (propanal, butanal) of the third PC of 17 October data set is similar to the PCf from 16 October data set. This component, i.e., PCf, indicates that fossil fuel including coal combustion emission explains 21.5% variance of the whole data set. The fourth component of 17 October data set with crotonaldehyde as indicative species is similar to PCc from 16 October data set, indicating contribution from cooking activity. PCc explains 12.1% variance of the whole data set. The fifth PC with formaldehyde and acetaldehyde as indicative species may be compared to PCph of 16 October data set. This PC, related to residual photochemical formation of VOCs during previous daytime, explains 8.3% variance of the whole data set. The last PC in the 17 October data set is denoted as PCoth1&oth2, as it show high relevance with respect to the key species of both PCoth1 (m&p-tolualdehyde) as well as PCoth2 (isovalaraldehyde and dimethylbenzaldehyde) corresponding to 16 October data set. Although the exact nature of PCoth1 and PCoth2 cannot be explained, for the purpose of this study, the similarity between pre-Diwali and post-Diwali event with respect to these sources indicates that the contribution of these sources are same for both the data set. However, the most interesting result was observed in case of 17 October data set. The third PC that is denoted as PCD explained 14.6% of total variance of the data set. The key species with high relevance to this component are acetone, benzaldehyde, isomers of tolualdehyde, and isomers of valaraldehyde. No component from 16 October data set corresponds to this component; hence, it can be deduced that this relates to the emission due to the fireworks and other anthropogenic activities typical to the night of Diwali.
Conclusion
The festival season in general is marked by unregulated anthropogenic activities different than usual practices. It is expected that the effect of this alteration will influence the mixing ratio for species of both oxygenated and nonoxygenated VOCs. Post-festival season shows higher level of oxygenated VOCs as compared with pre-festival season in most of the sites of Kolkata City. Formaldehyde to acetaldehyde ratio was altered from pre- to post-festival season, indicating variation in source composition between these two periods. Diurnal variation also indicates alteration in source characteristics as well as in source strength. Ozone-forming potential of the oxygenated VOCs indicate that the altered characteristics of anthropogenic activities specific to the festival season also have a significant effect on formation of secondary pollutants such as ozone. In case of the event of Diwali night, the VOCs sources were studied using principal component analysis. The effect of activities typical to the event of Diwali night, especially the extensive use of pyrotechnics, was clearly evident in the principal component analysis as the third major source, with 14.6% variance, on the night of the event, after vehicular emission and fossil fuel combustion. This study indicates that unregulated anthropogenic activities during festival period in Kolkata metropolitan city contribute to considerable extent towards elevated oxygenated and nonoxygenated volatile organic compounds in ambient air. Especially, the display of fireworks has marked impact on these organic pollutants. This study finding emphasizes that it is required to generate awareness among the community and also some regulations are required in place to minimizing these uncontrolled emissions.
Acknowledgment
The authors thank Mr. Shubhadeep Neogi and Mr. Anindya Mukherjee (Project Assistant) for participating in air sampling.
Funding
The authors sincerely thank the China Section of the Air & Waste Management Association for the generous scholarship received to cover the cost of page charges and make the publication of this paper possible. This study was carried out as part of the project funded by Central Pollution Control Board, Ministry of Environment and Forest, India.
Additional information
Funding
Notes on contributors
Dipanjali Majumdar
Dipanjali Majumdar is a scientist, Bipasha Dinda Chakraborty was a project fellow, Padma S. Rao is a senior principal scientist, and Anjali Srivastava is a chief scientist working at the National Environmental Engineering Research Institute, India.
Padma S. Rao
Dipanjali Majumdar is a scientist, Bipasha Dinda Chakraborty was a project fellow, Padma S. Rao is a senior principal scientist, and Anjali Srivastava is a chief scientist working at the National Environmental Engineering Research Institute, India.
Bipasha Dinda Chakraborty
Dipanjali Majumdar is a scientist, Bipasha Dinda Chakraborty was a project fellow, Padma S. Rao is a senior principal scientist, and Anjali Srivastava is a chief scientist working at the National Environmental Engineering Research Institute, India.
Anjali Srivastava
Dipanjali Majumdar is a scientist, Bipasha Dinda Chakraborty was a project fellow, Padma S. Rao is a senior principal scientist, and Anjali Srivastava is a chief scientist working at the National Environmental Engineering Research Institute, India.
References
- Atkinson, R. 2000. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 34:2063–2101. doi:10.1016/S1352-2310(99)00460-4
- Cakmak, S., R.E. Dales, and V.C. Blanco. 2007. Air pollution and mortality in Chile: Susceptibility among the elderly. Environ. Health Perspect. 115:524–527. doi:10.1289/ehp.9567
- Carter, W. 1994. Development of ozone reactivity scales for volatile organic compounds. J. Air Waste Manage. Assoc. 44:881–889. doi:10.1080/1073161X.1994.10467290
- Carter, W.P.L. 2009. Updated maximum incremental reactivity scale and 505 Hydrocarbon bin reactivities for regulatory applications. Prepared for California Air Resources Board, Riverside, CA, Contract 07–339.
- Cerqueira, M., L. Gomes, L. Tarelho, and C. Pio. 2013. Formaldehyde and acetaldehyde emissions from residential wood combustion in Portugal. Atmos. Environ. 72:171–176. doi:10.1016/j.atmosenv.2013.02.045
- Chang, S.C., T.H. Lin, C.Y. Young, and C.T. Lee. 2011. The impact of ground-level fireworks (13 km long) display on the air quality during the traditional Yanshui Lantern Festival in Taiwan. Environ Monit. Assess. 172:463–479. doi:10.1007/s10661-010-1347-1
- Cheng, Y., S.C. Lee, Y. Huang, K.F. Ho, S.S.H. Ho, P.S. Yau, P.K.K. Louie, and R.J. Zhang. 2014. Diurnal and seasonal trends of carbonyl compounds in roadside, urban, and suburban environment of Hong Kong. Atmos. Environ. 89:43–51. doi:10.1016/j.atmosenv.2014.02.014
- Central Pollution Control Board (CPCB). 2010. Study of Urban Air Quality in Kolkata for Source Identification and Estimation of Ozone, Carbonyls, NOx and VOC Emissions. Control of Urban Pollution Series: CUPS/72/20010-11. Delhi, India: Central Pollution Control Board.
- Croteau, G., R. Dills, M. Beaudreau, and M. Davis. 2010. Emission factors and exposures from ground-level pyrotechnics. Atmos. Environ. 44:3295–3303. doi:10.1016/j.atmosenv.2010.05.048
- Drewnick, F., S.S. Hings, J. Curtius, G. Eerdekens, and J. Williams. 2006. Measurement of fine particulate and gas-phase species during the New Year’s fireworks 2005 in Mainz, Germany. Atmos. Environ. 40:4316–4327. doi:10.1016/j.atmosenv.2006.03.040
- Duane, M., B. Poma, D. Rembges, C. Astorga, and B.R. Larsen. 2002. Isoprene and its degradation products as strong ozone precursors in Insubria, northern Italy. Atmos. Environ. 36:3867–3879. doi:10.1016/S1352-2310(02)00359-X
- Dutta, C., D. Som, A. Chatterjee, A.K. Mukherjee, T.K. Jana, and S. Sen. 2009. Mixing ratios of carbonyls and BTEX in ambient air of Kolkata, India and their associated health risk. Environ. Monit. Assess. 148:97–107. doi:10.1007/s10661-007-0142-0
- Edgerton, S.A., M.W. Holdren, and D.I. Smith. 1989. Inter urban comparison of ambient volatile organic compound concentration in U.S. cities. J. Air Pollut. Cont. Assoc. 39:729–732. doi:10.1080/08940630.1989.10466561
- Estrellan, C.R., and F. Iino. 2010. Toxic emissions from open burning. Chemosphere 80:193–207. doi:10.1016/j.chemosphere.2010.03.057
- Fleischer, O., H. Wichmann, and W. Lorenz. 1999. Release of polychlorinated dibenzo-p-dioxins and dibenzofurans by setting off fireworks. Chemosphere 39:925–932. doi:10.1016/S0045-6535(99)00019-3
- Hecht, S.S., A. Seow, M. Wang, R. Wang, L. Meng, W.P. Koh, S.G. Carmella, M. Chen, S. Han, M.C. Yu, and J.M. Yuan. 2010. Elevated levels of volatile organic carcinogen and toxicant biomarkers in Chinese women who regularly cook at home. Cancer Epidemiol. Biomarkers Prev. 19(5):1185–92. doi:10.1158/1055-9965.EPI-09-1291
- Joumard, R., J.-M. Andre, L. Paturel, and F. Cazier. Exhaust emissions of regulated and unregulated pollutants of passenger cars. Presented at 10th CONAT Automotive and Future Technologies, Brasov, Romania, October 2004.
- Kerns, W.D., K.L. Parkov, D.J. Donofrio, E.J. Gralla, and J.A. Swenberg. 1983. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Res. 43:4382–4392.
- Kulshrestha, U.C., T.R. Nageswara, S. Azhaguvel, and M.J. Kulshrestha. 2004. Emissions and accumulation of metals in the atmosphere due to crackers and sparkles during Diwali festival in India. Atmos. Environ. 38:4421–4425. doi:10.1016/j.atmosenv.2004.05.044
- Lee, S.-C., and B. Wang. 2004. Characteristics of emissions of air pollutants from burning of incense in a large environmental chamber. Atmos. Environ. 38:941–951. doi:10.1016/j.atmosenv.2003.11.002
- Lemieux, P.M., C.C. Lutes, and D.A. Santoianni. 2004. Emissions of organic air toxics from open burning: A comprehensive review. Prog. Energy Combust. Sci. 30:1–32. doi:10.1016/j.pecs.2003.08.001
- Majumdar, D., and A. Srivastava. 2012. Volatile organic compound emissions from municipal solid waste disposal sites: A case study of Mumbai, India. J. Air Waste Manage. Assoc. 62(4):398–407. doi:10.1080/10473289.2012.655405
- Majumdar, D., S. Ray, S. Chakraborty, P.S. Rao, A.B. Akolkar, M. Chowdhury, and A. Srivastava. 2014. Emission, speciation, and evaluation of impacts of nonmethane volatile organic compounds from open dump site, J. Air Waste Manage. Assoc. 64:834–845. doi:10.1080/10962247.2013.873747
- Mandal, R., B.K. Sen, and S. Sen. 1997. Impact of fireworks on our environment. Indian J. Environ. Protect. 17:850–853.
- Moussa, S.G., M. El-Fadel, and N.A. Saliba. 2006. Seasonal, diurnal and nocturnal behavoiursbehaviors of lower carbonyl compounds in the urban environment of Beirut, Lebanon. Atmos. Environ. 40:2459–2468. doi:10.1016/j.atmosenv.2005.12.031
- Nishanth, T., K.M. Praseed, K. Rathnakaran, K.M.K. Satheesh, R. Ravi Krishna, and K.T. Valsaraj. 2012. Atmospheric pollution in a semi-urban, coastal region in India following festival seasons. Atmos. Environ. 47:295–306. doi:10.1016/j.atmosenv.2011.10.062
- Pang, X., and Y. Mu. 2006. Seasonal and diurnal variations of carbonyl compounds in Beijing ambient air. Atmos. Environ. 40:6313–6320. doi:10.1016/j.atmosenv.2006.05.044
- Perry, K.D. 1999. Effects of outdoor pyrotechnic displays on the regional air quality of western Washington state. J. Air Waste Manage. Assoc. 49:146–155. doi:10.1080/10473289.1999.10463791
- Ravindra, K., S. Mor, and C.P. Kaushik. 2003. Short-term variation in air quality associated with firework events: A case study. J. Environ. Monit. 5:260–264. doi:10.1039/b211943a
- Rodrigues, M.C., L.L.N. Guarieiro, M.P. Cardoso, L.S. Carvalho, G.O. Rocha, and J.B. Andrade. 2012. Acetaldehyde and formaldehyde concentrations from sites impacted by heavy-duty diesel vehicles and their correlation with the fuel composition: Diesel and diesel/biodiesel blends. Fuel 92:258–263. doi:10.1016/j.fuel.2011.07.023
- Seo, Y.-K., and S.-O. Baek. 2011. Characterization of carbonyl compounds in the ambient air of an industrial city in Korea. Sensors 11:949–963. doi:10.3390/s110100949
- U.S. Environmental Protection Agency. 1997. Compendium Method TO-17: Determination of Volatile Organic Compounds in Ambient Air Using Active Sampling onto Sorbent Tubes. Washington, DC: U.S. Environmental Protection Agency.
- U.S. Environmental Protection Agency. 1999. Compendium Method TO-11A: Determination of Formaldehyde in Ambient Air Using Adsorbent Cartridge Followed by High Performance Liquid Chromatography (HPLC) [Active Sampling Methodology]. Washington, DC: U.S. Environmental Protection Agency.
- Wang, B., S.C. Lee, K.F. Ho, and Y.M. Kang. 2007. Characteristics of emissions of air pollutants from burning of incense in temples, Hong Kong. Sci. Total Environ. 377:52–60. doi:10.1016/j.scitotenv.2007.01.099
- Yerramsetti, V.S., A.R. Sharma, N.N. Gauravarapu, V. Rapolu, N.S. Dhulipala, and P.R. Sinha. 2013 The impact assessment of Diwali fireworks emissions on the air quality of a tropical urban site, Hyderabad, India, during three consecutive years. Environ. Monit. Assess. 185:7309–7325. doi:10.1007/s10661-013-3102-x
- Yu, J., Y. Zhang, X. Wang, Z. Zhang, S. Lü, M. Shao, and F.S.C. Lee. 2013. Species profiles and normalized reactivity of volatile organic compounds from gasoline evaporation in China. Atmos. Environ. 79:110–118. doi:10.1016/j.atmosenv.2013.06.029
- Ziping, B., H. Jianqun, and H. Fuzhong. 1994. Identification and mechanism of formation of certain mutagenic components in the volatile condensates from oxidized and heated edible oils. J. Environ. Sci. 6:200–205.