ABSTRACT
The study presents the measurement of carbonyl, BTEX (benzene, toluene, ethyl benzene, and xylene), ammonia, elemental/organic carbon (EC/OC), and greenhouse gas emissions from modern heavy-duty diesel and natural gas vehicles. Vehicles from different vocations that included goods movement, refuse trucks, and transit buses were tested on driving cycles representative of their duty cycle. The natural gas vehicle technologies included the stoichiometric engine platform equipped with a three-way catalyst and a diesel-like dual-fuel high-pressure direct-injection technology equipped with a diesel particulate filter (DPF) and a selective catalytic reduction (SCR). The diesel vehicles were equipped with a DPF and SCR. Results of the study show that the BTEX emissions were below detection limits for both diesel and natural gas vehicles, while carbonyl emissions were observed during cold start and low-temperature operations of the natural gas vehicles. Ammonia emissions of about 1 g/mile were observed from the stoichiometric natural gas vehicles equipped with TWC over all the driving cycles. The tailpipe GWP of the stoichiometric natural gas goods movement application was 7% lower than DPF and SCR equipped diesel. In the case of a refuse truck application the stoichiometric natural gas engine exhibited 22% lower GWP than a diesel vehicle. Tailpipe methane emissions contribute to less than 6% of the total GHG emissions.
Implications: Modern heavy-duty diesel and natural gas engines are equipped with multiple after-treatment systems and complex control strategies aimed at meeting both the performance standards for the end user and meeting stringent U.S. Environmental Protection Agency (EPA) emissions regulation. Compared to older technology diesel and natural gas engines, modern engines and after-treatment technology have reduced unregulated emissions to levels close to detection limits. However, brief periods of inefficiencies related to low exhaust thermal energy have been shown to increase both carbonyl and nitrous oxide emissions.
Introduction
Mobile source air toxics (MSAT) are defined as pollutants emitted by off-road equipment and on-road motor vehicles that pose serious health and environmental hazards. According to estimates from the U.S. Environmental Protection Agency (USEPA), nearly 44% of the total air toxic emissions are the result of activity from mobile sources (Winebrake, Wang, et al., Citation2001). The EPA also projects that nearly 50% of the air toxics related to cancer risk are emitted by mobile sources (Health Effects Institute [HEI], 2008). The majority of MSAT emissions emitted by internal combustion engines are by-products of the in-cylinder combustion process of fuel. Although the chemistry behind the formation of the individual MSAT compounds is complex and not well understood, the general theory is that incomplete combustion of localized fuel-rich zones in the combustion chamber may lead to the formation of intermediate hydrocarbon species such as carbonyls, polyaromatic hydrocarbons (PAHs), and other higher chain hydrocarbons. Combustion of lubrication oil, engine wear, exhaust after-treatment systems, and fuel composition also contribute to MSAT emissions in the form of metals and inorganic ions (Verma, Shafer et al., Citation2010; Lloyd and Cackette, Citation2011; Thiruvengadam, Besch et al., Citation2014).
The MSAT emissions characteristics of diesel and of compressed natural gas (CNG) vehicles are significantly different from one another. The primary reason for this contrast is due to the differences in fuel properties and engine technologies. Diesel engines, which operate on the principle of compression ignition of a liquid fuel, are different from natural gas engines operating on a spark-ignition platform. Natural gas engines are generally characterized by higher levels of carbonyl emissions, while diesel engines are characterized by higher PAH emissions (Kado, Okamoto et al., Citation2005). The differences in chemical composition of the exhaust are primarily due to the fuel properties. Natural gas is primarily methane, which is characterized by a high octane number, which makes natural gas fuel highly resistant to autoignition. In an internal combustion engine, the efficiency of combustion depends on the ability of a fuel to combust spontaneously with the propagating flame that originated from the primary ignition event. However, the high-octane property of natural gas resists this spontaneous combustion process and therefore contributes to partial fuel oxidation, which directly contributes to the formation of intermediate species such as carbonyls. In addition, all modern internal combustion engines utilize exhaust gas recirculation (EGR) as a combustion control approach to lower combustion temperatures and reduce NOx formation. EGR acts as a combustion retardant that lowers combustion efficiency and therefore reduces high-temperature combustion processes that result in the formation of NOx. The combination of EGR and the high-octane property of natural gas fuel resists spontaneous combustion, thereby resulting in the in-cylinder formation of MSAT compounds.
Recent emissions standards implemented by the USEPA have required engine manufacturers to employ catalyzed exhaust after-treatment systems to reduce emissions of oxides of nitrogen (NOx) and particulate matter (PM). The use of oxidation catalysts is the most effective pathway to control MSAT emissions from internal combustion engines, as opposed to combustion optimization pathways. A study by Khalek et al. shows that the total unregulated emissions, which included inorganic ions, PAH, nitro-PAH, carbonyls, metals, elements and dioxins emitted from a model year (MY) 2007, oxidation catalyst-equipped diesel engine were close to 99% lower than a MY 2004 uncontrolled diesel engine (Khalek, Bougher et al., Citation2011).
In the case of natural gas engines, Yoon et al. showed that current technology (engines compliant to USEPA 2010 emissions regulation) natural gas engines equipped with a three-way catalyst (TWC) reduce emissions of gas phase volatile organic compounds, carbonyls, organic carbon (OC), and elemental carbon (EC) by close to 99% compared to older model, lean-burn technology engines (Yoon, Hu et al., Citation2014).
Since 2007, the USEPA emissions standards have been subject to an 80% reduction in NOx emissions from 1.2 g/bhp-hr to 0.2 g/bhp-hr in 2010. This new NOx standard required engine manufacturers to use selective catalytic reduction (SCR) technology for diesel engines and TWC for stoichiometric engines to achieve certification limits.
During phase two of the recent Advanced Collaborative Emissions Study (ACES) it was found that emissions of PAH, nitro-PAH, carbonyls, inorganic ions, metals, elements, and other volatile organic compounds were reduced by close to 99% relative to the uncontrolled MY 2004 diesel engine technology (Khalek, Blanks et al., Citation2013). The ACES study used an FTP engine dynamometer cycle and a custom 16-hr duration transient cycle to collect samples for exhaust chemical speciation. The testing of engines on an engine dynamometer using extended-duration driving cycles may not capture the thermal inefficiencies in an after-treatment system that may be encountered during real-world driving conditions. Nevertheless, the results of the ACES study still provide a wealth of information on the detailed chemical characteristics of exhaust from advanced technology heavy-duty diesel engines. The results shown in this paper present unregulated emissions data for heavy-duty diesel and natural gas engines operational in different vocations while tested using driving cycles representative of in-use operation.
Ammonia emissions
Gas-phase ammonia is a potent precursor to secondary inorganic PM formation, as it readily neutralizes sulfuric and nitric acid to form ammonium sulfate and nitrates, respectively (Renner and Wolke, Citation2008). Ammonia emissions from internal combustion engines are a characteristic of stoichiometric spark-ignited engines equipped with a three-way catalyst (TWC) (Huai, Durbin et al., Citation2003; Suarez-Bertoa, Zardini et al., Citation2014). Traditionally, the ammonia emissions from diesel trucks were not quantifiable due to the absence of ammonia formation chemistry in diesel engine technology. Andrew et al. have shown that motor vehicle emissions of ammonia measured in California highway tunnels are dominated by light-duty gasoline vehicles (Andrew, Littlejohn et al., Citation2008). The heavy-duty (HD) vehicle population prior to 2010 is assumed to be dominated by diesel vehicles; therefore, the contribution of HD vehicles to ammonia emissions is considered to be minimal. Since the promulgation of the USEPA 2010 emissions standards, the HD vehicle population has included a mix of both natural gas engines equipped with TWC, and diesel engines using ammonia (through aqueous urea injection) as a reductant for NOx control after-treatment systems. These new standards, and their consequent technologies, enable HD vehicles to be catalogued into ammonia emissions inventories.
Ammonia in TWC is formed due to a secondary reaction pathway that includes CO, water vapor, and hydrocarbons in the presence of high exhaust temperatures (Defoort, Olsen et al., Citation2003; Bielaczyc, Szczotka et al., Citation2012). Ammonia emissions peak during slightly rich air-to-fuel ratios, which coincidentally result in the best NOx reduction performance of a TWC (Bielaczyc, Szczotka et al., Citation2012). Engines frequently operate in the fuel-rich zone to achieve lower tailpipe NOx emissions. A study by Thiruvengadam et al. has shown the superior NOx benefits of stoichiometric natural gas engines over SCR-equipped engines irrespective of the driving cycle (Thiruvengadam, Besch et al., Citation2015). Economic incentives and mandates for use of clean alternative fuel technology have resulted in greater penetration of natural gas-fueled vehicles into urban vehicle vocations such as transit and refuse trucks. Therefore, it is important to assess the emission rates of ammonia from HD natural gas vehicles. Ammonia emissions from SCR-equipped diesel engines are controlled by optimizing urea dosing and ammonia storage in the SCR catalyst (Koebel, Elsener et al., Citation2001). Inefficiencies in the operation of SCR after-treatment systems, however, can result in elevated ammonia emissions.
Greenhouse gas emissions
With recent emphasis on global climate change, reducing greenhouse gas (GHG) emissions has become an increasing priority. Carbon-dioxide (CO2), nitrous oxide (N2O), and methane (CH4) are the potent GHG pollutants emitted from internal combustion engines fueled by diesel and natural gas. N2O and CH4 have radiative forcing potentials equal to 298 times and 25 times that of CO2 over a 100-year time horizon, respectively (Intergovernmental Panel on Climate Change [IPCC], Citation2007). Beginning in 2017, USEPA GHG emissions regulation for heavy- and medium-duty engines will regulate emissions of CO2, CH4, and N2O at 460, 0.1, and 0.1 g/bhp-hr, respectively, over the FTP or the supplemental emissions test (SET) depending on the vocation of the engine (USEPA, Citation2011). While CO2 is an inevitable consequence of fossil fuel combustion, the impetus toward CO2 reduction is to reduce fuel consumption by developing more efficient engines. CH4 is a by-product of incomplete combustion in natural gas-fueled engines, while N2O is a by-product of inefficiencies in catalytic reactions over both TWC and SCR after-treatment systems.
Nitrous oxide (N2O)
In spark-ignited engines, N2O emissions are primarily observed during cold start operation. High N2O emissions are observed from TWC during the initial warm-up period of the catalyst. Depending upon the catalyst formulation, a TWC after-treatment system will produce N2O emissions between the temperatures of 300 and 500°C (Ball, Moser, et al., Citation2013). In most cases, a TWC that has achieved light-off (exhaust gas temperature at which catalytic activity of the after-treatment system is initiated) conditions does not produce any N2O emissions unless deterioration of the catalyst is observed. Therefore, a similar trend is expected in current technology natural gas engines that are equipped with TWC. The warm-up period of the vehicle, or extended idling, can bring local catalyst temperatures to within the range of N2O production. Kamasamudram et al. have shown diesel oxidation catalyst (DOC), SCR, and ammonia slip catalyst (ASC) can all contribute to N2O formation in a heavy-duty diesel engine. The study shows that N2O formation in DOC is primarily due to the reaction of NOx in the presence of hydrocarbons, known as lean-NOx catalysis or hydrocarbon SCR reactions (Kamasamudram, Henry, et al., Citation2012). Through this mechanism, however, N2O emissions occur during a very narrow temperature window and are not prevalent during diesel engine operation. A more prevalent source for N2O emissions occurs over the SCR after-treatment system as a result of both ammonia oxidation and decomposition of ammonium nitrate species formed over the catalyst surface. Kamasamudram et al. show that ammonium nitrate formation increases with elevating NO2/NOx ratios, which in turn results in increased N2O emissions formed by the decomposition of nitrates. Catalyst technology, urea dosing, engine operating conditions, and after-treatment aging are multiple factors that can alter the real-world emissions factor of N2O. Therefore, it is important to study the N2O emissions profile from both natural gas and heavy-duty diesel vehicles under real-world driving cycles.
Methane (CH4)
Methane is a clean-burning, gaseous fuel that is characterized by soot-free combustion in an internal combustion engine (Holmen and Ayala, Citation2002; Yoon, Collins, et al., Citation2013). Methane as a fuel is highly resistant to autoignition and is characterized by an octane number of over 100. Therefore, dedicated natural gas engines are by nature spark ignited, and compression ignition can only be achieved with a pilot fuel injection such as diesel. The combination of EGR and highly stable chemical properties of methane results in the incomplete combustion of natural gas. Consequently, methane emissions are characteristics of natural gas engines (Hajbabaei, Karavalakis, et al., Citation2013). Furthermore, oxidation catalysts require high exhaust gas temperatures to oxidize methane. Studies have shown that light-off temperatures for hydrocarbons decrease with higher hydrocarbon chains, while with CH4, the lowest hydrocarbon chain is characterized by higher light-off temperatures (Mizutani, Okawa, et al., Citation1998). Earlier model, lean-burn natural gas engines were characterized by higher degrees of incomplete combustion, lower exhaust gas temperatures, and consequently higher methane emissions. Emissions sampled from a transit bus equipped with a lean-burn natural gas engine show that 64% of the engine-out hydrocarbon emissions consist of CH4. Furthermore, a previous study shows that an oxidation catalyst rich in palladium was able to reduce the engine-out methane emissions by 57% (Thiruvengadam, Carder, et al., Citation2011).
Since methane is a potent greenhouse gas, regulating methane emissions from mobile sources is important. Current technology stoichiometric natural gas engines operating with a TWC are characterized by higher exhaust gas temperatures that may be conducive for methane oxidations. A lack of oxygen in the exhaust, however, could lower the ability of TWC to effectively lower CH4 emissions. Furthermore, dual-fuel engines operating with a conventional DOC could have insufficient thermal exhaust conditions for methane oxidation (Besch, Israel, et al., Citation2015).
Experimental method
The study was conducted using West Virginia University’s transportable heavy-duty chassis dynamometer and the Transportable Emissions Measurement System (TEMS). The TEMS is equipped with a full-scale constant volume sampling (CVS) dilution tunnel. Exhaust from the test vehicles was diluted in the CVS system prior to being sampled in respective media for unregulated speciation. The schematic of the sampling system is shown elsewhere (Thiruvengadam, Besch, et al., Citation2015). The tested heavy-duty vehicles belonged to four broad engine technology categories, namely, (1) stoichiometric natural gas with TWC; (2) dual-fuel high-pressure direct injection (HPDI) equipped with DPF and SCR; (3) USEPA 2007 compliant HD diesel with DPF; and (4) USEPA 2010 emissions compliant HD diesel with a DPF and SCR. Furthermore, the vehicle test matrix included vehicles from different vocations, namely, transit buses, refuse trucks, and goods movement (long-haul and drayage applications). shows the vehicle test matrix for the study.
Table 1. List of test vehicle vocations and engine technology category.
Vehicles were tested on the chassis dynamometer using driving cycles that are representative of real-world driving patterns for the respective vehicle vocations. lists the vocation-specific driving cycles used in this study. The study used drayage driving cycles to represent heavy-duty vehicle serving the ports of Los Angeles and Long Beach. The statistically representative drayage driving cycles were created from analyzing real-world activity data from port drayage vehicles (Couch, Citation2011). also lists the aerodynamic drag and tire rolling coefficients. The study used inertial test weights of 69,000, 35,000, and 56,000 lbs for goods movement, transit bus, and refuse truck applications, respectively. The refuse trucks tested as part of this study were of the type employed in residential refuse collection vehicles. The vehicles tested in this study were semiautomated side loaders. The SCAQMD refuse truck cycle is representative of residential refuse collection vehicle activity in Los Angeles, CA. shows the vehicle specifications of the test vehicles used in this study. Details of chassis dynamometer test procedure, driving cycle characteristics, and sampling setup are explained elsewhere (Thiruvengadam, Besch, et al., Citation2015). The study collected diluted exhaust samples for carbonyl, elemental carbon (EC), organic carbon (OC), and benzene, toluene, ethyl benzene, and xylene (BTEX). 2, 4-Dinitrophenylhydrazine (DNPH) cartridges were used for carbonyl speciation, passivated SUMMA canisters (electropolished, passivated stainless-steel vacuum containers) were used to collect samples for BTEX analysis, and prefired quartz filters were used for PM sample collection for EC/OC analysis. The analysis of BTEX and carbonyl species was conducted by Southwest Research Institute (SWRI). Gas chromatography with mass spectrometry (GC/MS) was used to analyze for the target species. The analysis procedures documented in the EPA method TO-15 were followed (USEPA, Citation1999). The laboratory detection limit for this study was 10 ppbv for BTEX volatile organic compounds (VOCs). BTEX concentrations below 10 ppbv were reported as not detected. Detection limits for carbonyl species varied depending on target analyte. The detection limits were 0.07 µg/sample for formaldehyde, 0.10 µg/sample for acetaldehyde, and 0.12 µg/sample for acetone. The analysis for EC/OC fractions was conducted by Desert Research Institute (DRI). A thermal/optical carbon analysis method based on the IMPROVE_A protocol was used to quantify four different OC fractions and three different EC fractions. Details of the analysis procedures and calculations of uncertainty are explained elsewhere (Chow, Watson, et al., Citation2007). Sampling for unregulated emissions was conducted over two consecutive hot starts. Sampling time over two consecutive hot starts was performed to increase sample loading on the respective media and to therefore improve the detection capability of the analytical methods for species that are low in exhaust gas concentration. This study also employed the MKS 2030 HS Fourier-transform infrared (FTIR) analyzer for real-time measurement of tailpipe concentrations of ammonia, nitrous oxide, and formaldehyde. The FTIR is capable of continuous measurements of 20 gas species at 5 Hz.
Table 2. Vocation specific test cycles and chassis dynamometer test parameters.
Table 3. Vehicle specification of test vehicles.
Results
The results of regulated emissions from the study show that modern natural gas goods movement vehicles exhibit significantly lower NOx emissions than their diesel counterparts over all driving cycles (Thiruvengadam, Besch, et al., Citation2015). The stoichiometric, spark-ignited engine platform with the use of TWC offers superior NOx reduction capabilities compared to diesel engines with SCR that are plagued by low exhaust temperatures at low engine load conditions. Results in this paper illustrate the differences in emission rates of unregulated pollutants, GHG, and ammonia emissions from the same vehicles for which data are published elsewhere (Thiruvengadam, Besch, et al., Citation2015).
EC/OC
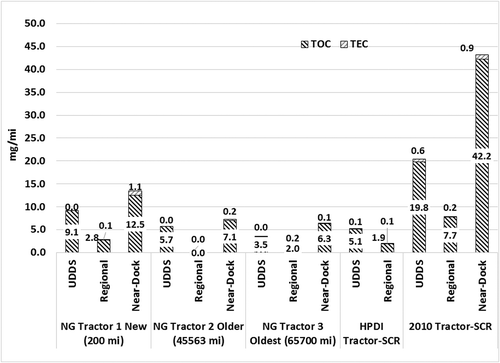
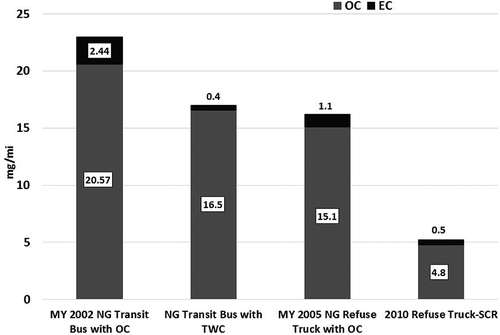
shows the EC/OC emission rates from varying heavy-duty vehicle technologies operating in a goods movement vocation over four different driving cycles. The results show that the PM from dedicated natural gas engines, dual-fuel engines, and diesel engines are dominated by the OC fraction. This is primarily due to the fact that natural gas internally combusts soot-free, while the dual-fuel and diesel engines are fitted with a DPF that traps soot emissions from the engine, and therefore emit only volatile carbon compounds that could form particulate matter upon dilution and cooling of exhaust (Herner, Hu, et al., Citation2011; Liu, Vasys, et al., Citation2012). For natural gas engines operating as goods movement vehicles, the OC fraction constitutes between 97 and 100% of the total carbon emissions measured over the prefired quartz filters. The diesel engines on the other hand, show EC fraction between 2 and 20%, depending on the fill state of DPF. Passive and active regenerations of the DPF can result in breakthrough of soot from the DPF (Liu, Thurow, et al., Citation2005; Thiruvengadam, Besch, et al., Citation2011). A large fraction of OC measured through the analysis of a prefired quartz filter can be associated with positive artifacts contributed by gas-phase absorption of hydrocarbons to the filter material. Therefore, an overestimation of OC mass measured from the prefired quartz filters is possible. To avoid overestimation of OC due to absorption of gas-phase hydrocarbon species, a backup filter is used in series with the primary filter (Khalek, Citation2007). The OC fraction measured from the backup filter is subtracted from the primary filter to correct for gas-phase absorption. This procedure, however was not used in this study, and therefore the OC emission rates of natural gas vehicles reported here could be positively biased with gas-phase hydrocarbon species. All gravimetric filter materials exhibited varying degrees of gas-phase adsorption of hydrocarbons (Liu, Vasys, et al., Citation2012), of which prefired quartz demonstrates the greatest affinity toward hydrocarbon species. shows the EC/OC emission rates for the transit bus and refuse truck applications over the UDDS cycle. The figure shows the EC/OC emissions trend of natural gas vehicles from MY 2002 to present day. Legacy natural gas engines were designed to operate as lean-burn engines with an oxidation catalyst. Therefore, depending on the duty cycle, the exhaust temperatures significantly affected the tailpipe emissions of nonmethane hydrocarbons and the subsequent formation of volatile PM during exhaust gas dilution.
The results from the current study show that OC emissions from a transit bus powered by a stoichiometric engine with a TWC catalyst is close to 20% lower OC emissions when compared to an MY 2002 lean-burn, oxidation catalyst-equipped transit bus, while in the refuse truck application the stoichiometric platform with a TWC showed close to 68% lower OC emission rates compared to a lean-burn platform with oxidation catalyst. Natural gas-fueled transit buses and refuse trucks exhibit higher EC fraction compared to trucks in goods movement applications. This can be attributed to a frequent stop-and-go type operation that could possibly result in combustion of entrained lubrication oil in the combustion chamber. Furthermore, the transit bus tested in this study had accumulated 116,232 miles. Higher vehicle miles also increase lubrication oil consumption by the engine through worn piston seals, valve train, and turbocharger seals. A study by Buchholz et al. shows that up to 4% of carbon in PM measured from a diesel engine was contributed by lubrication oil (Buchholz, Dibble, et al., Citation2003). However, in soot-free natural gas engines, carbon emissions could be entirely attributed to lubrication oil combustion.
Goods movement applications, on the other hand, typically experience lower transients and longer freeway type operation that provides better lubrication oil control. Duty cycle-based engine aging could possibly lead to combustion of lubrication oil, which in turn will result in higher tailpipe EC emissions. EC emission rates from current technology transit bus and refuse trucks are on average 54% lower than the emission rates observed from MY 2002 engine technology.
Carbonyls
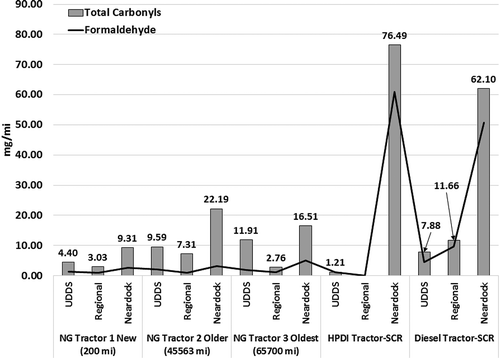
Carbonyl emissions are indicative of the level of incomplete combustion of fuel in an internal combustion engine. Most oxidation catalysts, however, are highly efficient in reducing tailpipe emissions of carbonyls. Of the different carbonyl species detected in the tailpipe emissions, formaldehyde is observed to be the most dominant species (Kado, Okamato, et al., Citation2005; Yoon, Hu, et al., Citation2014). Natural gas-fueled engines typically exhibit higher concentrations of carbonyls when compared to diesel engines (Kado, Okamato, et al., Citation2005). Carbonyl are intermediate species formed during the incomplete oxidation of fuel during in-cylinder combustion. It is expected that combustion of a high-octane fuel such as natural gas will be associated with higher levels of incomplete combustion compared to lean-burn compression ignition diesels. The duty cycle of an engine and its effect of oxidation catalyst light-off temperatures can contribute to higher emission rates of carbonyls. shows the total carbonyl and formaldehyde emissions from natural gas and diesel goods movement vehicles over the UDDS and the three port drayage cycles. The natural gas engines equipped with a three-way catalyst show carbonyl emissions between 4.40 mg/mile and 11.91 mg/mile over the UDDS cycle, with 3 times higher carbonyl emissions for a new truck compared with the oldest truck tested over the UDDS cycle in the study. The near-dock port cycle with extended idle and creep mode operation results in the highest total carbonyl and formaldehyde emissions. Acetone and acetaldehyde emissions were detected in the same magnitude as formaldehyde from the exhaust natural gas vehicles. In the case of the higher mileage, natural-gas-powered vehicles had acetone emissions that were measured to be two to three times greater than formaldehyde emissions. Carbonyl emissions from diesel trucks equipped with DPF and SCR were similar in magnitude to that observed from natural gas engines equipped with TWC. Carbonyl emissions from the HPDI vehicle were close to 89% lower than for the stoichiometric natural gas vehicle. The near-dock driving cycle resulted in the highest total carbonyl and formaldehyde emissions from all technology vehicles, with the dual-fuel and diesel engines equipped with DPF and SCR showing total carbonyl emissions that were three times higher than natural gas engines equipped with TWC. Increased EGR rates are often used as the primary in-cylinder NOx control strategy during idle and creep mode operation. EGR simply reduces combustion efficiency and lowers combustion temperatures. Therefore, the resulting lower combustion efficiency could possibly contribute to high engine-out emissions of carbonyls. In addition to high EGR, low exhaust temperatures associated with low load engine operation could lead to a lower oxidation catalyst activity in reducing formaldehyde emissions.
Figure 3. Total carbonyl and formaldehyde emission rates from diesel and natural gas good movement vehicles over UDDS and port drayage cycles.
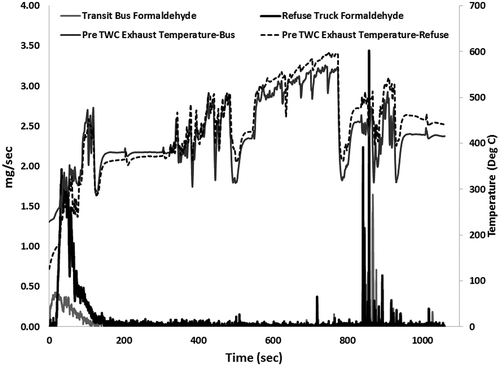
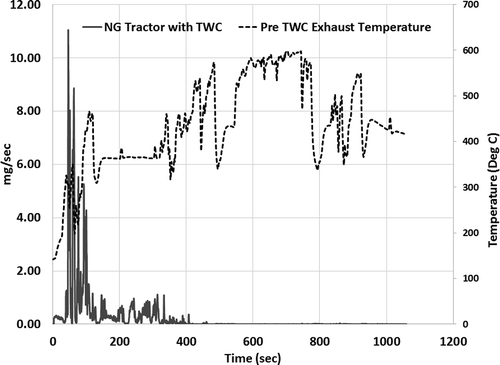
shows the real-time tailpipe formaldehyde emissions measured from a TWC equipped natural gas transit bus and refuse truck over a cold-start UDDS driving cycle. The test was conducted after an overnight soak to simulate cold-start operation of the natural gas vehicles. The results show that the bulk of the formaldehyde emissions occur within the first 200 sec of the cold start operation, after which the TWC achieves light-off conditions. The results also show the differences in formaldehyde emissions between a transit bus and refuse truck as a result of duty-cycle-based aging of the engine and after-treatment system. The refuse truck with lower accumulated miles emitted two times as much formaldehyde emissions as did the transit bus with two times the accumulated miles. The duty cycle of refuse haulers in general is characterized by rapid acceleration, deceleration, extended idling, and creep mode operation. The South Coast Air Quality Management District refuse truck driving cycle, developed by West Virginia University, which is used in this study, is representative of refuse hauler operation in Los Angeles, CA. The vehicle operation simulated in this driving cycle is characterized as an aggressive duty cycle, due to multiple acceleration and deceleration events of magnitude 2.5 mph/s and –4 mph/s, respectively. In addition to the highly transient garbage transportation mode, the garbage pick-up activity is also characterized by extended idle/creep time. A case study by the U.S. Department of Energy on CNG refuse fleets show that on average a refuse truck stops 460 times a day and idles close to 60 minutes on a 9-hr average use per day (Laughlin and Burnham, Citation2014). Rapid changes in engine load, extended idle, and creep can contribute to insufficient lubrication of engine components, reduced lubrication oil life, and low exhaust temperatures leading to catalyst deactivation due to hydrocarbon condensation. Day-to-day operation of vehicles over duty cycles such as one similar to the refuse truck can result in accelerated aging of engine components and after-treatment systems.
BTEX
shows the results of the BTEX emission rates measured for the natural gas and diesel vehicles over the UDDS driving cycle. BTEX emissions were below the 10-ppbv detection limit for all vehicles, over all driving cycles. The main source of BTEX emissions from natural gas engines is typically from excessive lubrication oil combustion; however, results from this study indicate that the high exhaust temperature activity of the TWC catalyst helps reduce BTEX emissions to levels below detection of the analytic method. Higher chain hydrocarbons have the lowest catalytic light-off temperatures (Mizutani, Okawa, et al., Citation1998). The exhaust gas temperatures entering the DOC are most often conducive for oxidation of higher chain hydrocarbons; therefore, it is expected that the tailpipe signature of BTEX from current-technology diesel engines would be below detection limits. Similar findings are also presented by a study conducted by Khalek et al. (Khalek, Blanks, et al., Citation2013). Furthermore, SCR after-treatment systems exhibit affinity toward adsorption of hydrocarbons (Luo, Yezerets, et al., Citation2012); therefore, a hydrocarbon slip through the DOC would most likely be adsorbed by the SCR.
Table 4. Carbonyl and BTEX emissions results from natural gas and diesel vehicles.
Ammonia
shows the distance-specific ammonia emissions from natural-gas and heavy-duty diesel vehicles for different vehicle vocations. For the goods movement application, the NG tractor 1 with the lowest mileage emitted the lowest distance-specific ammonia emissions over all driving cycles. Communication with the engine manufacturer indicated that typically a degreening period lasts for 4000 miles. Therefore, NG tractor 1 was still in the degreening period of the engine during which the catalyst has not achieved its peak activity, which could be the underlying reason for lower ammonia production. Both NG tractors 2 and 3 emitted an average of two to five times higher ammonia than NG tractor 1, depending on the driving cycle. The NG transit bus emitted distance-specific ammonia emissions of 0.89 and 0.95 g/mile over the UDDS and OCTA cycle, respectively, while the NG refuse truck emitted 1.17 and 1.09 g/mile over the UDDS and refuse truck cycle, respectively.
Table 5. Distance-specific ammonia emission rates from different vocation natural gas and diesel vehicles.
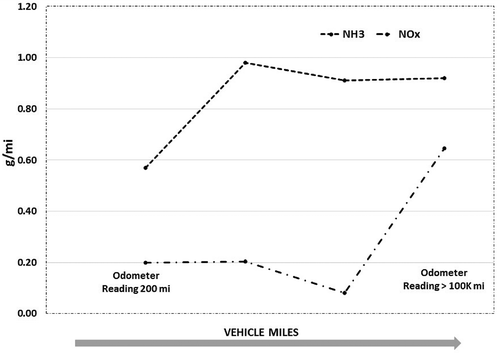
shows the effect of vehicle mileage on the NOx and ammonia emissions from natural gas vehicles. Results are indicative of an increasing trend in NOx emissions with higher vehicle miles. However, the results of this trend are not conclusive due to the limited data available and warrant a larger data set of vehicles of different mileage to derive a conclusive relationship between NOx and ammonia emissions as a function of vehicle age. Nevertheless, the results do indicate a trend that could be contributed by both chemical and thermal aging of the catalyst with increased vehicle miles. Thermal and chemical aging of catalysts will contribute to reduction in active catalyst surface area that would contribute to an increase in tailpipe emissions of criteria pollutants. The closed-loop feedback control from the tailpipe oxygen sensor is a critical parameter in maintaining optimum air-fuel ratio for the simultaneous reduction of CO, THC, and NOx (Thomas, Soltis, et al., Citation1999). Ammonia production and NOx conversion from a TWC are dependent on the dithering frequency of the changes in air–fuel ratio between lean and rich operation. A study by Carriero et al. shows that deterioration of oxygen sensors may alter the dithering frequency and sensor response, causing the operating air–fuel ratio of engines to be leaner or richer (Massimo, Mauro, et al., Citation1998). An air–fuel ratio shift to the richer side will increase ammonia emissions, while an overly lean mixture will lower ammonia and increase NOx emissions (Defoort, Olsen, et al., Citation2003).
Greenhouse gas emissions
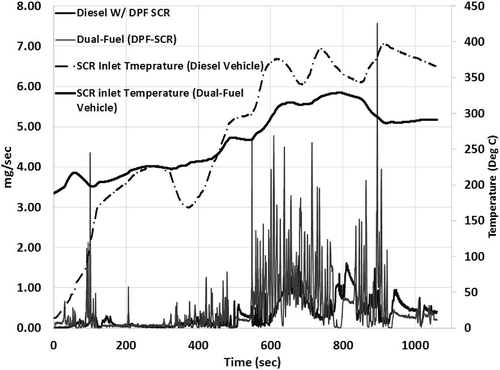
N2O and CH4 emissions were measured in real-time as raw emissions samples from the tailpipe using a high-speed FTIR (MKS 2030 HS). shows the instantaneous N2O emissions from NG tractor, while details the instantaneous N2O emissions from diesel and dual-fuel tractors over the UDDS cycle. N2O emissions from the NG tractor are observed only during the warm-up phase of the catalyst, with instantaneous emissions of up to 10 mg/sec observed during the first 200 sec of the UDDS cycle. The results show that a thermally functioning TWC does not produce any N2O emissions. Results show that N2O formation from both diesel and dual-fuel vehicles equipped with a DPF and SCR are dominant during the vehicle operation that coincides with high exhaust temperature conditions. This could be possibly attributed to decomposition of ammonium nitrate species formed in the SCR after-treament (Hallstrom, Voss, et al., Citation2013). shows an order of magnitude higher N2O emissions from the dual-fuel tractor compared to the diesel tractor over the port drayage cycle (Kamasamudram, Henry, et al., Citation2012).
Table 6. Distance-specific emission rates of N2O and CH4 from natural gas, diesel, and dual-fuel vehicles of different vocations.
Figure 7. Instantaneous nitrous oxide emissions over UDDS cycle from diesel and dual-fuel tractor equipped with DPF and SCR.
Methane emissions were observed only from the natural gas vehicles. More than 95% of the total hydrocarbon emissions composition was measured to be methane. Catalytic oxidation of methane is highly challenging, as it requires the highest catalytic light-off temperatures. Although temperatures on stoichiometric engine platforms often exceed the light-off conditions for methane oxidation, the delicate balance in dithering air–fuel ratio for optimizing NOx conversion and oxygen storage for NMHC reduction in TWC could possibly result in lower methane oxidation. A study by Deffort et al. shows that an air–fuel ratio corresponding to maximum NOx reduction also results in lowest methane oxidation or highest methane slip (Defoort, Olsen, et al., Citation2003). shows methane emissions averaged from all the NG tractors over the UDDS cycle to be 1.74 g/mile. Methane emissions were lowest over the regional cycle consisting of extended freeway operation. The highest methane emission rates are observed from the transit bus and refuse truck applications with 6.86 and 6.41 g/mile, respectively. The results are indicative of an increasing methane emissions trend with the increased mileage of the respective vehicle. The high methane emissions from the refuse truck application could possibly be attributed to the accelerated deterioration of engine and after-treatment components contributed by a rigorous refuse truck duty cycle.
As expected, the methane emissions from the HPDI dual-fuel goods movement vehicle were higher than for the stoichiometric NG tractor. Although the HPDI engine employs an advanced direct injection of natural gas technology, the high-octane fuel properties results in methane slip through the combustion chamber. In addition, the lean-burn compression ignition results in lower exhaust temperatures that negatively affect the catalytic oxidation of methane. The highest distance-specific tailpipe methane emissions were observed over the local and near-dock cycle at 4.71 g/mile and 5.61 g/mile, respectively. Results from the study show that vehicle operation over the local cycle resulted in exhaust temperatures below 250°C for 59% of cycle duration, while near-dock cycle resulted in 88% of the vehicle operation resulting in temperatures below 250°C (Thiruvengadam, Besch, et al., Citation2015). Exhaust temperatures during the near-dock and local cycle are well below those of the light-off conditions for methane and therefore resulted in high methane emissions over these two cycles.
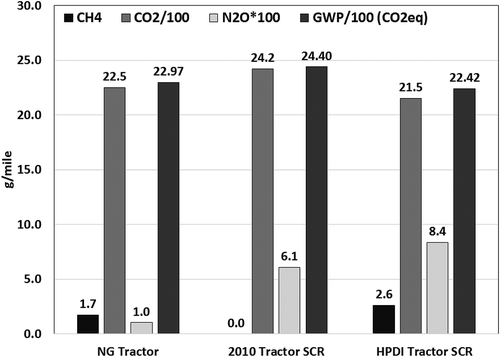
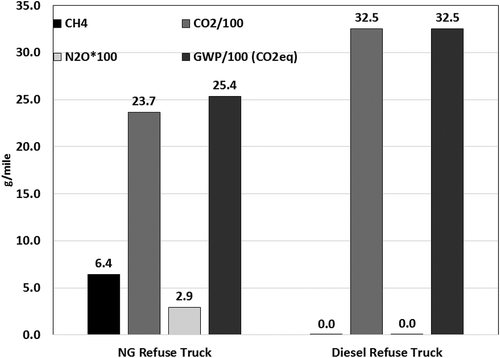
and show the comparison of distance-specific global warming potential (GWP) of diesel and natural gas vehicles in goods movement and refuse truck applications, respectively. The GWP is expressed for the UDDS driving cycle in equivalent CO2 (CO2eq) units. CH4 and N2O emissions were converted to CO2eq by assuming a 100-year GWP of 25 and 298 times GWP of CO2, respectively. The GWP values of stoichiometric natural gas vehicles with TWC are on average 6% lower than the diesel truck with SCR, while the GWP of the dual-fuel HPDI vehicle with SCR was 8% lower than the diesel truck with SCR. CH4 emissions from both the stoichiometric and the HPDI goods movement vehicles contributed to less than 3% of the total GWP of the tailpipe emissions. N2O emissions do not contribute significantly to the GWP of the tailpipe emissions of both diesel and natural gas vehicles. For the refuse truck application, the GWP of the natural gas vehicle with TWC was 22% lower than for the diesel refuse truck with SCR. Diesel refuse trucks equipped with SCR frequently employ a thermal management strategy to improve SCR activity. Strategies to increase exhaust temperatures will invariably result in increased fuel consumption, leading to a net increase in CO2 emissions. CH4 emissions from a natural gas refuse truck contributed 6% of the total tailpipe GWP emissions. The relatively small contribution of CH4 to total GWP of natural gas vehicles was also previously shown by Yoon et al. in a study conducted on transit buses equipped with engines similar to those tested in this study (Yoon, Collins, et al., Citation2013).
Summary
Current-technology heavy-duty diesel and natural gas engines are dependent on complex engine controls and after-treatment systems to achieve low NOx and PM emissions. The stoichiometric natural gas engine with TWC has shown low tailpipe NOx emissions characteristics over all driving cycles. The unregulated emissions results show that the high exhaust temperatures and the subsequent activity of the TWC is effective in lowering all BTEX emissions to levels below detection. Formaldehyde emissions were detected only during the brief warm-up period of the TWC during cold start operations, while the dual-fuel HPDI with diesel-like operation resulted in no BTEX emissions. However, high carbonyl emissions were observed during driving cycles characterized by extended idle and creep mode operation.
Stoichiometric natural gas engines with TWC are characterized with high levels of ammonia emissions in the order of 1 g/mile. An air–fuel ratio slightly rich of stoichiometric conditions provide the maximum NOx reduction from the TWC. This operating condition is also the most conducive to ammonia production from TWC. Since NOx reduction is the priority in criteria pollutant reduction, ammonia emissions from stoichiometric engines are an inevitable byproduct. As ammonia is a potent secondary PM precursor, further research into the pathways required for ammonia abatement from stoichiometric engines is needed. The results of the study indicate a trend of higher emission rates of carbonyls and methane with higher vehicle mileage. However, due to the limited data available, to correlate vehicle age and corresponding emission rates, the findings are merely indicative and cannot be considered conclusive. There is a knowledge gap in the literature that documents the effect of engine and after-treatment aging on the emissions rates of unregulated pollutants.
Natural gas vehicles exhibit a greater GWP advantage compared to diesel vehicles in the refuse truck application rather than on the goods movement application. This can be attributed to the fact that thermal management strategies that increase fuel consumption are much more aggressive in refuse truck application than on goods movement tractor application.
Additional information
Notes on contributors
Arvind Thiruvengadam
Arvind Thiruvengadam an assistant professor at West Virginia University, Mechanical and Aerospace Department at Morgantown, WV.
Marc Besch
Marc Besch is researcher at the Center for Alternative Fuels, Engine and Emissions (CAFEE) at West Virginia University at Morgantown, WV.
Daniel Carder
Daniel Carder serves as the director of CAFEE at West Virginia University at Morgantown, WV.
Adewale Oshinuga
Adewale Oshinuga is a project manager in the Science and Technology Advancement Office at South Coast Air Quality Management District, at Diamond Bar, CA.
Randall Pasek
Randall Pasek works as a manger in the off-road section in South Coast Air Quality Management District, at Diamond Bar, CA.
Henry Hogo
Henry Hogo is the assistant deputy executive officer for the Mobile Source Division in the office of Science and Technology Advancement, at Diamond Bar, CA.
Mridul Gautam
Mridul Gautam serves as the Vice President for Research and Economic Development at the University of Nevada, Reno.
References
- Andrew, K., D. Littlejohn, et al. 2008. Trends in on-road vehicle emissions of ammonia. Atmos. Environ. 43(8): 1565–70.
- Ball, D., D. Moser, et al. 2013. N2O emissions of low emissions vehicle. SAE Int. J. Fuels Lubricants 6(2): 450–56. doi:10.4271/2013-01-1300
- Besch, C.M., J. Israel, et al. 2015. Emissions characterization from different technology heavy-duty engines retrofitted for CNG/diesel dual-fuel operation. SAE Int. J. Engines 8(3). 1342–1358. doi:10.4271/2015-01-1085
- Bielaczyc, P., A. Szczotka, et al. 2012. Comparison of ammonia emission factors from light-duty vehicles operating on gasoline, liquefied petroleum gas (LPG) and compressed natural gas (CNG). SAE 2012-01-1095.
- Buchholz, B., R. Dibble, et al. 2003. Quanitfying the contribution of lubrication oil carbon to particulate emissions from a diesel engine. SAE 2003-01-1987.
- Chow, C. J., G J. Watson, et al. 2007. The IMPROVE_A temperature protocol for thermal/optical carbon analysis: Maintaining consistency with a long-term database. J. Air Waste Manage. Assoc. 57:1014–23. doi:10.3155/1047-3289.57.9.1014
- Couch, P. 2011. Development of a drayage truck chassis dynamometer test cycle. Irvine, CA: TIAX LLC.
- Defoort, M., D. Olsen, et al. 2003. The effect of air–fuel ratio control strategies on nitrogen compound formation in three-way catalysts. Int. J. Engine Res. 5(1): 115–22. doi:10.1243/146808704772914291
- Hajbabaei, M., G. Karavalakis, et al. 2013. Impact of natural gas fuel composition on criteria, toxic and particle emissions from transit buses equipped with lean-burn and stoichiometric engines. Energy 62: 425–34. doi:10.1016/j.energy.2013.09.040
- Hallstrom, K., K. Voss, et al. 2013. The formation of N2O on the SCR catalyst in a heavy-duty US 2010 emission control system. SAE 2013-01-2463.
- Health Effects Institute. 2008. Mobile-source air toxics: A critical review of the literature on exposure and health effects. A. T. R. Panel. Special Report 16. Boston, MA: Health Effects Institute.
- Herner, D.J., S. Hu, et al. 2011. Effect of advanced aftertreatment for PM and NOx reduction on heavy-duty diesel engine ultrafine particle emissions. Environ. Sci. Technol. 45(6): 2413–19. doi:10.1021/es102792y
- Holmen, B.A., and A. Ayala. 2002. Ultrafine PM emissions from natural gas, oxidation catalyst diesel and particulate-trap diesel heavy-duty transit buses. Environ. Sci. Technol. 36(23): 5041–5050. doi:10.1021/es015884g
- Huai, T., T. Durbin, et al. 2003. Investigation of NH3 Emissions from New Technology Vehicles as a Function of Vehicle Opearting Conditions. Environmental Science and technology 37(21): 4841–4847. doi:10.1021/es030403+
- Intergovernmental Panel on Climate Change. 2007. IPCC fourth assessment report: Climate change 2007. https://www.ipcc.ch/publications_and_data/publications_ipcc_fourth_assessment_report_synthesis_report.htm. Retrieved March 21, 2014.
- Kado, N., R. Okamato, et al. 2005. Emissions of toxic pollutants from compressed natural gas and low sulfur diesel-fueled heavy-duty transit buses tested over multiple driving cycles. Environ. Sci. Technol. 39: 7638–49. doi:10.1021/es0491127
- Kamasamudram, K., C. Henry, et al. 2012. N2O formation and mitigation in diesel aftertreatment systems. SAE Int. J. Engines 5(2): 688–98. doi:10.4271/2012-01-1085
- Khalek, A.I. 2007. 2007 Diesel particulate measurement research—Final report, Project E-66 Phase 3. Alpharetta, GA: Coordinating Research Council.
- Khalek, A.I., M. Blanks, et al. 2013. Phase 2 of the Advanced Collaborative Emissions Study. Southwest Research Institute. http://www.crcao.org/reports/recentstudies2013/ACES%20Ph2/03-17124_CRC%20ACES%20Phase2-%20FINAL%20Report_Khalek-R6-SwRI.pdf
- Khalek, A.I., T. Bougher, et al. 2011. Regulated and unregulated emissions from highway heavy-duty diesel engines complying with U.S Environmental Protection Agency 2007 emissions standards. J. Air Waste Manage. Assoc. 61(4): 427–42. doi:10.3155/1047-3289.61.4.427
- Koebel, M., M. Elsener, et al. 2001. Recent advances in the development of urea-SCR for automotive applications. SAE Technical Paper 2001-01-3625.
- Laughlin, M. and A. Burnham. 2014. Case study—Compressed natural gas refuse fleets. U.S. Department of Energy. http://www.afdc.energy.gov/uploads/publication/casestudy_cng_refuse_feb2014.pdf
- Liu, G. Z., E. M. Thurow, et al. 2005. Transient performance of diesel particulate filters as measured by an engine exhuast particle size spectrometer. SAE 2005-01-0185.
- Liu, G. Z., V. N. Vasys, et al. 2012. Comparison of strategies for the measurement of mass emissions from diesel engines emitting ultra-low levels of particulate matter. Aerosol Sci. Technol. 43(11): 1142–52. doi:10.1080/02786820903219035
- Lloyd, C.A., and T. Cackette. 2011. Diesel engines: Environmental impact and control. J. Air Waste Manage. Assoc. 51(6): 809–47. doi:10.1080/10473289.2001.10464315
- Luo, J.-Y., A. Yezerets, et al. 2012. Hydrocarbon poisoning of Cu–zeolite SCR catalysts. SAE SAE-2012-01-1096.
- Massimo, C., M. Mauro, et al. 1998. Poisoning of lambda sensor: An experimntal method to measure lambda sensor switch velocity and its effect on air–fuel ratio excursion. SAE 982647.
- Mizutani, A., T. Okawa, et al. 1998. Oxygen sensor applications as ULEV or tighter emission vehicle. SAE 980264.
- Renner, E., and R. Wolke. 2008. Formation of secondary inorganic aerosols by high ammonia emissions simulated by LM/MUSCAT. In Air Pollution Modeling and Its Application XIX, ed. C. Borrego and A. Miranda, 522–29. Dordrecht, The Netherlands: Springer.
- Suarez-Bertoa, R., A.A. Zardini, et al. 2014. Ammonia exhaust emissions from spark ignition vehicles over the New European Driving Cycle. Atmos. Environ. 97 (November):43–53. doi:10.1016/j.atmosenv.2014.07.050
- Thiruvengadam, A., M. Besch, et al. 2011. Influence of real-world engine load conditions on nanoparticle emissions from a DPF and SCR equipped heavy-duty diesel engine. Environ. Sci. Technol. 46(3): 1907–13. doi:10.1021/es203079n
- Thiruvengadam, A., M. Besch, et al. 2015. Emission rates of regulated pollutants from current technology heavy-duty diesel and natural gas goods movement vehicles. Environ. Sci. Technol. 49(1): 5236–44. doi:10.1021/acs.est.5b00943
- Thiruvengadam, A., M. Besch, et al. 2014. Characterization of particulate matter emissions from a current technology natural gas engine. Environ. Sci. Technol. 48(1): 8235–42. doi:10.1021/es5005973
- Thiruvengadam, A., D.K. Carder, et al. 2011. Effect of an economical oxidation catalyst formulation on regulated and unregulated pollutants from natural gas fueled heavy duty transit buses. Transport. Res. D Transport Environ. 16(6): 469–73. doi:10.1016/j.trd.2011.04.003
- Thomas, J.A., R.E. Soltis, et al. 1999. Laboratory and engine studies of the effect of NOx on the response of heated exhaust gas oxygen sensors. SAE 1999-01-1079.
- USEPA. 1999. Compendium method TO-15—Determination of volatile organic compounds (VOCs) in air collected in specially-prepared canisters and analyzed by gas chromatography/mass spectrometry (GC/MS). Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air. Cincinnati, OH: USEPA.
- USEPA. 2011. Final Rulemaking to establish greenhouse gas emissions standards and fuel efficiency standards for medium- and heavy-duty engines and vehicles. USEPA. Office of Transportation and Air Quality. EPA-420-R-11-901. https://www3.epa.gov/otaq/climate/documents/420r11901.pdf
- Verma, V., M. M. Shafer, et al. 2010. Contribution of transition metals in the reactive oxygen species activity of PM emissions from retrofitted heavy-duty vehicles. Atmos. Environ. 44: 5165–73. doi:10.1016/j.atmosenv.2010.08.052
- Winebrake, J., M. Wang, et al. 2001. Toxic emissions from mobile sources: A total fuel-cycle analysis for conventional and alternative fuel vehicles. J. Air Waste Manage. Assoc. 51: 1073–86. doi:10.1080/10473289.2001.10464325
- Yoon, S., J. Collins, et al. 2013. Criteria pollutant and greenhouse gas emissions from CNG transit buses equipped with three-way catalysts compared to lean-burn engines and oxidation catalyst technologies. J. Air Waste Manage. Assoc. 63(8): 926–33. doi:10.1080/10962247.2013.800170
- Yoon, S., S. Hu, et al. 2014. Chemical and toxicological properties of emissions from CNG transit buses equipped with three-way catalyst compared to lean-burn engines and oxidation catalyst technologies. Atmos. Environ. 83(1): 220–28. doi:10.1016/j.atmosenv.2013.11.003