ABSTRACT
Passive samplers are used in air quality monitoring for many years to compete in terms of being economical with continuous measurement systems. In this study, different amounts of single-wall carbon nanotubes (SWCNTs) were added in the impregnation solution of the filters of passive samplers and the effect on the absorption of ozone studied. The results of the measurement of ozone with varying amounts of SWCNTs added to the impregnation solution of the filters of the passive samplers were compared with the results of the continuous ozone measurement system (CS). Measurements were performed for 7 days and 14 days at two different exposure times. The increase of the amount of SWCNTs on the filters of the passive samplers, however, did not have an effect on the measurement of ozone. The measurement results of the passive samplers of the 14-day exposure periods, alternating with the 7-day exposure periods, were lower considerably than the results of the 7-day exposure.
Implications: The accuracy and the use of passive samplers in SWCNTs are expected to provide high measurement results. Observing the effect of the change in the amount of diffusion of pollutants held in the SWCNT is also one of the expected implications.
Introduction
In urban and regional air pollution, a tropospheric ozone is a persistent pollutant. This problem has many critical effects in large urban areas that are associated with the wind. Critical meteorological conditions such as solar radiation and temperature, nitrogen oxide emissions, and volatile organic compounds may lead to the increase in ambient concentrations of ozone (National Research Council [NRC], 1991).
Monitoring of tropospheric ozone with passive samplers is used as a measurement method, and therefore many commercial samplers have been developed (Sanz et al., Citation2007). This method was an alternative in continuous measurement systems for networks related to high-density measurements where necessary. Although the uncertainties associated with passive sampler standards still continue, it has been seen that continuous measurement systems reveal results with high correlations (Helaleh et al., Citation2002; Caballero, Citation2007). A summary of impregnation solutions used in developed passive samplers and measurement performances is given in .
Table 1. Absorbents/adsorbents and analysis techniques for ozone pollutant though passive sampling.
The amount of sample in a passive sampler is checked by physical procedures, including diffusion and permeability. The basis for the approach is that the transfer of a gas through a passive sampler can be expressed using the equation for a diffusion flux (Palmes and Gunnison, Citation1973; Koutrakis et al., Citation1993):
An average ozone concentration:
where J is the mass flux of ozone (µg/cm2/sec), D is the coefficient of diffusion (cm2/sec), dc/dx is the concentration gradient (moles/cm2/sec), A is the cross-sectional area of diffusion zone (cm2), L is the length of the diffusion zone (cm), M is the net NO3− concentration (µg/mL), V is the extraction volume (mL), is the molecular weight of ozone (µg/µmol), S is the collection rate (cm3/min)·(m3/106 cm3), K = 0.0409 (µmol/ppb·m3), T is sampling time (min), C0 is the concentration within the chamber (ppm), and Ca is ambient ozone concentration (ppm).
A total weighted average (TWA) constitutes the basis in passive samplers, and a calibration is of great importance in order to achieve reliable results. Many environmental factors such as temperature, turbulence, and humidity in the ambient air may affect the calibration (Ouyang and Pawliszyn, Citation2007). In the lower temperatures, which can be tolerated by plastic substrates, and at room temperatures, ozone wears single-wall carbon nanotubes down and has high reactivity to C=C double bonds (Peng et al., Citation2011). The diffusion is determined by a static air duct. The type of sampler tube is a limitation in the air duct. This limitation eliminates the effect of wind speed and reduces the sensitivity of the sample. Additionally, the diffusion is determined by the pore size of the membrane. Therefore, the diffusion filter has an effect on the amount of adsorption. Nitrogen dioxide may interfere due to the impregnation solution used (Helaleh et al., Citation2002; Tatsiana et al., 2007). The temperature, precursor ozone concentration, and other meteorological parameters, especially pressure and solar radiation, may interfere in the ozone production (Brown, Citation2000; Hauser et al., Citation2015). In studies on the changes in the structure of carbon nanotubes exposed to ozone, it has been observed that oxidated nanofilms caused structural deteriorations (Akdim et al., Citation2007). Correspondingly, a significant decrease was observed in the nanotube length during mechanical procedures (Tang et al., Citation2011).
In recent years, the research shows that carbon nanotubes (CNTs) are a good adsorbent. It is assumed that single-wall carbon nanotubes (SWCNTs) would absorb tropospheric ozone in the atmosphere due to adsorbent properties. This study investigated whether or not there is an effect on the determination of ozone using added SWCNTs to the impregnation liquid of the filters of passive samplers.
Materials and methods
Continuous system analyzer
The continuous ozone measurement device used was Environnement SA O3 41M (Poissy, France). The analyzer has a detection limit of 2 μg/m3 and is accurate to ±1% in the 0−2000 μg/m3 range. The accuracy of the analyzer is ±1%. The continuous ozone analyzer (CS) (Environnement SA, O3 41M) is calibrated once a year according to the U.S. Environmental Protection Agency (EPA) calibration protocols (EPA, 1979, 2013).
Field study
The measurements were performed in the organized industrial zone located 12 km northeast of Samsun city center. The coordinates of the measurement point is 41°14′24″N, 36°26′03″E, and the height of measurement point is 8 m above sea level. The measuring point is located 1.60 km from the Samsun-Ordu Highway. The continuously measuring probe and passive samplers on top of the mobile air quality monitoring vehicle are at 2.5 m height from the ground level. There are no trees or buildings near the same height, as the measurement point is in a garden in the small and medium business development and support administration (KOSGEB).
Nanomaterial
The SWCNTs were purchased from Sigma-Aldrich, Inc. (xx, xx). The properties of SWCNTs are >90% carbon, ≥77% carbon as SWCNTs, 0.7–1.4 nm diameter, 0.091 g/cm3 bulk density, and 1.7–1.9 g/cm3 at 25 °C. The manufacturing method of the nanomaterial is a catalytic chemical vapor deposition (CVD), and the quality factor is ≥0.940.
Preparation of impregnation solution
The filter that would be impregnated was washed in 500 mL pure water (TKA GenPure; Niederelbert, Germany) and dried in an oven at 100 °C for 1 hr, then it was cooled in a desiccator for 15 min. Exposure to light during these procedures was reduced to minimum. The impregnation solution was obtained with the addition of 0.3 g NaNO2, 0.28 g KCO3, and 1 mL glycerol to 100 mL with ultrapure water. The SWCNTs were added to the impregnation solution by dissolving respectively 0, 0.1, 0.5, 1, and 5 mg into 100 mL impregnation solution. This solution was used to impregnate the filters of the passive samplers. Each sample was stirred in a sonicator with a 75% amplitude for 5 min. Overall, five sets of the impregnation solution were prepared containing respectively 0, 1, 5, 10, and 50 mg/L SWCNTs and kept at 4 °C in the refrigerator.
Preparation of the passive sampler
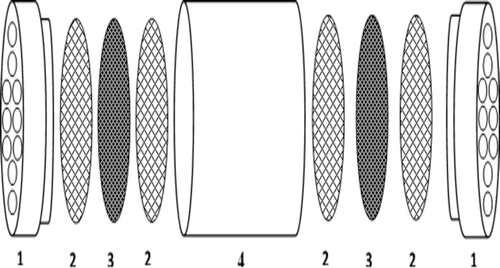
Impregnated solutions were stirred in a light blind closed cabin, using a sonicator (Bandalin HD 2070; Berlin, Germany) at 60% amplitude for 30 min, then 0.5 mL solution taken from the mixture was added dropwise onto the filter (Pall Grass Fabric Filters, 1 µm; New York, NY, USA) that was be impregnated and left to drying in a dark environment. The glass fabric filter was made of borosilicate glass microfibers with acrylic binder. High particulate-holding capacity makes the discs efficient depth filters and allows for filtration of large volumes of solutions. The properties of the filter were aerosol retention (0.3 µm di-octyl phthalate (DOP)) 99.97%, diameter 47 mm, thickness 330 µm, and air flow rate 21 mL/min/cm2 (0.7 bar) (catalog no: 515-0008.VWR.2009; Atlanta, GA, USA). The dried filter, as is shown in , was placed into the passive sampler.
Figure 1. Components of the Ogawa passive sampler. 1, diffuser end caps; 2, stainless screen; 3, impregnated filter with single-wall carbon nanotubes (SWCNTs); 4, body. Modified from Mulik et al. (Citation1991).
Data analysis
At the end of 1- and 2-week exposures, filter and the other apparatuses (grill, ring, and supporting plastic) in the samplers were placed in a dark bottle containing 5 mL of ultrapure water in a dark environment. To ensure the transition of nitrate into the ultrapure water, each sample was stirred in a sonicator with a 100% amplitude for 15 min. At the end of the procedure, the sample was filtered with a syringe filter (Millew-HA Filter, 0.45 µm; MCE, Darmstadt, Germany) and analyzed in ion chromatography devices (Ogawa & Co., Citation2001). The measurements for samplers and reference passive samplers were performed in two different time periods in two different ion chromatography devices due to the technical fault. The technical information of ion chromatography analyses is given in .
Table 2. The technical information of different ion chromatography analyses.
The sampler was exposed to an average ozone concentration determined by following equation (Ogawa & Co., Citation2001):
Simplified:
where passive sampling rate for ozone is 21.08 mL/min.
Results and discussion
It was noted that the ozone concentrations as measured with the continuous ozone measurement system (CS) were lower than those measured with the passive filters. In 2007, in the same location, research had been carried out with the same measurement device (CS). The ozone measurement results of this study were similar to those in 2007. According to the research, the wind speeds in November 2006, December 2006, January 2007, February 2007, and April 2007 were respectively 0.78, 0.68, 1.17, 1.06, and 0.92 m/sec. The solar radiations were respectively 57.3, 49.1, 148.5, 135.4, and 107.6 W/m2. The ambient temperatures were respectively 10.8, 6.2, 9.36, 6.74, and 10.56 °C (Akdemir, Citation2007). The environmental conditions during the sampling periods were not different from the usual wind velocities and temperatures and thus not affecting the transport of ozone to the filters. Nevertheless, an average correlation of R2 = 0.57 between the results of the passive samplers (PS) and the results of the continuous ozone measurement system (CS) was determined, with P < 0.001. The correlation coefficient was r = 0.75; this value is usual for passive samplers (). But the correlation between each other for passive samplers was low due to sampling error and interferences. The correlations between specific concentrations of SWCNTs and the CS may be accidental. More research is necessary to confirm the observations of the specific correlations.
The results of ozone measurements for the consecutive measuring periods at ambient environmental exposures of passive samplers prepared with the addition of different amounts of SWCNTs are displayed in .
Figure 2. The course from November 2013 to May 2014 of the mean ground-level ozone concentrations as measured with passive samplers with the addition of respectively 0, 5, 10, and 50 mg/L single-wall carbon nanotubes (SWCNTs) on the absorption filters.
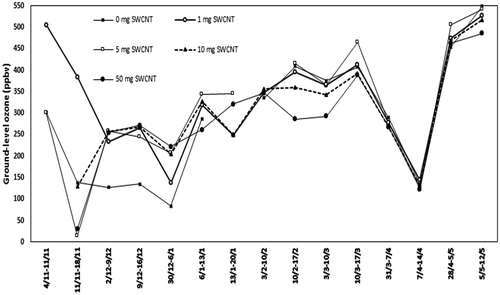
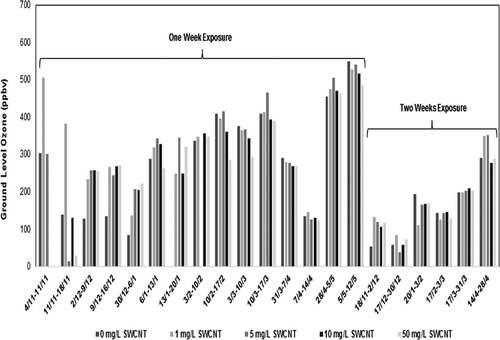
Figure 3. Comparison per exposure period of the ozone concentration of the passive samplers as measured with the addition of respectively 0, 1, 5, 10, and 50 mg/L single-wall carbon nanotubes (SWCNTs) on the absorption filters.
The variation in measurement results within a measuring period tended to become smaller when the concentration of ozone increased. This phenomenon can be attributed to the fact that small analytical inaccuracies have a relatively lesser effect on the measuring results of high concentrations of ozone than on low concentrations. In , the ozone values started to increase with the increase in temperature. The increase in ozone values by May, especially, supported the studies in the literature (Caballero et al., Citation2007); the increase in ozone at this region, which is close to the shore, during summer season due to the solar radiation is effective in this result.
It should be understood that in the future, sampling experiments with the passive samplers must be conducted excluding interferences. This research is necessary, as the interferences have too much influence on the results of the passive samplers. Experiments in the future with added SWCNTs on the filters of the passive samplers should not be hampered by the interference of other pollutants.
It is conspicuous that the 14-day measuring results are lower than the measuring results of the 7-day exposures (see ). If the amount of ozone on the filter reaches a saturation level, then the concentrations of the measurements should be at least as high as the last periods of the 7-day exposures. A possible reason can be that the solution of glycerol/water mixture during the exposure gradually evaporates water, and therefore the nitrite ions cannot properly react with ozone. Also, other unknown interferences can cause the difference. The only solution to this problem must be extra research by simultaneously measuring 7-day and 14-day exposures.
SWCNTs can adsorb gases such as ammonia, nitrogen, sulfur gases, aromatic structures, and ethylene of double bond. Additionally, troposheric ozone can trigger the formation of hydroxyl radical and thus may result in changing the structure of the oxidized SWCNTs. It should be noted that these interferences were not measured and are unknown. The turbulence that occurs with a contribution of wind can cause a shortening of the effective diffusion path of the passive sampler. However, the environmental conditions during the sampling periods were not specifically different (Gair and Penkett, Citation1995; Heal and Cape, Citation1997). The glass fabric filter that was used for the experiments with the passive samplers is used in many studies (Levin et al., Citation1989; Brown, Citation1993; Occupational Safety and Health Administration [OSHA], 2008) and is not believed to be a source of interfering substances. However, as O3 is analyzed as nitrate, particulate nitrate compounds may positively interfere in the analysis and sulfur dioxide may negatively interfere. The changing of the analytical conditions (measurement using different types of ion chromatography) may interfere. Conductivity when analyzed may be affected by interferences. However, the possible sources of interferences, e.g., nitrate particles and OH• radicals and their effects on the results of the measurements have not been measured and are unknown.
If ozone reacts preferably with the added SWCNTs on the impregnated filters of the passive samplers, then the measurement values at the bank must have the highest concentration of ozone for all measuring periods. This hypothesis could be tested using a chi-square test for differences. Excluded in the evaluation of results were the first two periods due to starting-up problems.
According to chi-square test, there was not a significant influence of the added SWCNTs on the ozone measurement results with the passive filters (n = 15 with n = 18). The reason for not having an effect of the added SWCNTs on the ozone measurement can be a too low concentration of the added SWCNTs on the filter. Another reason can be that the SWCNTs are embedded in the glycerol solution and thus not available for ozone. The nitrite, however, is dissolved in a solution of glycerol and water and is available for easy oxidation.
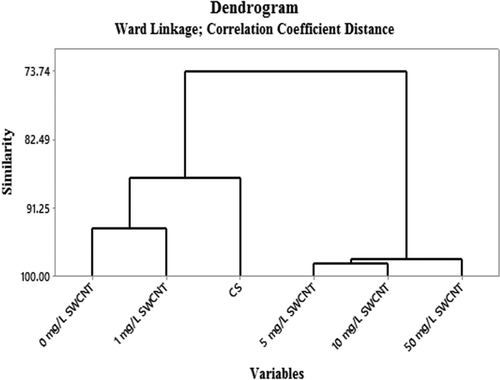
A clustering graph regarding ozone measurement results is given in . A hierarchical dendrogram shows two large separations: the 0 and 1 mg/L SWCNTs to the CS with a similarity of 89%, and the cluster from 5 to 50 mg/L SWCNTs with a similarity of 98% to the CS. The overall similarity with the CS monitor was 73%. These results may indicate a systematic difference in the reaction with ozone on the filters due to added SWCNTs. However, the differences in correlations may be accidental, and more research is necessary for confirmation of these phenomena.
Conclusions
This study investigated whether or not there is an effect of added SWCNTs on the measurement of ozone on the absorption filters of passive samplers. A significant difference between the concentration of ozone measured with the passive sampler with 0 mg/L (the blank) added SWCNTs on the absorption filter and with any of the respectively 1, 5, 10, and 50 mg/L SWCNT-impregnated absorption filters could not be detected. A reason for not measuring an effect of the added SWCNTs on the ozone measurement can be a too low concentration of the added SWCNTs on the filter and that the SWCNT tubes are embedded in the glycerol solution and thus not available for ozone.
The lower ozone concentrations measured with the passive samplers for a 14-day exposure with respect to the results of the 7-day exposure could not be explained. A gradual evaporation of water out of the solution of the glycerol/water mixture on the filter during the elongated exposure time resulting in a gradual diminishing capacity of the nitrite ions to react with ozone and unknown interferences of air pollutants could be caused by the lower concentration of the 14-day exposure. More research is necessary, as the periods of the 7-day and 14-day exposures in this study were not coinciding but alternating. The correlation coefficient, r, of the relation between the average results of the five passive samplers per period of 7-day exposure and the reference ozone monitor (CS) was 0.75 and is usual for passive sampling systems. Excluded in the evaluation of results were the first two periods due to starting-up problems. A hierarchical dendrogram showed two large separations, the 0 and 1 mg/L SWCNTs with a similarity of 89% to the CS, and the cluster from 5 to 50 mg/L SWCNTs with a similarity of 98% to the CS. The overall similarity with the CS monitor was 73%. These results may indicate a systematic difference in the reaction with ozone on the filters due to added SWCNTs. Environmental conditions, such as wind velocity and temperature, during the sampling periods were normal. Interferences of air pollutants such as nitrate particulates and OH• radicals were not measured and are unknown. Deviations in the response of the reference ozone monitor (CS) could not be detected. However, the differences in correlations may be accidental, and more research is necessary for confirmation of these phenomena.
Acknowledgment
The author would like to thank Hüseyin K. Ozcan, Serdar Aydın, Eren Bagislar, and Bora Filiz for their helpful comments and discussion.
Funding
The research was funded by Turkish Scientific and Technical Research Council (TUBITAK), project no: 113Y034.
Additional information
Funding
Notes on contributors
A. Akdemir
A. Akdemir is an assistant professor in the Department of Environmental Engineering, Ondokuz Mayis University, in Samsun, Turkey.
References
- Akdemir, A. 2007. Investigation and monitoring of air quality parameters in organized industrial region of Samsun. Ph.D. dissertation, Ondokuz Mayis University, Samsun Turkey.
- Akdim, B., T. Kar, X. Duan, and R. Patcher, 2007. Density functional theory calculations of ozone adsorption on sidewall single-wall carbon nanotubes with stone-wales defects. Chem. Phys. Lett. 445:281–287.
- Bernard, N.L., M.J. Gerber, C.M. Astre, and M.J. Saintot, 1999. Ozone measurement with passive samplers: Validation and use for ozone, pollution assessment in Montpellier France. Environ. Sci. Technol. 33:217–222. doi:10.1021/es971140k
- Brauer, M., and J.R. Brook, 1995. Personal and fixed-site ozone measurements with a passive sampler. J. Air Waste Manage. Assoc. 45:529–533. doi:10.1080/10473289.1995.10467384
- Brown, R.H. 1993. The use of diffusive samplers for monitoring of ambient air. Pure Appl. Chem. 65:1859–1874. doi:10.1351/pac199365081859
- Brown, R.H. 2000. Monitoring the ambient environment with diffusive samplers: Theory and practical considerations. J. Environ. Monit. 2:1–9. doi:10.1039/a906404d
- Caballero, S., N. Galindo, C. Pastor, M. Varea, and J Crespo. 2007. Estimated tropospheric ozone levels on the southeast Spanish Mediterranean coast. Atmos. Environ. 41: 2881–2886. doi:10.1016/j.atmosenv.2006.12.047
- Campos, V.P., L.P.S. Cruz, R.H.M. Godoi, A.F.L. Godoi, and T.M. Tavares, 2010. Development and validation of passive samplers for atmospheric monitoring of SO2, NO2, O3 and H2S in tropical areas. Microchem. J. 96:132–138. doi:10.1016/j.microc.2010.02.015
- Gair, A.J., and S.A. Penkett, 1995. The effect of wind speed and turbulence on the performance of diffusion tube samplers. Atmos. Environ. 29:2529–2533. doi:10.1016/1352-2310(95)91998-8
- Grosjean, D., E.L. Williams, and E. Grosjean, 1995 Monitoring ambient ozone with a network of passive samplers: A feasibility study. Environ. Pollut. 88:267–273. doi:10.1016/0269-7491(95)93439-7
- Hauser, C.D., A. Buckley, and J. Porter, 2015. Passive sampler and community science in regional air quality measurement, education and communication. Environ. Pollut. 203:243–249.
- Hauser, T.R., and D.W. Bradley, 1966. Specific spectrophotometric determination of ozone in the atmosphere using 1,2-di-(4-pyridyl)ethylene. Anal. Chem. 38:1529–1532. doi:10.1021/ac60243a018
- Heal, M.R., and J.N. Cape, 1997. A numerical evaluation of chemical interferences in the measurement of ambient nitrogen dioxide by passive diffusion samplers. Atmos. Environ. 13:1911–1923. doi:10.1016/S1352-2310(97)00025-3
- Helaleh, M.I.H., S. Ngudiwaluyo, T. Korenaga, and K. Tanaka 2002. Development of passive sampler technique for ozone monitoring. estimation of indoor and outdoor ozone concentration. Talanta 58:649–659. doi:10.1016/S0039-9140(02)00375-2
- Koutrakis, P., J.M. Wolfson, A. Bunyaviroch, S.E. Froehlich, K. Hirano, and J.D. Mulik, 1993. Measurement of ambient ozone using a nitrite-coated filter. Anal. Chem. 65:209–214. doi:10.1021/ac00051a004
- Levin, J.O., R. Lindahl, and K. Andersson, 1989. Monitoring of parts-per-billion levels of formaldehyde using a diffusive sampler. JAPCA 39:44–47. doi:10.1080/08940630.1989.10466506
- Manning, W.J., S.V. Krupa, C.J. Bergweiler, and K.I. Nelson, 1996. Ambient ozone (O3) in three Class I wilderness areas in the northeastern USA: Measurement with Ogawa passive sampler. Environ. Pollut. 91:399–404. doi:10.1016/0269-7491(95)00075-5
- Mulik, J.D., J.L. Varns, P. Koutrakis, D.D. Wolfson A. Bunyaviroch, D.D. Williams, and K.G. Kronmiller, 1991. Using passive sampling devices to measure selected air volatiles for assessing ecological change. In Proceedings of the 1991 US EPA/A&WMA International Symposium, Measurement of Toxic and Related Air Pollutants, 1–6. Pittsburgh, PA: Air and Waste Management Association.
- National Research Council, Committee on Troposferic Ozone Formation and Measurement. 1991. Rethinking the Ozone Problem in Urban and Regional Air Pollution, Washington, DC: The National Academies Press.
- Occupational Safety and Health Administration. 2008. Ozone in Workplace Atmospheres (Impregnated Glass Fiber Filter). ID-214. Washington, DC: Occupational Safety and Health Administration.
- Ogawa & Co. 2001. Ogawa &Co Protocol for Ozone Measurement Using Passive Sampler Badge. Pompano Beach, FL: Ogawa & Co. ogawausa.com/wp-content/uploads/2014/04/proozone.pdf (accessed 2016).
- Ouyang, G., and J. Pawliszyn, 2007. Configurations and calibration methods for passive sampling techniques. J. Chromatogr. 1168:226–235. doi:10.1016/j.chroma.2007.01.133
- National Research Council, Committee to Review and Assess the Health and Productivity Benefits of Green Schools. 2006. Green Schools: Attributes for Health and Learning. Washington, DC: National Research Council.
- Palmes, E.D., and A.F. Gunnison, 1973. Personal monitoring device for gaseous contaminants. Am. Ind. Hyg. Assoc. J. 34:78–81. doi:10.1080/0002889738506810
- Peng, K., L.Q Liu, H. Li, H. Meyer, and Z. Zhang, 2011. Room temperature functionalization of carbon nanotubes using an ozone/water vapor mixture. Carbon 49:70–76. doi:10.1016/j.carbon.2010.08.043
- Rusina, T.P., F. Smedes, J. Klanova, K. Booij, and I. Holoubek, 2007. Polymer selection for passive sampling: A comparison of critical properties. Chemosphere 68:1344–1351. doi:10.1016/j.chemosphere.2007.01.025
- Salem, A.A., A.A. Soliman, and I.A el-Haty. 1999. Determination of nitrogen dioxide, sulfur dioxide, ozone, and ammonia in ambient air using the passive sampling and potentiometric analyses. Air Qual. Atmos. Health 2:133–145.
- Sanz, M.J., V. Calatayud, and S.P. Pena, 2007. Measures of ozone concentrations using passive sampling in forest of south western Europe. Environ. Pollut. 145:620–628. doi:10.1016/j.envpol.2006.02.031
- Surgi, M.R., and J.A. Hodgeson, 1985. 10,10’-Dimethyl-9,9’-biacridylidene impregnated film edge dosimeters for passive ozone sampling. Anal. Chem. 57:1737–1740. doi:10.1021/ac00285a052
- Tang, L.C., H. Zhang, J.H. Han, X.P Wu, and Z. Zhang, 2011. Fracture mechanisms of epoxy filled with ozone functionalized multi-wall carbon nanotubes. Composites Sci. Technol. 72:7–13. doi:10.1016/j.compscitech.2011.07.016
- U.S. Environmental Protection Agency. 1979. Technical Assistance Document for the Calibration of Ambient Ozone Monitors. EPA-600/4-79-057. Research Triangle Park, NC: U.S. Environmental Protection Agency.
- U.S. Environmental Protection Agency. 2013. Transfer Standards for Calibration of Ambient Air Monitoring Analyzers for Ozone. EPA-454/B-13-004. Research Triangle Park, NC: U.S. Environmental Protection Agency.
- Yamada, E., M Kimura, K. Tomozawa, and Y. Fuse, 1999. Sample analysis of atmospheric NO2, SO2, and O3 in mountains by using passive samplers. Environ. Sci. Technol. 33:4141–4145. doi:10.1021/es981163e
- Zhou, J., and S. Smith, 1997. Measurement of ozone concentrations in ambient air using a badge-type passive monitor. J. Air Waste Manage. Assoc. 47:697–703. doi:10.1080/10473289.1997.10463932