ABSTRACT
A series of iron–manganese oxide catalysts supported on TiO2 and titanium nanotubes (TNTs) were studied for low temperature selective catalytic reduction (SCR) of NO with NH3 in the presence of SO2. The results showed that the specific surface area and the amount of Brønsted acid sites were highly correlated. The results also demonstrated that higher Mn4+/Mn3+ ratios and larger specific surface areas might be the main reasons for the excellent performance of MnFe-TNTs catalyst after SO2 poisoning. The SO2 poisoning effect could be minimized by reducing the GHSV, increasing the reaction temperature, or increasing the [NH3]/[NO] molar ratio. The results also indicated that the formation of ammonium sulfate had a stronger effect on the NO conversion efficiency as compared to the formation of metal sulfate. Thus operating the low temperature SCR at above 230 oC to avoid the formation of ammonium sulfate would be the priority choice when SO2 poisoning is a concerned issue. Implications: Low-temperature selective catalytic reduction (SCR) has attracted increasing attention due to that it can reduce the energy consumption for the SCR process employed in industries such as steel plants and glass manufacturing plants. However, it also suffers from the sulfur dioxide (SO2) poisoning problem. This study investigates the possibility of using titania nanotubes (TNTs) as the support of Mn/Fe bimetal oxide catalysts for low-temperature SCR to reduce the SO2 poisoning. The results indicated that the MnFe-TNT catalyst can tolerate SO2 for a longer time as compared with the MnFe-TiO2 catalyst.
Introduction
Nitrogen in fuels or air combines with oxygen to yield nitrogen oxides (NOx) from stationary sources such as power plants, iron and steel industry, and glass manufacturing plants is an important air pollution issue, with combustion processes typically emitting NOx mixtures of around 95% nitric oxide (NO) and 5% nitrogen dioxide (NO2) (Gupta, Citation2000; Busca et al., Citation1998). And selective catalytic reduction (SCR) with NH3 is one of the most efficient and economic technologies for the removal of NOx from stationary sources. The general SCR reaction is 4NO + 4NH3 + O2 → 4N2 + 6H2O. And the most commonly used catalysts in conventional SCR systems are usually mixed vanadium-tungsten/titanium oxide (V2O5–WO3/TiO2)–based catalysts, which can retain high NOx conversion efficiencies at 300~400 °C (Li, Citation2014; Cha et al., Citation2013; Kompio et al., 2012; Lee et al., Citation2012a; Balle et al., Citation2009). However, the conventional SCR catalysts have several problems, including the high oxidation rate of sulfur dioxide (SO2) to sulfur trioxide (SO3) with vanadium(V) oxide (V2O5) catalyst, and the low N2 selectivity at high temperatures (Lu et al., Citation2014). Furthermore, the flue gas stream has to be reheated when used at flue gas temperature of lower than 300 °C, which consumes a lot of energy. Therefore, researchers have been interested in the development of low-temperature SCR catalysts in recent years (Yao et al., Citation2010; Jiang et al., Citation2009; Wu et al., Citation2008; Smirniotis et al., Citation2006; Park et al., Citation2001).
Literature data showed that Mn-based catalysts had good activity for low-temperature SCR with NH3 (Lee and Bai, Citation2016; Liu et al., Citation2014a, Citation2014b; Su et al., Citation2013; Xu et al., Citation2012a; Lee et al., Citation2012b; Tang et al., Citation2007; Marban et al., Citation2001). However, the presence of SO2 could result in poisoning effect on the catalysts at low temperatures. There have been researchers studying the SO2 poisoning at temperatures of 150~250 °C (Shen et al., Citation2014; Tong and Huang, Citation2012; Liu et al., Citation2011a; Yu et al., Citation2010; Wu et al., Citation2009; Huang et al., Citation2008). And the catalyst could be deactivated because of the ammonium sulfate particles that occupied the active sites (Cheng et al., Citation2010; Yu et al., Citation2010; Huang et al., Citation2008).
It was demonstrated that TiO2 had chemical stability and a higher resistance ability to sulfur poisoning, so TiO2 has been commonly used as the support of SCR catalysts (Ettireddy et al., Citation2007; Wallin et al., Citation2004). And there have been a few studies that used titania nanotubes (TNTs) instead of TiO2 as the support for low-temperature SCR. Wang et al. (Citation2011) showed that Ce/TNTs had a higher SCR activity than Ce/TiO2(P25). Chen et al. (Citation2007) synthesized Cu-loaded TNTs that had high SCR activity, and they pointed out that the layered structure of the titanate allowed intercalation of metal ions. The nanotubes not only improved the ion dispersion on the support but also inhibited the agglomeration of the loaded species during the subsequent calcination or reaction. Castillo et al. (2014) demonstrated that the addition of vanadium and tungsten to titanic acid nanotubes (TANs) provided stronger and more active Brønsted and Lewis acid sites. Pappas et al. (Citation2016) used manganese confined to different TiO2 and TNT supports for the low-temperature SCR of NO. They indicated that the high activity of the manganese-confined titania nanotube catalysts was attributed to the high surface area of the support.
Literature results demonstrated the unique tubular structures of TNTs, which provided higher surface area in their interior spaces as well as better SCR efficiency (Xiong et al., Citation2010; Min et al., Citation2012; Chen et al., Citation2007). Nonetheless, to the authors’ knowledge, there has been no work on SO2 poisoning effect via the comparison of TNT and TiO2 supports. And there has been no direct evidence to discuss on the correlation between NO conversion and the specific surface area under SO2 poisoning. In this study, Mn and Fe metal oxides supported on three types of TiO2 (P25, ST01, and TiO(OH)2) and TNTs (made from P25, ST01, and TiO(OH)2) were prepared at different calcination temperatures and tested for their low-temperature SCR of NO in the presence of SO2. These catalysts were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), inductively coupled plasma optical emission spectroscopy (ICP-OES), Brunauer-Emmett-Teller (BET) method, ammonia (NH3) temperature-programmed desorption (NH3-TPD), X-ray photoelectron spectroscopy (XPS), and thermogravimetric analysis (TGA) to evaluate the crystal phase, metal contents, surface structure, and surface acidity of the catalysts as well as to identify the sulfate species that poisons the catalysts.
Experimental
Synthesis of TNTs
The TNT supports used in this study were synthesized by hydrothermal method. In a typical procedure, 6 g of TiO2 (P25, ST01, or TiO(OH)2) powder was mixed and sonicated with 180 mL of 10 N NaOH solution, followed by hydrothermal treatment at 135 °C for 24 hr in an autoclave. The produced slurry was filtered then sonicated in 1 L of HNO3 solution at pH 1.6. Then the suspension was filtered and washed with deionized (DI) water several times until the rinsed pH value reached 6.5–7. Finally, the material was dried at 120 °C for 12 hr. The TNTs prepared by different TiO2 sources of P25, ST01, and TiO(OH)2 were named TNT(P25), TNT(ST01), and TNT(TiO(OH)2), respectively.
Synthesis of SCR catalysts
Mn and Fe metal oxides were respectively supported on TiO2 (P25, ST01, or TiO(OH)2) or TNTs (P25, ST01, or TiO(OH)2) by the co-precipitation method. In a typical procedure, TiO2 or TNTs (8 g), ferric nitrate 9-hydrate (11.57 g), manganese(II) acetate tetrahydrate (7.13 g), and DI water (76 mL) were mixed and then adjusted to pH = 10 with ammonia solution (25 wt%) to form a precipitate. The precipitate was filtrated and washed using DI water, and dried at 120 °C for 12 hr. Finally, it was calcined at 350, 450, or 550 °C for 6 hr in air.
SCR activity test
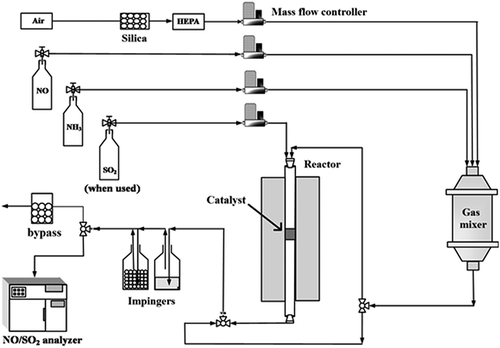
A schematic diagram of the experimental setup for low-temperature SCR reaction is shown in . Pelletized catalysts were sieved (16–30 mesh) and used for the activity tests. The total flow rate for the SCR activity test was 1200 mL/min, and gas concentrations were 220 ppmv for NO, 100 ppmv for SO2 (when used), and 15 vol% for O2. The baseline condition for [NH3]/[NO] molar ratio was 0.91, which is the typical injection ratio of many commercialized SCR system to avoid ammonia slip. And the baseline condition for gas hourly space velocity (GHSV) and SCR reaction temperature were 20,000 hr−1 and 150 °C, respectively. However, the [NH3]/[NO] molar ratios, GHSVs, and SCR temperatures were varied in the ranges of 0.91~1.82, 10,000~40,000 hr−1, and 150~300 °C, respectively, for evaluating the operation parameter effects. The feed gases were mixed in a gas mixer. And the catalysts were preheated in the SCR reactor (150 °C) for 90 min to ensure that it reached an isothermal reaction temperature. During the SCR test, the NO concentrations at the inlet and outlet of the reactor were monitored by a NO analyzer (Ultramat 23; Siemens, Taiwan). The NO conversion is defined by
Characterization
The morphology of the catalysts was obtained from a high-resolution scanning electron microscope (SEM) (S-4700I; Hitachi). The metal contents were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) using an Agilent 700 series instrument. X-ray diffraction (XRD) measurements were carried out on a computerized Rigaku D/MAX-2500X with Cu Kα radiation. The diffraction data were collected in the 2θ range between 10° and 80°. The XRD phases of the catalyst samples were identified using the Joint Committee on Powder Diffraction Standards (JCPDS) powder data files.
The Brunauer-Emmet-Teller (BET) specific surface area and pore volume of the catalysts were measured by N2 adsorption at liquid nitrogen temperature using a Micromeritics ASAP2020 instrument. Prior to the N2 adsorption, the samples were degassed at 300 °C for 12 hr. The surface areas were determined by a BET equation in 0.05–0.30 partial pressure range. The pore volume distributions were determined by the Barrett-Joyner-Halenda (BJH) method from the desorption branch of the isotherms.
The NH3 temperature-programmed desorption (NH3-TPD; Micromeritics AutoChem II 2920) was used to detect the surface acidity of catalysts. Prior to the TPD experiments, 0.1 g samples were pretreated at 250 °C in a flow of He (25 mL/min) for 30 min, and cooled down to 50 °C. Then the samples were exposed to a flow of 15% NH3/He at 50 °C for 1 hr, followed by He purged for another hour. Finally, the TPD measurement was carried out from 50 to 900 °C at a ramping rate of 10 °C/min. The amount of NH3 desorbed from the catalysts was monitored by the TCD.
X-ray photoelectron spectroscopy (XPS) measurements were performed on a VG Scientific Microlab 350 with Al Kα radiation (1486.6 eV). Binding energies (BEs) of Mn 2p were calibrated using C 1s peak (BE = 284.6 eV) as the standard. Thermogravimetric analysis (TGA) was conducted to determine the sulfate species forming on the surface of catalysts with a NETZSCH TG 209 F1 apparatus. The heating program was carried out under an airflow of 10 mL/min, with a heating rate of 10 °C/min from room temperature to 900 °C.
Results and discussion
Catalyst characterization
A total of 18 SCR catalyst samples were prepared, with 9 samples of MnFe-TiO2–based catalysts and 9 samples of MnFe-TNT–based catalysts. The specific surface areas of the 18 samples of MnFe-TiO2 and MnFe-TNT catalysts are listed in . The catalysts supported by TNTs (made from P25, ST01, and (TiO(OH)2)) calcined at 350 °C had specific surface areas of 243~305 m2/g, which were 2~4 times higher than their pristine TiO2 (70~140 m2/g). The surface area decreased when the calcination temperature was increased from 350 to 550 °C due to catalyst aggregation and sintering.
Table 1. Characterization of the MnFe-TiO2 and MnFe-TNT catalysts.
The Mn and Fe contents on the TiO2 and TNT supports are also listed in . It can be seen that regardless of the TiO2 or TNT supports, all catalysts had similar Mn and Fe contents of 10.8–12.0 and 12.0–13.4 wt%, respectively. Even though the TNT supports usually had much higher specific surface area, they did not accommodate more metals on the supports. And this observation was also true for the MnFe-TNT(TiO(OH)2) samples calcined at different temperatures. Therefore, the calcination temperature did not affect the metal content either. As a result, the following discussion was made under the same amount of active metals coating on each catalyst.
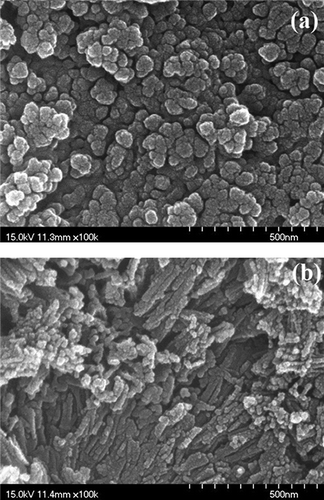
The SEM images of MnFe-TiO(OH)2 and MnFe-TNT(TiO(OH)2) are shown in and , respectively. It is observed in that MnFe-TiO(OH)2 had particle sizes of around 50 nm. On the other hand, MnFe-TNT(TiO(OH)2) was in cylindrical shape, with diameters of around 10–30 nm and lengths of around 100~300 nm, as shown in . The cylindrical shape of the MnFe-TNT catalysts indicated that the tubular structure of TNT supports was not destroyed during the co-precipitation and calcination procedures of catalyst preparation.
Figure 2. SEM images of catalysts prepared at calcination temperature 350 °C: (a) MnFe-TiO(OH)2; (b) MnFe-TNT(TiO(OH)2).
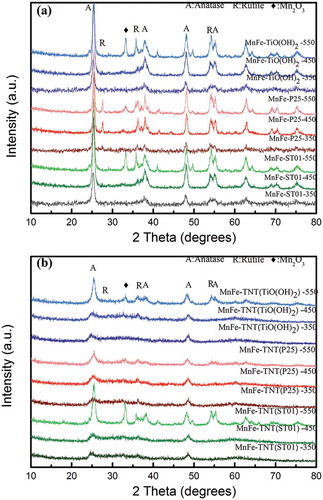
The crystal phases of the MnFe-TiO2 and MnFe-TNT catalysts calcined at different temperatures were revealed by the XRD results shown in and , respectively. The peaks located at 25.4, 37.8, 48.0, and 54.5 corresponded to the (101), (004), (200), and (105 and 211) planes of the anatase phase (JCPDS 21-1272), and the peaks located at 27.5, 36.1, and 54.4 corresponded to the (110), (101), and (211) planes of the rutile phase (JCPDS 21-1276). The peaks located at 32.9, 38.2, 45.2, 49.3, 55.2, and 65.8 corresponded to the (111), (200), (220), (311), (222), and (400) planes of the Mn2O3 (JCPDS 41-1442), respectively. No clear peaks of Mn2O3 were observed at 350 and 450 °C, which implied that manganese could be at a highly dispersive state that might enhance the catalytic activity (Liu et al., Citation2009, Citation2014c; Saqer et al., Citation2011). But at the calcination temperature of 550 °C, all the catalysts showed Mn2O3 phase, and a part of the anatase phase was converted to the rutile phase.
Figure 3. XRD patterns of catalysts prepared under different calcination temperatures: (a) MnFe-TiO2; (b) MnFe-TNTs.
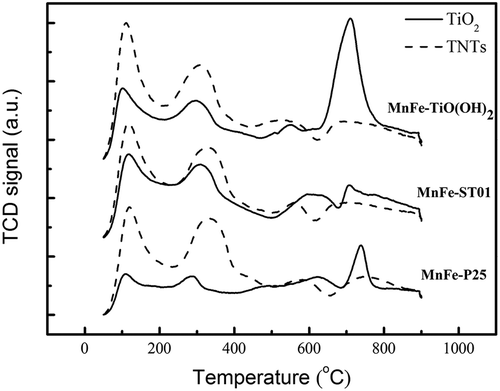
The NH3-TPD analysis was performed to investigate the amounts of Brønsted acid and Lewis acid sites on the MnFe-TiO2 and MnFe-TNT catalysts. From the literature (Jin et al., Citation2010; Mhamdi et al., Citation2009), the peaks at around 50~200 °C were due to physical desorption whereas the broad desorption peaks spanned at around 200~500 °C were attributed to NH3 desorbed by Brønsted acid sites. The chemisorbed NH3 molecules released at desorption temperatures of about 500~850 °C were referred to Lewis acid sites. And the total acidity was obtained from the sum of the peaks at 200~500 and 500~850 °C. shows the NH3-TPD curves for catalysts calcined at 350 °C. It is observed that the peaks of Lewis acid sites decreased whereas those of Brønsted acid sites increased when the supports of TiO2 were replaced by TNTs.
Figure 4. NH3-TPD patterns of MnFe-TiO2 and MnFe-TNT catalysts at calcination temperature of 350 °C. The solid lines represent for the results of MnFe supported on TiO2, whereas the dash lines are for those on TNTs.
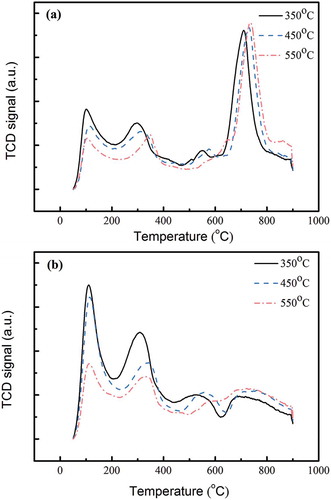
The NH3-TPD curves of MnFe-TiO2 and MnFe-TNTs under different calcination temperatures are shown in and , respectively. It can be clearly seen that Brønsted acid sites of MnFe-TNTs decreased with increasing calcination temperature. But the amounts of Lewis acid sites of MnFe-TNTs did not change significantly with calcination temperature. On the other hand for the MnFe-TiO2 catalyst, both the amounts of Brønsted acid sites and Lewis acid sites did not change much with the calcination temperature.
Figure 5. NH3-TPD patterns of (a) MnFe-TiO2 and (b) MnFe-TNTs (both made from TiO(OH)2) calcined at different temperatures.
The variation in the acid site amount with respect to the calcination temperature of the catalysts can be seen more clearly from : they are listed at different temperature ranges of 200~500 and 500~850 °C. Take the catalysts calcined at 350 °C as examples, the results showed that the NH3-TPD desorption amounts at the lower temperature range (200~ 500 °C) by catalysts supported on TiO2 of P25, ST01, and TiO(OH)2 were 0.23, 0.40, and 0.44 mmol/g, respectively. And they were 0.92, 0.84, and 0.88 mmol/g for catalysts supported on TNTs of P25, ST01, and TiO(OH)2, respectively. Thus, it is obvious that the amounts of Brønsted acid sites for all TNT-supported metal catalysts were higher than those of the TiO2-based catalysts, and among the former the MnFe-TNT(P25) catalyst had the highest amount of Brønsted acid sites. On the other hand, the NH3-TPD desorption amount at the higher temperature range (500–850 °C) by catalysts supported on P25, ST01, and TiO(OH)2 as calcined at 350 °C were 0.42, 0.31, and 0.89 mmol/g, respectively. And they were 0.30, 0.26, and 0.35 mmol/g for catalysts supported on TNT(P25), TNT(ST01), and TNT(TiO(OH)2), respectively. The results showed that the MnFe-TNT catalysts had slightly smaller amounts of Lewis acid sites than those of the MnFe-TiO2. And the MnFe-TiO(OH)2 catalyst had the highest amount of Lewis acid sites.
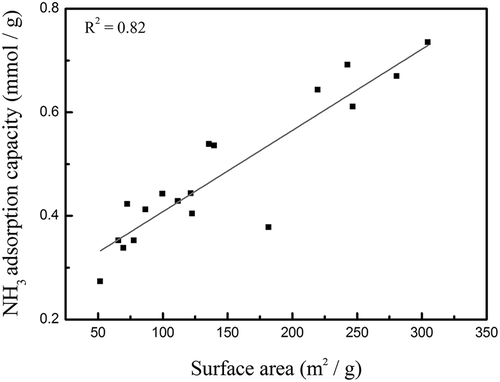
In order to understand the correlation between the specific surface areas of the catalysts and their NH3-TPD desorption amounts, linear regression analyses were performed for both of the amounts of Brønsted acid sites and Lewis acid sites using a total of 18 catalyst data listed in . The Lewis acid sites, however, showed no correlation with the specific surface area. Thus, only the correlation result between the Brønsted acid site amount and the specific surface area is shown in . It can be seen that the specific surface area and the Brønsted acid site amount were linearly correlated, with R2 value of 0.82. The correlation could be even better (R2 = 0.90) if excluding the two data of MnFe-P25-550 and MnFe-TNT(ST01)-550, both were calcined at 550 °C. Although MnFe-P25-550 and MnFe-TNT(ST01)-550 had specific surface areas of 52 and 182 cm2/g, respectively, they didn’t have high amounts of Brønsted acid sites as expected according to the linear regression result. This may be explained by that MnFe-P25-550 had the most significant rutile phase at the major peak of 2θ = 27° (Thamaphat et al., Citation2008), and by that MnFe-TNT(ST01)-550 had the highest peak intensity of Mn2O3. This might indicate that in addition to the specific surface area, the TiO2 crystal phase and the well dispersion of the metal particles could also play a role in the amount of Brønsted acid sites on the catalyst surface.
SCR activity
The low-temperature SCR catalysts can be active at temperatures of below 300 °C (Choi et al., Citation2013; Shen et al., Citation2011; Richter et al., Citation2002). In this study, the test range of low-temperature SCR was from 150 to 300 °C. The tests were conducted without introducing SO2 for the first 3 hr. Then the SO2 cylinder was turned on at the end of the third hour to introduce SO2 poisoning effect. shows the results of NO conversion as a function of operation time over the MnFe-TiO2 and MnFe-TNT (both TiO(OH)2 based) catalysts calcined at 350 °C. During the first 3 hr, both MnFe-TiO2 and MnFe-TNTs showed high NO conversion efficiencies of 92.5% and 97.5%, respectively. It reveals that although the inlet [NH3]/[NO] ratio was only 0.91, the NO conversion could be higher than 91%. This maybe because that a portion of NO was oxidized into NO2 at low temperatures instead of directly reduced to N2 by NH3. The formation of NO2 from NO would facilitate the SCR reaction at low temperatures and is usually referred as the “fast SCR” reaction (Lee and Bai, Citation2016; Xu et al., Citation2012b).
Figure 7. Comparison of NO conversion between TiO2- and TNT- (both made from TiO(OH)2) based MnFe catalysts calcined at 350 °C in the NH3-SCR reaction. Reaction conditions: reaction temperature = 150 °C, [NO] = 220 ppm, [NH3] = 200 ppm, [SO2] = 100 ppm, [O2] = 15%, balanced with air, and GHSV = 20,000 hr−1.
![Figure 7. Comparison of NO conversion between TiO2- and TNT- (both made from TiO(OH)2) based MnFe catalysts calcined at 350 °C in the NH3-SCR reaction. Reaction conditions: reaction temperature = 150 °C, [NO] = 220 ppm, [NH3] = 200 ppm, [SO2] = 100 ppm, [O2] = 15%, balanced with air, and GHSV = 20,000 hr−1.](/cms/asset/75a5b445-94cb-44bb-8494-7d3dfe1b3f7c/uawm_a_1231144_f0007_b.gif)
In Taiwan and some developed countries, the large industrial sources are often required to reduce their SO2 emission concentrations to be less than a few tens of ppm by environmental regulations. However, the SO2 concentration used in this study was 100 ppm in order to speed up the SO2 poisoning test. When SO2 was added at the end of the third hour, the NO conversion efficiency of MnFe-TiO2 and MnFe-TNTs decreased to 72% and 86%, respectively, after 1 hr of SO2 poisoning test as observed in . And they remained roughly stable for around 2–3 hr, then the NO conversion efficiencies decreased again for both catalysts. At the end of tests (i.e., after 5 hr of SO2 poisoning), the NO conversion efficiencies of MnFe-TiO2 and MnFe-TNTs further decreased to 50% and 75%, respectively. The results also showed that when using TNTs as the support, the NO conversion efficiency was less affected by SO2 poisoning as compared with the TiO2-based catalyst.
It is well known that the calcination temperature has great effects on the metal crystal size and the specific surface area of the catalysts, which is an indication on the thermal stability of the catalysts. Thus, the calcination temperature effect on the SCR activity was studied and compared in for TiO2- and TNT- (both TiO(OH)2 based) supported MnFe catalysts. The data tested without SO2 were shown by averaging over 3 hr of SCR operation time, and the data tested with SO2 were obtained after 5 hr of SO2 poisoning time. And to understand variation of results during sample preparation and SCR performance measurement, the MnFe-TiO(OH)2 and MnFe-TNT(TiO(OH)2) samples were prepared and tested on their SCR activity for two to three times, and the deviations for the three duplicated samples are shown by error bars. shows that when SO2 was not present, the deviations in NO conversion efficiency of MnFe-TiO(OH)2 and MnFe-TNT(TiO(OH)2) were only within 1~2%. On the other hand, after SO2 poisoning, the deviations in NO conversion efficiency of MnFe-TiO(OH)2 and MnFe-TNT(TiO(OH)2) slightly increased to 2~4.5%. This reveals that the catalysts had good reproducibility in NO conversion of within 2% deviation in the absence of SO2 and within 4.5% deviation in the presence of SO2.
Figure 8. NO conversion with and without the presence of SO2 in the NH3-SCR reaction over MnFe-TiO2 and MnFe-TNT (both made from TiO(OH)2) catalysts at different calcination temperatures. Reaction conditions: reaction temperature = 150 °C, [NO] = 220 ppm, [NH3] = 200 ppm, [SO2] = 100 ppm, [O2] = 15%, balanced with air, and GHSV = 20,000 hr−1.
![Figure 8. NO conversion with and without the presence of SO2 in the NH3-SCR reaction over MnFe-TiO2 and MnFe-TNT (both made from TiO(OH)2) catalysts at different calcination temperatures. Reaction conditions: reaction temperature = 150 °C, [NO] = 220 ppm, [NH3] = 200 ppm, [SO2] = 100 ppm, [O2] = 15%, balanced with air, and GHSV = 20,000 hr−1.](/cms/asset/20936dac-52a3-4fb3-b392-46b35903f263/uawm_a_1231144_f0008_b.gif)
One can see from that for the MnFe-TNTs, the NO conversion had slight changes at different calcination temperatures in the absence of SO2. It was reduced by only 10%, from 98% at 350 °C to 88% at 550 °C. But the NO conversion decreased more significantly for MnFe-TiO2–based catalysts when calcination temperature was increased from 350 °C (93%) to 550 °C (63%). This might be due to that the high-temperature aggregation of metal catalysts was more significant with the TiO2 support as compared with that with the TNT support (Chen et al., Citation2007).
On the other hand, when the catalyst was exposed to 5 hr of SO2 poisoning, the NO conversion was 76% for MnFe-TNT catalyst calcined at 350 °C. And it decreased to 55% when the calcination temperature was increased to 550 °C. For MnFe-TiO2 catalyst calcined at 350 °C, the NO conversion efficiency was already low at 50% under the presence of SO2. But it was even lower at only 17% when the catalyst was calcined at 550 °C. The variation in the NO conversion at different calcination temperatures revealed that TNT support had a better thermal stability under both conditions of with and without SO2 poisoning as compared with that of the TiO2 support.
In order to identify the key factor of the SCR, the regression analyses of both NO conversion versus Brønsted acid sites and NO conversion versus specific surface areas were evaluated. The correlation between Brønsted acid sites and NO conversion is lower than the correlation between specific surface area and NO conversion. Thus, only the correlation result between the NO conversion and the specific surface area was shown in this study. shows the correlation between NO conversion and the specific surface area with and without the presence of SO2 poisoning. For the case of without SO2 poisoning, the specific surface area had a minor effect on the NO conversion. And except for the lowest NO conversion of 64% at the specific surface area of 66 m2/g (MnFe-TiO(OH)2-550), all other data show high NO conversions of 84~98%. Even for MnFe-P25-550, which had the lowest specific surface area of 52 m2/g, it also had a high NO conversion of 87% when SO2 was not introduced. Thus, one can conclude from the results shown in and that the low NO conversion for MnFe-TiO(OH)2-550 was not mainly due to its small specific surface area or less Brønsted acid sites, instead, it could be due to the large agglomerate size of the metal particles at high calcination temperature of 550 °C.
Figure 9. The effect of specific surface area of catalysts on the NO conversion at SCR temperature of 150 °C with and without the presence of SO2. The data of without SO2 were averaged over 3 hr of operation time, whereas the data with SO2 was after 5 hr of SO2 poisoning.
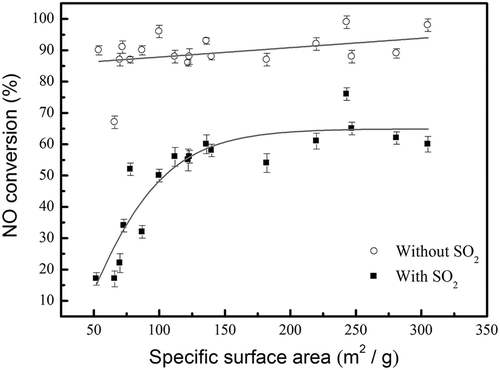
After the SCR system was exposed to 5 hr of SO2, one can see from that the effect of specific surface area on NO conversion became stronger, especially for the lower values of specific surface area. The NO conversion efficiency and specific surface area were highly correlated when the specific surface areas were less than around 150 m2/g. And the NO conversion efficiencies remained roughly the same of 62 ± 7% for specific surface areas of 150–305 m2/g after 5 hr of SO2 exposure. This is consistent with the result of Liu et al. (Citation2013), who indicated that the promoted activity of MnCe (ST) catalyst could be attributed to the high surface area and the smaller active metal particles.
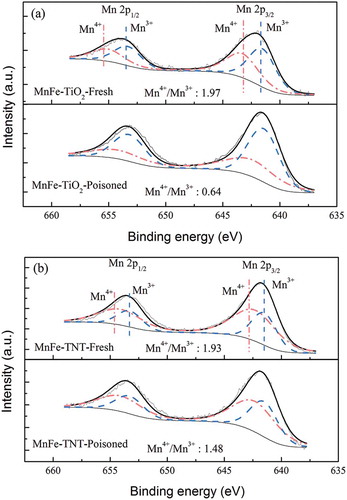
The XPS analysis was performed to further elucidate the metal species present on the surface of the catalysts. and show the Mn 2p spectra of MnFe-TiO2 and MnFe-TNTs, respectively, before and after SO2 poisoning. The XPS results showed that the Mn 2p region consisted of a spin-orbit doublet with Mn 2p1/2 and Mn 2p3/2, which had binding energies of 641.5 and 642.3 eV and corresponded to Mn3+ and Mn4+ (Thirupathi and Smirniotis, Citation2011). The Mn4+/Mn3+ ratios in MnFe-TiO2 and MnFe-TNT catalysts were 1.97 and 1.93, respectively, before SO2 poisoning. And after 5 hr of SO2 poisoning, the Mn4+/Mn3+ ratios decreased to 0.64 and 1.48, respectively, for MnFe-TiO2 and MnFe-TNT catalysts. Liu et al. (Citation2009) and Boningari et al. (Citation2015) indicated that the oxidation of NO to NO2 would increase by increasing Mn4+/Mn3+ ratio, which was beneficial to promote the fast SCR reaction at low temperatures. This indicated that when TNTs were used as the supports, the oxidation state of manganese was less affected by SO2 poisoning as compared with the TiO2-based catalysts. Thus, MnFe-TNT catalysts could maintain higher NO conversion efficiencies.
Effects of operating conditions of MnFe-TNT(TiO(OH)2)
MnFe-TNT(TiO(OH)2) calcined at 350 °C was used to evaluate the SO2 poisoning effect at different values of gas hourly space velocity (GHSV), reaction temperature, and [NH3]/[NO] inlet ratio, with the results shown in –.
Figure 11. Effect of gas hourly space velocity (GHSV) on the NO conversion over the MnFe-TNT(TiO(OH)2)(350) catalyst. Reaction conditions: reaction temperature = 150 °C, [NO] = 220 ppm, [NH3] = 200 ppm, [SO2] = 100 ppm, [O2] = 15%, balanced with air.
![Figure 11. Effect of gas hourly space velocity (GHSV) on the NO conversion over the MnFe-TNT(TiO(OH)2)(350) catalyst. Reaction conditions: reaction temperature = 150 °C, [NO] = 220 ppm, [NH3] = 200 ppm, [SO2] = 100 ppm, [O2] = 15%, balanced with air.](/cms/asset/f4e13f64-a52c-44ee-9265-7b413ea23ab0/uawm_a_1231144_f0011_b.gif)
Figure 12. Effect of reaction temperature on the NO conversion over the MnFe-TNT(TiO(OH)2)(350) catalyst. Reaction conditions: [NO] = 220 ppm, [NH3] = 200 ppm, [SO2] = 100 ppm, [O2] = 15%, balanced with air, and GHSV = 20,000 hr−1.
![Figure 12. Effect of reaction temperature on the NO conversion over the MnFe-TNT(TiO(OH)2)(350) catalyst. Reaction conditions: [NO] = 220 ppm, [NH3] = 200 ppm, [SO2] = 100 ppm, [O2] = 15%, balanced with air, and GHSV = 20,000 hr−1.](/cms/asset/40f4f8f7-3110-4475-8035-4bd12a4686c0/uawm_a_1231144_f0012_b.gif)
Figure 13. Effect of [NH3]/[NO] ratio on the NO conversion over the MnFe-TNT(TiO(OH)2)(350) catalyst. Reaction conditions: reaction temperature = 150 °C, [NO] = 220 ppm, [NH3] = 200~400 ppm, [SO2] = 100 ppm, [O2] = 15%, balanced with air, and GHSV = 20,000 hr−1.
![Figure 13. Effect of [NH3]/[NO] ratio on the NO conversion over the MnFe-TNT(TiO(OH)2)(350) catalyst. Reaction conditions: reaction temperature = 150 °C, [NO] = 220 ppm, [NH3] = 200~400 ppm, [SO2] = 100 ppm, [O2] = 15%, balanced with air, and GHSV = 20,000 hr−1.](/cms/asset/89dbefb9-ff3d-464e-bc5b-b6c7ad3d54f6/uawm_a_1231144_f0013_b.gif)
The values of GHSV tested in the literature ranged from 2000 to 30,000 hr−1 for the SCR system employed by power plants (Zhao et al., Citation2015; Zhou et al., Citation2015; Buzanowski and Yang, Citation1990). In this experiment, the GHSVs were from 10,000 to 40,000 hr−1 for the poisoning tests, which were in the high range of operating condition to ensure that the catalysts can maintain their high performance for field application in the future. shows the results of NO conversion over the MnFe-TNT catalyst with different values of GHSV under SO2 poisoning. The results revealed that MnFe-TNTs had similar NO conversion efficiencies of over 95% with the values of GHSV between 10,000 and 40,000 hr−1 before the input of SO2 gas. However, after SO2 poisoning, the NO conversion for GHSV of 40,000 hr−1 significantly decreased to only 66%. But at lower GHSV of 10,000 hr−1, it could remain around 90% NO conversion even after 5 hr of SO2 poisoning. The results indicated that a lower space velocity could inhibit the SO2 poisoning. This is due to that a lower value of space velocity means more catalyst amount, which can thus tolerate more sulfate compounds after the SO2-NH3 or SO2-metal reaction. In addition, as the reaction between SO2 and NH3 is faster, the NO might have no chance to react with NH3 under short residence time. Thus, the longer residence time at a lower space velocity may also provide enough time for the chemical reaction between NO and NH3.
shows the SO2 poisoning effect on the NO conversion of MnFe-TNT catalyst at different SCR reaction temperatures. It is observed that when the SCR reaction temperature was 150 °C, the NO conversion decreased to 76% after 5 hr of SO2 poisoning. But the SO2 effect became negligible upon increasing the reaction temperature. When the reaction temperatures were 250 and 300 °C, the NO conversion efficiencies remained almost the same as those without SO2 poisoning. The NO conversion efficiencies of the MnFe-TNT catalyst were 90, 94, and 95% when reaction temperatures were 200, 250, and 300 °C, respectively, after 5 hr SO2 poisoning. The SO2 poisoning decreased when the reaction temperature was increased. And at reaction temperatures of above 250 °C, the SO2 poisoning effect could be negligible. There were two main reasons for the inhibition on SO2 poisoning. The first one is due to that ammonia sulfate is not easily formed on the catalyst surface at higher temperatures. The second reason is attributed to that the catalyst follows the Eley-Rideal mechanism at temperatures of above 250 °C, which is different from the Langmuir-Hinshelwood mechanism at temperatures of less than 250 °C. Under the Eley-Rideal mechanism, the SO42− on the catalyst surface could increase NH3 adsorption and promotes reaction of NH3 with NO (Huang et al., Citation2002).
The [NH3]/[NO] ratio is an important factor for achieving high SCR performance. The [NH3]/[NO] ratios used in this study were from 0.91 to 1.82 to ensure that the SCR system had sufficient NH3 for reaction with both NO and SO2. However, under conventional SCR process, the [NH3]/[NO] ratio has to be less than 1.0 for avoiding the ammonia slip problem. Thus, the outlet concentration of NH3 was continuously monitored to understand whether ammonia slip is of concern. shows the SO2 poisoning effect on the NO conversion at different [NH3]/[NO] ratios over the MnFe-TNT catalyst. One can see that the NO conversion efficiencies of MnFe-TNT(TiO(OH)2) after 5 hr of SO2 poisoning were 76, 89, and 98%, when the [NH3]/[NO] ratios were 0.91, 1.365, and 1.82, respectively. When the [NH3]/[NO] ratio was 1.82, the SO2 poisoning effect could be minimized and the NO conversion remained almost the same as those without SO2 poisoning. However, about 70 ppm of NH3 slip was also detected at the outlet gas stream. On the other hand, under [NH3]/[NO] ratio of 1.365, there was negligible NH3 slip at the reactor outlet. This indicates that SO2 would compete with NO to react with NH3. And the decrease in the NO conversion efficiency was initially due to that NO could not react with NH3 instead of due to the deposition of ammonium sulfate. Hence, if the SCR system could provide a sufficient amount of NH3 to react with both SO2 and NO, then the NO conversion in the SCR process will not be affected by the presence of SO2.
From the above results, one knows that the SO2 poisoning effect could be minimized by reducing the GHSV, increasing the reaction temperature, or increasing the [NH3]/[NO] ratio. By increasing the [NH3]/[NO] ratio, the SO2 poisoning effect can be inhibited for a certain time by providing enough NH3 for both the NH3-NO reaction and the NH3-SO2 reaction. Similarly by decreasing the value of GHSV, the catalyst can also sustain its NO removal efficiency for a longer time. But the catalysts would still be deactivated in the long run no matter by increasing the [NH3]/[NO] ratio or reducing the GHSV, since the reaction between SO2 and NH3 is unavoidable. One the other hand, by increasing the reaction temperature to above the decomposition temperature of ammonium sulfate (230 °C) (Wu et al., Citation2009), the SO2 poisoning effect can be significantly reduced. Thus, it is suggested that operating the low-temperature SCR at >230 °C shall be the priority choice when SO2 poisoning is a concerned issue.
TGA analysis of NH3-SO2 reaction products
The TGA analysis was performed on MnFe-TiO2 and MnFe-TNTs (both prepared by TiO(OH)2) after 5 and 21 hr of SO2 poisoning. shows the TGA results and shows the results of differential thermograms (DTGs). From the weight loss profiles of the TGA, one can identify different sulfate compounds on the catalyst surface. It was observed that the first weight loss at 50~150 °C was due to water desorption on the catalyst surface, which appeared for the fresh catalysts. And the second and third weight losses at 160~450 and 670~900 °C were ascribed to the decomposition of ammonium sulfate and metal sulfates, respectively (Shu et al., Citation2014; Huang et al., Citation2008), as can be observed for the catalysts after SO2 poisoning. This indicates the presence of both ammonium sulfate and metal sulfates on the catalyst surfaces.
Figure 14. (a) TGA spectra and (b) DTG spectra of fresh and poisoned catalysts. Reaction conditions: reaction temperature = 150 °C, [NO] = 220 ppm, [NH3] = 200 ppm, [SO2] = 100 ppm, [O2] = 15%, balanced with air, and GHSV = 20,000 hr−1.
![Figure 14. (a) TGA spectra and (b) DTG spectra of fresh and poisoned catalysts. Reaction conditions: reaction temperature = 150 °C, [NO] = 220 ppm, [NH3] = 200 ppm, [SO2] = 100 ppm, [O2] = 15%, balanced with air, and GHSV = 20,000 hr−1.](/cms/asset/845bf885-df9f-4fd3-bc63-eb51a4cc7ea3/uawm_a_1231144_f0014_b.gif)
It can also be seen from that the weight losses due to the decomposition of metal sulfates (670~900 °C) over both MnFe-TiO2 and MnFe-TNTs increased when poisoned time was increased. Besides, the decomposition temperature of metal sulfates shifted to a relatively lower temperature for the MnFe-TNT catalyst as compared with that for the MnFe-TiO2 catalyst. This indicated that the TNT support might provide a relatively lower chemical binding energy between the metal catalyst and the sulfate species.
lists the amounts of ammonium sulfate and metal sulfates after SO2 poisoning. The specific surface areas of these two catalysts after 8 hr of test (3 hr without SO2 and then 5 hr with SO2) were measured, and the data are also shown in . By comparing with the specific surface areas of the fresh catalysts listed in , one can see that the specific surface areas of MnFe-TiO(OH)2 decreased from 100 to 53 m2/g, and that of MnFe-TNT(TiO(OH)2) decreased from 243 to 155 m2/g. This result clearly revealed that the sulfate species occupied the surface of catalyst and caused significant decreases in the specific surface area.
Table 2. The specific surface areas after SO2 poisoning and the amounts of SO2 poisoning products on the MnFe-TiO(OH)2 and MnFe-TNT(TiO(OH)2) catalysts.
As observed in , the amounts of ammonium sulfate increased to 1.49 and 1.10 wt% for the MnFe-TiO2 and MnFe-TNT catalysts, respectively, after 24 hr of SO2 poisoning test. This indicates that SO2 tended to continuously react with NH3 and deposited more on the MnFe-TiO2 catalyst than on the MnFe-TNT catalyst. On the other hand, the amount of metal sulfates increased to 4.69 and 5.81 wt% for the MnFe-TiO2 and MnFe-TNT catalysts, respectively, after 24 hr of SO2 poisoning test. It seemed that the SO2 tended to react with the metal catalyst and form metal sulfates more on MnFe-TNT catalyst than on MnFe-TiO2. Therefore, it may indicate that the formation of ammonium sulfate had a stronger negative effect on the NO conversion efficiency as compared with the formation of metal sulfates. However, this requires a further study to provide more evidence on the effects of these two sulfate species.
Although a complete SO2 poisoning mechanism on the MnFe/TiO2 and MnFe/TNT catalysts cannot be established at the present time, from the above results and the literature data, one can know that two SO2 poisoning reactions occurred during the low-temperature SCR process. One is that SO2 reacted with NH3 to form ammonium sulfate (Wu et al., Citation2009), the other is that SO2 reacted with metal catalysts to form metal sulfates. Wu et al. (Citation2009) doped Ce on the Mn/TiO2 catalyst and showed that the formation of Ti(SO4)2 and Mn(SO4)x was prevented and the depositions of (NH4)2SO4 and NH4HSO4 were significantly inhibited with the doping of ceria. Jiang et al. (Citation2010) carried out an in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFT) study on the SO2 poisoning, and the results showed that besides the deposition of ammonium sulfate, the competitive adsorption between SO2 and NO on the active sites of the catalysts also contributed to the poisoning effect of SO2 on the SCR reaction. In addition, from results shown in it is known that the Mn4+/Mn3+ ratio significantly decreased after SO2 poisoning, thus the transformation of Mn4+ into Mn3+ is one of the key factors to the NO conversion under SO2 poisoning. Detailed analysis on the above influencing parameters is required to provide more evidence for setting up the mechanism of SO2 poisoning under low-temperature SCR process.
Conclusion
In this study, Mn and Fe supported on TiO2 (P25, ST01, and TiO(OH)2) and TNTs (made from P25, ST01, and TiO(OH)2) were prepared at different calcination temperatures. Their low-temperature SCR performances were tested in terms of NO removal under SO2 poisoning. From the results of XRD, BET, and XPS analyses, it was found that higher Mn4+/Mn3+ ratios and larger specific surface areas might be the main reasons for the excellent performance of MnFe-TNT catalyst under SO2 poisoning. In addition, the crystal phase of the TiO2 and the well dispersion of the metal particles may affect the amount of Brønsted acid sites on the catalyst surface. The SO2 poisoning effect could be minimized by reducing the GHSV, increasing the reaction temperature, or increasing the [NH3]/[NO] molar ratio. The results also indicated that the formation of ammonium sulfate had a stronger effect on the NO conversion efficiency as compared with the formation of metal sulfates. Thus, operating the low-temperature SCR at above the decomposition temperature of ammonium sulfate (>230 °C) would be the priority choice when SO2 poisoning is an issue of concern. However, this requires a further study for providing more evidence to completely understand the SO2 poisoning mechanism for low-temperature SCR process.
Funding
The authors gratefully acknowledge the financial support from the Ministry of Science and Technology of the Republic of China through grant no. MOST 103-3113-E-009-003.
Additional information
Funding
Notes on contributors
TsungYu Lee
TsungYu Lee is a Ph.D. candidate at the Institute of Environ-mental Engineering, National Chiao Tung University, Hsinchu, Taiwan.
Sihyu Liou
Sihyu Liou received her master’s degree from the Institute of Environmental Engineering, National Chiao Tung University, Hsinchu, Taiwan. She is currently a research assistant at the same institute.
Hsunling Bai
Hsunling Bai is a professor at the Institute of Environmental Engineering, National Chiao Tung University, Hsinchu, Taiwan.
References
- Balle, P., B. Geiger, and S. Kureti. 2009. Selective catalytic reduction of NOx by NH3 on Fe/HBEA zeolite catalysts in oxygen-rich exhaust. Appl. Catal. B Environ. 85:109–119. doi: 10.1016/j.apcatb.2008.07.001.
- Boningari, T., D.K. Pappas, P.R. Ettireddy, A. Kotrba, and P.G. Smirniotis. 2015. Influence of SiO2 on MiTiO(2) (M = Cu, Mn, and Ce) formulations for low-temperature selective catalytic reduction of NOx with NH3: Surface properties and key components in relation to the activity of NOx reduction. Ind. Eng. Chem. Res. 54:2261–2273. doi: 10.1021/ie504709j.
- Busca, G., L. Lietti, G. Ramis, and F. Berti. 1998. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 18:1–36. doi: 10.1016/S0926-3373(98)00040-X.
- Buzanowski, M.A., and R.T. Yang. 1990. Simple design of monolith reactor for selective catalytic reduction of NO for power-plant emission control. Ind. Eng. Chem. Res. 29:2074–2078. doi: 10.1021/ie00106a015.
- Camposeco, R., S. Castillo, V. Mugica, I. Mejia-Centeno, and J. Marin. 2014. Role of V2O5-WO3/H2Ti3O7-nanotube-model catalysts in the enhancement of the catalytic activity for the SCR-NH3 process. Chem. Eng. J. 242:313–320. doi: 10.1016/j.cej.2014.01.002.
- Cha, W., E. Park, S. Chin, S.T. Yun, and J. Jurng. 2013. Effect of V2O5 loading of V2O5/TiO2 catalysts prepared via CVC and impregnation methods on NOx removal. Appl. Catal. B Environ. 140:708–715. doi: 10.1016/j.apcatb.2013.05.002.
- Chen, S.A., J.N. Nian, C.C. Tsai, and H. Teng. 2007. TiO2 nanotube-supported Cu as the catalyst for selective NO-reduction with NH3. J. Air Waste Manage Assoc. 57:600–605. doi: 10.3155/1047-3289.57.5.600.
- Cheng, Y. S., C. Lambert, D.H. Kim, J.H. Kwak, S.J. Cho, and C.H.F. Peden. 2010. The different impacts of SO2 and SO3 on Cu/zeolite SCR catalysts. Catal. Today 151:266–270. doi: 10.1016/j.cattod.2010.01.013.
- Choi, H.J., S.S. Kim, and S.C. Hong. 2013. Improving the activity of Mn/TiO2 catalysts through control of the pH and valence state of Mn during their preparation (vol 62, pg 362, 2012). J Air Waste Manage Assoc. 63:124. doi: 10.1080/10473289.2011.653515.
- Ettireddy, P.R., N. Ettireddy, S. Mamedov, P. Boolchand, and P.G. Smirniotis. 2007. Surface characterization studies of TiO2 supported manganese oxide catalysts for low temperature SCR of NO with NH3. Appl. Catal. B Environ. 76:123–134. doi: 10.1016/j.apcatb.2007.05.010.
- Gupta, R.C. 2000. Proceedings of the International Conference on Environmental Management in Metallurgical Industries (EMMI-2000). New Delhi, India: Allied Publishers.
- Huang, J.H., Z.Q. Tong, Y. Huang, and J.F. Zhang. 2008. Selective catalytic reduction of NO with NH3 at low temperatures over iron and manganese oxides supported on mesoporous silica. Appl. Catal. B Environ. 78:309–314. doi: 10.1016/j.apcatb.2007.09.031.
- Huang, Z.G., Z.P. Zhu, and Z.Y. Liu. 2002. Combined effect of H2O and SO2 on V2O5/AC catalysts for NO reduction with ammonia at lower temperatures. Appl. Catal. B Environ. 39:361–368. doi: 10.1016/S0926-3373(02)00122-4.
- Jiang, B.Q., Y. Liu, and Z.B. Wu. 2009. Low-temperature selective catalytic reduction of NO on MnOx/TiO2 prepared by different methods. J. Hazard. Mater. 162:1249–1254. doi: 10.1016/j.jhazmat.2008.06.013.
- Jiang, B.Q., Z.B. Wu, Y. Liu, S.C. Lee, and W.K. Ho, 2010. DRIFT study of the SO2 effect on low-temperature SCR reaction over Fe-Mn/TiO2. J. Phys. Chem. C 114:4961–4965. doi: 10.1021/jp907783g.
- Jin, R.B., Y. Liu, Z.B. Wu, H.Q. Wang, and T.T. Gu. 2010. Low-temperature selective catalytic reduction of NO with NH3 over Mn-Ce oxides supported on TiO2 and Al2O3: A comparative study. Chemosphere 78:1160–1166. doi: 10.1016/j.chemosphere.2009.11.049.
- Lee, S.M., S.S. Kim, and S.C. Hong. 2012a. Systematic mechanism study of the high temperature SCR of NOx by NH3 over a W/TiO2 catalyst. Chem. Eng. Sci. 79:177–185. doi: 10.1016/j.ces.2012.05.032.
- Lee, S.M., K.H. Park, S.S. Kim, D. Kwon, and S.C. Hong. 2012b. Effect of the Mn oxidation state and lattice oxygen in Mn-based TiO2 catalysts on the low-temperature selective catalytic reduction of NO by NH3. J. Air Waste Manage. Assoc. 62:1085–1092, doi: 10.1080/10962247.2012.696532.
- Lee, T.Y., and H.L. Bai. 2016. Low temperature selective catalytic reduction of NOx with NH3 over Mn-based catalyst: A review. AIMS Environ. Sci. 3:249–260. doi: 10.3934/environsci.2016.2.249.
- Li, L., Y.F. Diao, and X. Liu. 2014. Ce-Mn mixed oxides supported on glass-fiber for low-temperature selective catalytic reduction of NO with NH3. J. Rare Earth 32:409–415. doi: 10.1016/S1002-0721(14)60086-7.
- Liu, F.D., K, Asakura, H. He, W.P. Shan, X.Y. Shi, C.B. Zhang. 2011a. Influence of sulfation on iron titanate catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 103:369–377. doi: 10.1016/j.apcatb.2011.01.044.
- Liu, F.D., H. He, Y. Ding, and C.B. Zhang. 2009. Effect of manganese substitution on the structure and activity of iron titanate catalyst for the selective catalytic reduction of NO with NH3. Appl. Catal. B Environ. 93:194–204. doi: 10.1016/j.apcatb.2009.09.029.
- Liu, F.D., H. He, C.B. Zhang, W.P., Shan, and X.Y. Shi. 2011b. Mechanism of the selective catalytic reduction of NOx with NH3 over environmental-friendly iron titanate catalyst. Catal. Today 175:18–25. doi: 10.1016/j.cattod.2011.02.049.
- Liu, L., X. Gao, H. Song, C.H. Zheng, X.B. Zhu, Z.Y. Luo, M.J. Ni, and K. F. Cen. 2014a. Study of the promotion effect of iron on supported manganese catalysts for NO oxidation. Aerosol Air Qual. Res. 14:1038–1046. doi: 10.4209/aaqr.2013.04.0136.
- Liu, Z.M., Y. Li, T.L. Zhu, S. Hang, and J. Zhu. 2014b. Selective catalytic reduction of NOx by NH3 over Mn-promoted V2O5/TiO2 catalyst. Ind. Eng. Chem. Res. 53:12964–12970. doi: 10.1021/ie501887f.
- Liu, Z.M., Y. Yi, S.X. Zhang, T.L. Zhu, J.Z. Zhu, and J.G. Wang. 2013. Selective catalytic reduction of NOx with NH3 over Mn-Ce mixed oxide catalyst at low temperatures. Catal. Today 216:76–81. doi: 10.1016/j.cattod.2013.06.009.
- Liu, Z.M., J.Z. Zhu, J.H. Li, L.L. Ma, and S.I. Woo. 2014c. Novel Mn-Ce-Ti mixed-oxide catalyst for the selective catalytic reduction of NOx with NH3. ACS Appl. Mater. Interfaces 6:14500–14508. doi: 10.1021/am5038164.
- Lu, X.N., C.Y. Song, C.C. Chang, Y.X. Teng, Z.S. Tong, and X.L. Tang. 2014. Manganese oxides supported on TiO2-graphene nanocomposite catalysts for selective catalytic reduction of NOx with NH3 at low temperature. Ind. Eng. Chem. Res. 53:11601–11610. doi: 10.1021/ie5016969.
- Marban, G., and A. Fuertes. B. 2001. Low-temperature SCR of NOx with NH3 over Nomex (TM) rejects-based activated carbon fibre composite-supported manganese oxides: Part II. Effect of procedures for impregnation and active phase formation. Appl. Catal. B Environ. 34:55–71. doi: 10.1016/S0926-3373(01)00197-7.
- Mhamdi, M., S. Khaddar-Zine, and A. Ghorbel. 2009. Influence of the cobalt salt precursors on the cobalt speciation and catalytic properties of H-ZSM-5 modified with cobalt by solid-state ion exchange reaction. Appl. Catal. A Gen. 357:42–50. doi: 10.1016/j.apcata.2008.12.036.
- Min, Y.L., K. Zhang, W. Zhao, F.C. Zheng, Y.C. Chen, and Y.G. Zhang. 2012. Enhanced chemical interaction between TiO2 and graphene oxide for photocatalytic decolorization of methylene blue. Chem. Eng. J. 193:203–210. doi: 10.1016/j.cej.2012.04.047.
- Pappas, D.K., T. Boningari, P. Boolchand, and P.G. Smirniotis. 2016. Novel manganese oxide confined interweaved titania nanotubes for the low-temperature Selective Catalytic Reduction (SCR) of NOx by NH3. J. Catal. 334:1–13. doi: 10.1016/j.jcat.2015.11.013.
- Park, T.S., S.K. Jeong, S.H. Hong, and S.C. Hong. 2001. Selective catalytic reduction of nitrogen oxides with NH3 over natural manganese ore at low temperature. Ind. Eng. Chem. Res. 40:4491–4495. doi: 10.1021/ie010218+.
- Richter, M., A. Trunschke, U. Bentrup, K.W. Brzezinka, E. Schreier, M. Schneider, M.M. Pohl, and R. Fricke. 2002. Selective catalytic reduction of nitric oxide by ammonia over egg-shell MnOx/NaY composite catalysts. J. Catal. 206:98–113. doi: 10.1006/jcat.2001. 3468.
- Saqer, S.M., D.I. Kondarides, and X.E. Verykios. 2011. Catalytic oxidation of toluene over binary mixtures of copper, manganese and cerium oxides supported on gamma-Al2O3. Appl. Catal. B Environ. 103:275–286. doi: 10.1016/j.apcatb.2011.01.001.
- Shen, B.X., Y.Y. Wang, F.M. Wang, and T. Liu. 2014. The effect of Ce-Zr on NH3-SCR activity over MnOx(0.6)/Ce0.5Zr0.5O2 at low temperature. Chem. Eng. J. 236:171–180. doi: 10.1016/j.cej.2013.09.085.
- Shen, B.X., Y. Yao, H.Q. Ma, and T. Liu. 2011. Ceria modified MnOx/TiO2-pillared clays catalysts for selective catalytic reduction of NO with NH3 at low temperature. Chin. J. Catal. 32:1803–1811. doi: 10.1016/S1872-2067(10)60269-0.
- Shu, Y., T. Aikebaier, X. Quan, S. Chen, and H.T. Yu. 2014. Selective catalytic reaction of NOx with NH3 over Ce-Fe/TiO2-loaded wire-mesh honeycomb: Resistance to SO2 poisoning. Appl. Catal. B Environ. 150:630–635. doi: 10.1016/j.apcatb.2014.01.008.
- Smirniotis, P.G., P.M. Sreekanth, D.A. Pena, and R.G. Jenkins. 2006. Manganese oxide catalysts supported on TiO2, Al2O3, and SiO2: A comparison for low-temperature SCR of NO with NH3. Ind. Eng. Chem. Res. 45:6436–6443. doi: 10.1021/ie060484t.
- Su, Y.X., B.X. Fan, L.S. Wang, Y.F. Liu, B.C. Huang, M.L. Fu, L.M. Chen, and D.Q. Ye. 2013. MnOx supported on carbon nanotubes by different methods for the SCR of NO with NH3. Catal. Today 201:115–121. doi: 10.1016/j.cattod.2012.04.063.
- Tang, X.L., J. M, Hao., H. H. Yi, and J. H. Li. 2007. Low-temperature SCR of NO with NH3 over AC/C supported manganese-based monolithic catalysts. Catal. Today 126:406–411. doi: 10.1016/j.cattod.2007.06.013.
- Thamaphat, K., P. Limsuwan, and B. Ngotawornchai. 2008. Phase characterization of TiO2 powder by XRD and TEM. Kasetsart J. Nat. Sci. 42:357–361.
- Thirupathi, B., and P.G. Smirniotis. 2011. Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures. Appl. Catal. B Environ. 110:195–206. doi: 10.1016/j.apcatb.2011.09.001.
- Tong, H., and Y. Huang. 2012. The effects of manganese precursors on Mn-based/TiO2 catalysts for catalytic reduction of NO with NH3. J. Air Waste Manage. Assoc. 62:271–277. doi: 10.1080/10473289.2011.646350.
- Wallin, M., S. Forser, P. Thormahlen, and M. Skoglundh. 2004. Screening of TiO2-supported catalysts for selective NOx reduction with ammonia. Ind. Eng. Chem. Res. 43:7723–7731. doi: 10.1021/ie049695t.
- Wang, H.Q., X.B. Chen, X.L. Weng, Y. Liu, S. Gao, and Z.B. Wu. 2011. Enhanced catalytic activity for selective catalytic reduction of NO over titanium nanotube-confined CeO2 catalyst. Catal. Commun. 12:1042–1045. doi: 10.1016/j.catcom.2011.03.005.
- Wu, Z.B., R.B. Jin, Y. Liu, and H.Q. Wang. 2008. Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature. Catal. Commun. 9:2217–2220. doi: 10.1016/j.catcom.2008.05.001.
- Wu, Z.B., R.B. Jin, H.Q. Wang, and Y. Liu. 2009. Effect of ceria doping on SO2 resistance of Mn/TiO2 for selective catalytic reduction of NO with NH3 at low temperature. Catal. Commun. 10:935–939. doi: 10.1016/j.catcom.2008.12.032.
- Xiong, L., Y. Yang, J.X. Mai, W.L. Sun, C.Y. Zhang, D.P. Wei, Q. Chen, and J.R. Ni. 2010. Adsorption behavior of methylene blue onto titanate nanotubes. Chem. Eng. J. 156:313–320. doi: 10.1016/j.cej.2009.10.023.
- Xu, H.D., Z.T. Fang, Y. Cao, S. Kong, T. Lin, M.C. Gong, and Y.Q. Chen. 2012a. Influence of Mn/(Mn plus Ce) ratio of MnOx-CeO2/WO3-ZrO2 monolith catalyst on selective catalytic reduction of NOx with ammonia. Chin. J. Catal. 33:1927–1937. doi: 10.1016/S1872-2067(11)60467-1.
- Xu, H.D., Q.L. Zhang, C.T. Qiu, T. Lin, M.C. Gong, and Y.Q. Chen. 2012b. Tungsten modified MnOx-CeO2/ZrO2 monolith catalysts for selective catalytic reduction of NOx with ammonia. Chem. Eng. Sci. 76:120–128. doi: 10.1016/j.ces.2012.04.012.
- Yao, G.H., K.T. Gui, and F. Wang. 2010. Low-temperature de-NOx by selective catalytic reduction based on iron-based catalysts. Chem. Eng. Technol. 33:1093–1098. doi: 10.1002/ceat.201000015.
- Yu, J., F, Guo., Y.L. Wang, J.H. Zhu, Y.Y. Liu, F.B. Su, S.Q. Gao, and G.W. Xu. 2010. Sulfur poisoning resistant mesoporous Mn-base catalyst for low-temperature SCR of NO with NH3. Appl. Catal. B Environ. 95:160–168. doi: 10.1016/j.apcatb.2009.12.023.
- Zhao, B, X.W. Liu, Z.J. Zhou, H.Z. Shao, C. Wang, M.H. Xu. 2015. Mercury oxidized by V2O5-MoO3/TiO2 under multiple components flue gas: An actual coal-fired power plant test and a laboratory experiment. Fuel Process. Technol. 134:198–204. doi: 10.1016/j.fuproc.2015.01.034.
- Zhou, Z.J., X.W. Liu, B. Zhao, Z.G. Chen., H.Z, Shao., L.L. Wang, and M.H. Xu. 2015. Effects of existing energy saving and air pollution control devices on mercury removal in coal-fired power plants. Fuel Process. Technol. 131:99–108. doi: 10.1016/j.fuproc.2014.11.014.