ABSTRACT
The nitrogen oxides (NOx) reduction technology by combustion modification which has economic benefits as a method of controlling NOx emitted in the combustion process, has recently been receiving a lot of attention. Especially, the moderate or intense low oxygen dilution (MILD) combustion which applied high temperature flue gas recirculation has been confirmed for its effectiveness with regard to solid fuel as well. MILD combustion is affected by the flue gas recirculation ratio and the composition of recirculation gas, so its NOx reduction efficiency is determined by them. In order to investigate the influence of factors which determine the reduction efficiency of NOx in MILD coal combustion, this study changed the flow rate and concentration of nitrogen (N2), carbon dioxide (CO2) and steam (H2O) which simulate the recirculation gas during the MILD coal combustion using our lab-scale drop tube furnace and performed the combustion experiment. As a result, its influence by the composition of recirculation gas was insignificant and it was shown that flue gas recirculation ratio influences the change of NOx concentration greatly. Implications: We investigated the influence of factors determining the nitrogen oxides (NOx) reduction efficiency in MILD coal combustion, which applied high-temperature flue gas recirculation. Using a lab-scale drop tube furnace and simulated recirculation gas, we conducted combustion testing changing the recirculation gas conditions. We found that the flue gas recirculation ratio influences the reduction of NOx emissions the most.
Introduction
Nitrogen oxides (NOx) are one of the typical air pollutants that are emitted from stationary combustion sources such as power plants and incinerators. Prompt NOx, fuel NOx, and thermal NOx are the generation mechanisms of NOx that are produced in the combustion process. Prompt NOx is the name for the mechanism produced at a fast rate of less than 0.04 sec by the reaction of the functional groups of hydrogen cyanide (HCN) and hydrocarbons that are intermediate products of combustion (Fenimore et al., Citation1981). Fuel NOx refers to the NOx that is generated when the N–C bond is weak, from the nitrogens combined in the fuel. Thermal NOx is formed by the reaction of nitrogen and oxygen in the air either when the concentration of oxygen is high or when oxygen stays for a long time in the range of high combustion temperatures (Cooper and Alley, Citation2011).
There are two techniques that control NOx: combustion modification and flue gas treatment technique (Cooper and Alley, Citation2011; Mosca et al., Citation2014). The former is a technique controlling NOx formation by reducing the highest temperature in the flame region, residence time, and oxygen concentration. On the other hand, the latter is the technique controlling the NOx that has been already produced in the combustion process; selective catalytic reduction (SCR) and selective noncatalytic reduction (SNCR) are two typical techniques. Flue gas treatment generally has an advantage of its high efficiency of controlling NOx. However, due to its financial burdens and secondary pollution problems, the NOx reduction technique through combustion modification has had a lot of attention (Wang et al., Citation2006).
Some of the combustion modification technologies that are effective in reducing the production of NOx are off-stoichiometric combustion, reduced air preheat, flue gas recirculation, flame cooling, and reburning. Off-stoichiometric combustion goes through two stages: In the first stage, it mixes fuel and air rapidly in fuel-rich conditions so that NOx is reduced due to lack of oxygen and low temperatures, and in the second stage, it injects excess air to attain complete combustion. Reduced air preheat is a technology that lowers the combustion temperature by reducing the temperature of preheated air. However, due to the reduction of the combustion temperature, it decreases thermal NOx, but it can also decrease combustion efficiency, resulting in energy loss. Flue gas recirculation is a method that reinjects part of the exhaust gas by sending it back to the combustion system. As a result, both the temperature of the combustion and the concentration of oxygen in combustion furnace get lower, resulting in the reduction of NOx. Flame cooling recirculates exhaust gas or reduces NOx by injecting steam (H2O) and reducing the time it stays in the highest temperature in flame region. However, it can emit carbon monoxide (CO) because of its incomplete combustion and cause energy loss (Wunning and Wunning, Citation1997).
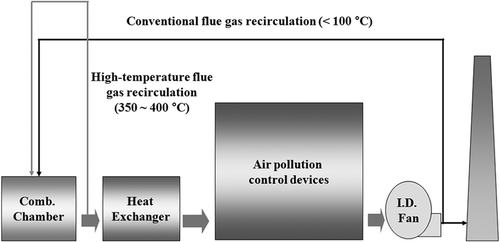
High-temperature flue gas recirculation has recently been studied in order to minimize energy loss, as well as to reduce the production of NOx efficiently. Through the internal high-temperature gas recirculation, combustion is made at a low concentration of oxygen and an extended reaction zone. In addition, through the recirculation of combustion products into the combustion zone, NOx can be effectively reburned under more uniform temperature and lower oxygen concentration conditions (Stadler et al., Citation2009). This creates a distributed reaction zone and reduced peak combustion temperature, which contribute to reducing the NOx production and increasing combustion efficiency. This method is called “flameless oxidation” (FLOX) because flames are not visible during combustion. It is also called “moderate or intense low oxygen dilution (MILD) combustion” or “high-temperature air combustion” (HTAC). The differences between the conventional flue gas recirculation and the high-temperature flue gas recirculation are shown in . Flue gas recirculation recirculates the exhaust gas of temperature that has already been cooled, whereas high-temperature flue gas recirculation recirculates to the combustion furnace the gas of high temperature that is emitted from the combustion furnace.
Figure 1. Schematic diagram of the conventional flue gas recirculation and the high-temperature flue gas recirculation.
Wunning et al. (Citation1997) discovered that the better the recirculation gas is mixed, the better the flameless oxidation occurs when the air preheated at 950°C is used. Weber et al. (Citation2005) also demonstrated the MILD combustion of natural gas and light fuel oil (LFO) in the combustion furnace at 1300°C. They suggested that the NOx reduction effect by MILD combustion is affected by physical factors such as flue gas recirculation ratio and the design of a combustion furnace and by chemical factors such as heat capacity of fuel, composition of fuel, and composition of injected air; therefore, the demonstration of MILD combustion using solid fuel is relatively difficult. By doing a modeling analysis that uses a computational fluid dynamics (CFD) program, they confirmed that through appropriate boiler design it is possible to produce combustion with solid fuel, but that they need a powerful recirculation gas at a very high speed. Using sawdust as fuel and also using in a lab-scale combustion furnace a variety of carrier gases, such as air, carbon dioxide (CO2), nitrogen (N2), and so on, and then injecting temperate air at 440°C, Dally et al. (Citation2010) demonstrated the MILD combustion. As a result, the reduction efficiency of NOx and CO varied depending on the composition of the combustion gases. Shim et al. (Citation2014, Citation2015) successfully demonstrated the MILD combustion, using dried sludge (waste fuels) in lab-scale and pilot-scale combustors, and confirmed the reduction efficiency of NOx emissions on a large scale. Also, with regard to coal, a typical solid fuel, research studies showing successful MILD combustion are being reported (Shim, Citation2014; Saha et al., Citation2014).
Putting all the results of previous research studies together, the NOx reduction efficiency of MILD combustion depends on flue gas recirculation ratio, composition of recirculation gas, and other factors. Therefore, in this study, we used a lab-scale drop tube furnace in order to investigate the factors determining the NOx reduction efficiency in the MILD combustion of coal. Using air combustion as a baseline condition, we investigated the NOx emissions produced when the flow of N2, CO2, and steam simulates the recirculation gas in the MILD combustion with the same excess air ratio and their concentrations are changed.
Experiment
Experimental apparatus
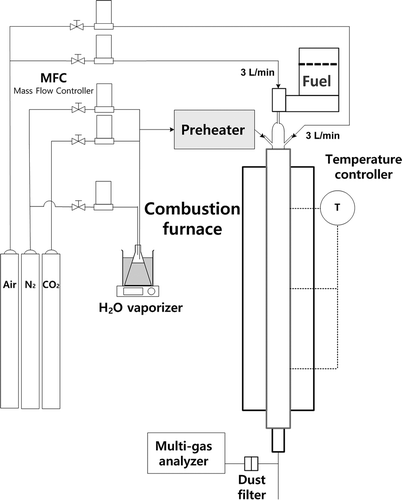
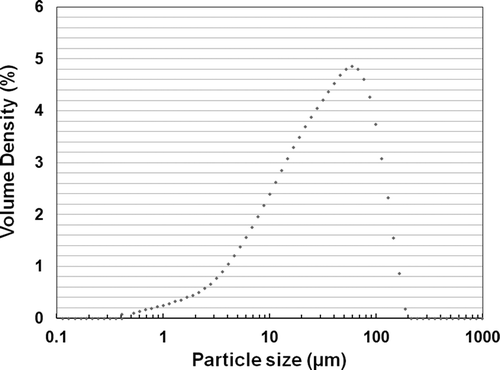
When high-temperature flue gas recirculation was applied, we prepared a lab-scale combustion system as shown in in order to investigate the reduction efficiency of NOx, depending on the composition of recirculation gases. The fuel used in the experiment was pulverized coal. When analyzed using a laser diffraction method, the size of the coal was 43 μm on average. The particle size distribution of the coal is shown in . Using ASTM D5373-08 (ASTM, Citation2008), we analyzed the carbon (C), hydrogen (H), and nitrogen (N) contained in the coal. We used ASTM D4239-12 (ASTM, Citation2012), which uses high-temperature tube furnace combustion, to analyze the sulfur (S). We also conducted proximate analysis using a thermogravimetric analyzer (SDT Q600, TA Instruments, USA). The results of the analysis are summarized in .
Table 1. Results of proximate and ultimate analysis for coal (unit %).
As an apparatus in the combustion experiment, we used a powder feeder (S06, DAEWON ENG, Korea) in order to continuously provide coal to the combustion furnace. The flow of N2, CO2, and steam used to change the conditions for combustion was controlled using mass flow controllers (5850E, Brooks Instruments, USA). Taking advantage of the difference in water vapor pressure depending on the temperatures, we put H2O in the water tank kept at constant temperature, using a triangular flask containing water and injecting gas in it. For the tube in which vapor flows around we added a heating tape in order to prevent water vapor from condensing. We constructed the combustion furnace using quartz material with 100 cm length and 4.4 cm internal diameter. We installed an electric furnace outside the combustion furnace to provide a temperature above the autoignition temperature of coal. We also installed temperature controllers (CT-100, KS ENG, Korea) at the top, middle, and bottom between the outside of the combustion furnace and the inside of the electric furnace. Also, in order to simulate high-temperature flue gas recirculation, we preheated the simulated recirculation gas with a preheater and then injected it into the combustion furnace. The drop tube combustion furnace was used to measure the NOx concentration right after the combustion of coal and to investigate the effects of the recirculation gas conditions on NOx emissions. The concentration of the combustion exhaust gas was measured using a gas analyzer (Vario Plus, MRU, Germany). Nitric oxide (NO) and nitrogen dioxide (NO2) were measured by electrochemical sensors inside the gas analyzer. NOx concentration was determined as the sum of the concentrations of NO and NO2.
Experimental procedure
With the condition that the combustion experiment should inject air at a flow rate of 6 L/min and burn coal at excess air ratio of 1.2, we did an experiment adding simulated recirculation gas to the baseline condition. We maintained the excess air ratio at 1.2 in order that we might apply the same conditions for combustion even under the condition that simulated recirculation gas being added. We performed the experiment, changing the composition of N2, CO2, and H2O, which are the components of simulated recirculation gas, and their flow rate. From the baseline condition, we used air at a flow rate of 3 L/min as a carrier gas to the coal and the remaining air at a flow rate of 3 L/min to the side of the fuel inlet. We injected coal at a rate of 0.61 g/min through the fuel supply system so that the excess air ratio of 1.2 was maintained throughout. The components of simulated recirculation gas are (1) steam, (2) N2, (3) 10% CO2/N2, and (4) 10% CO2/N2 + steam, as shown in . When high-temperature flue gas recirculation was applied, we simulated the temperature of recirculation gas and injected simulated recirculation gas to the combustion furnace after having it preheated at 350°C. Also, considering the composition of full-scale recirculation gas, we injected steam at a flow rate of 0.12, 0.24, and 0.36 L/min, which are 2, 4, and 6%, respectively, of the airflow of the baseline condition (6 L/min), and the whole simulated recirculation gas at a flow rate of 25 and 50% (v/v), which are 1.5 and 3 L/min, respectively. In order to investigate the influence according to the injected flow only, we added N2 at a flow rate of 1.5 and 3 L/min to the baseline condition and injected it. In addition, in order to investigate the influence of CO2, we made 10% CO2, a typical concentration in the combustion gas, and injected it at a flow rate of 1.5 and 3 L/min to perform the combustion experiment. Lastly, as a gas that simulates the composition of recirculation gas, we added steam at a flow rate of 0.24 L/min to 10% of CO2/N2 at a flow rate of 1.5 and 3 L/min, respectively. The experimental conditions are summarized in .
Table 2. Summary of experimental conditions.
Before performing the experiment, we maintained the outside temperature of the combustion furnace at 950°C after raising it, and we adjusted the temperature of the preheater so that simulated recirculation gas was injected to the combustion furnace at 350°C with regard to each condition. By injecting air first into the combustion furnace at a flow rate of 6 L/min and simulated recirculation gas according to each condition, we began the experiment. We continuously measured the concentration of CO2, CO, oxygen (O2), and NOx at an interval of 2 sec using a gas analyzer. The experiment period for each condition was 10 min in total, and we calculated the concentration of exhaust gas on the basis of the period in which combustion reached steady state and averaged it. We also performed each experiment twice according to each condition.
Results and discussion
Combustion stability test
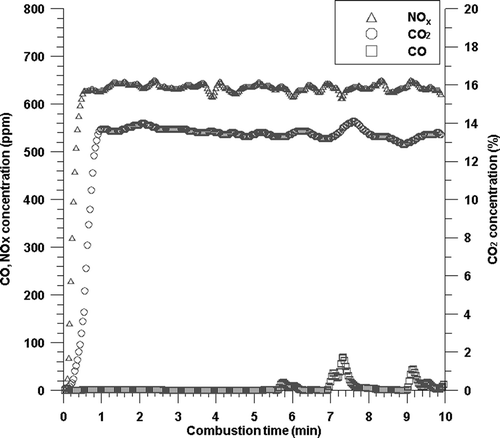
We conducted combustion tests under the baseline condition and analyzed the combustion stability. The concentration of each gas emitted after being burned under each condition was continuously recorded at an interval of 2 sec using a combustion gas analyzer. shows the concentrations of CO2, CO, and NOx, respectively, emitted over time when they are burned according to the baseline condition for combustion. As shown in the figure, the concentration of gases rapidly increases after combustion starts and reaches a steady state within 1 min and shows steady concentration. This shows steady emission concentration, which indicates that combustion conditions remain steady. Although the emission concentration of CO tended to increase temporarily 7 min after combustion, it maintained the very low emission concentration of CO, which indicates stable complete combustion. Also, the emission concentration of CO2 reaches more than 90% of the theoretical CO2 concentration. Under all the other conditions as well as the baseline condition, we were able to confirm complete combustion. Therefore, the experimental system of this study is considered appropriate for investigating the changes of NOx concentration according to the changes of combustion condition.
Combustion tests with the conditions of recirculation gas
In order to investigate the influence of the flow rate and composition of recirculation gases in MILD combustion, we made it a baseline condition that air at a flow rate of 6 L/min be injected and then coal be burned. We performed the experiment changing the composition and flow rate of N2, CO2, and H2O that become the components of recirculation gas. NOx emission concentrations measured at the outlet of the combustion furnace were converted to the amount of NOx emission per the amount of fuel injected (g NOx/kg fuel) to compare the results regardless of dilution effects, as presented in –. First, in order to investigate the influence according to the flow rate of recirculation gas only, we injected N2 at a flow rate of 1.5 and 3 L/min, respectively, in addition to the baseline condition. Each experiment was performed twice with regard to each condition. As shown in , as the injection of N2 increased, the NOx emission decreased. While the average NOx emission was 8.2 g NOx/kg fuel at the baseline condition, it decreased to 6.9 and 5.8 g NOx/kg fuel with injections of 1.5 and 3 L/min N2, respectively. With N2 injection, the effect of restraining the production of NOx turned out to be large.
Figure 5. NOx emissions with time from the combustion with baseline, 1.5 L/min N2, and 3 L/min N2 conditions.
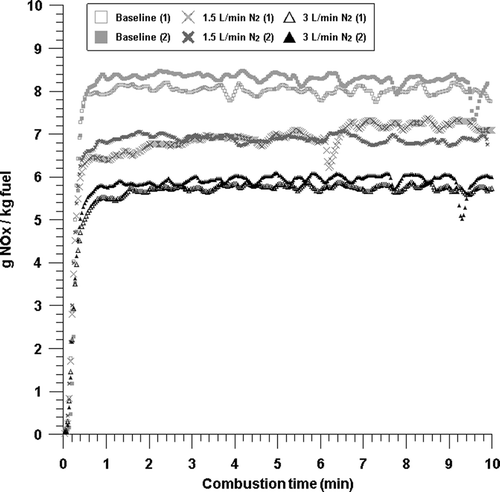
Figure 6. NOx emissions with time from the combustion with baseline, 1.5 L/min 10% CO2/N2, and 3 L/min 10% CO2/N2 conditions.
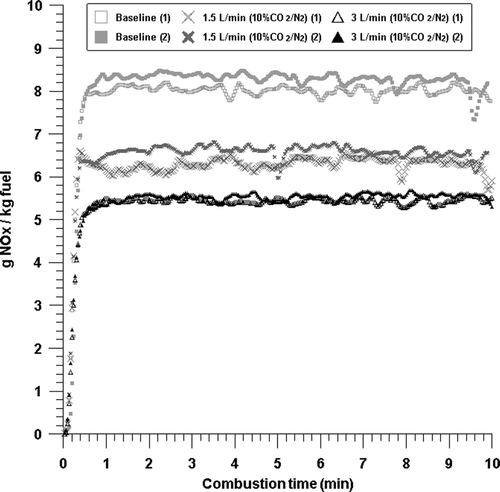
Figure 7. NOx emissions with time from the combustion with baseline, 0.12 L/min, 0.24 L/min, and 0.36 L/min steam conditions.
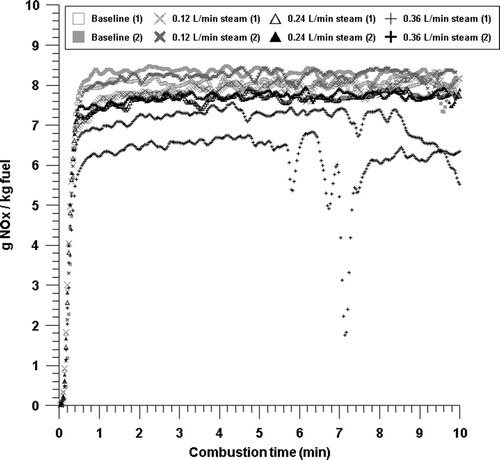
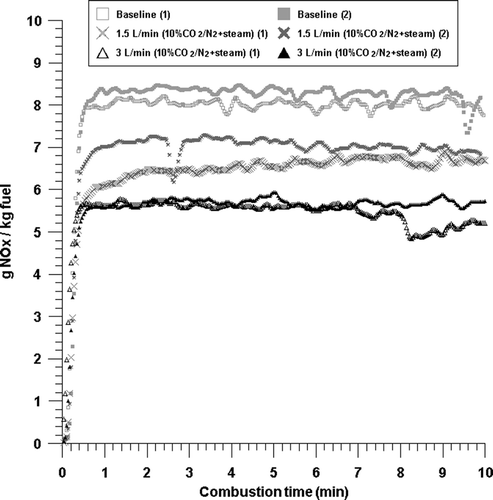
Figure 8. NOx emissions with time from the combustion with baseline, 1.74 L/min (10% CO2/N2 + steam), and 3.24 L/min (10% CO2/N2 + steam) conditions.
In order to examine the influence of CO2, we used 10% as a general concentration of CO2 in the combustion flue gas and then injected at a flow rate of 1.5 and 3 L/min, with results that are shown in . As in the case where only N2 was injected, as the injected flow rate increased, the NOx emission decreased. In addition, when compared to the case in which only N2 was injected, the NOx emission at the same flow rate decreased very slightly. Therefore, the influence of CO2 in the reduction of NOx emission is minimal.
In order to examine the influence of steam injection, we additionally injected steam at a flow rate of 0.12, 0.24, and 0.36 L/min, respectively, and performed the combustion experiment. The results are shown in . Compared to the average NOx emission of 8.2 g NOx/kg fuel under the baseline condition, when steam was injected at a flow rate of 0.12, 0.24, and 0.36 L/min, the average NOx emission was 8.1, 7.7, and 6.7 g NOx/kg fuel, respectively. When steam was injected at a flow rate of 0.12 and 0.24 L/min, the reduction in the NOx emission was minimal, whereas when steam was injected at a flow rate of 0.36 L/min, a relatively large reduction in the NOx emission appeared. However, when steam was injected at a flow rate of 0.36 L/min, the NOx emission changed greatly over time, which is considered to be the result of unstable combustion. Therefore, the effect of restraining the production of NOx according to steam injection is deemed to be very minimal.
Lastly, as the gas that simulates the composition of recirculation gas, steam at a flow rate of 0.24 L/min was additionally injected to 10% of CO2/N2 at a flow rate of 1.5 and 3 L/min, respectively, and the combustion experiment was performed. Therefore, the total flow rate of additionally injected simulated recirculation gas was 1.74 and 3.24 L/min, respectively, as the results in indicate. The NOx emission for each flow rate injection was 6.8 and 5.6 g NOx/kg fuel, respectively, as indicated in the figure. This shows that the reduction effect in NOx emission was large. However, considering that this is similar to the NOx emission, that is, 6.9 and 5.8 g NOx/kg fuel emitted when only N2 was injected at a flow rate of 1.5 and 3 L/min and burned, it was shown that the effect of restraining the production of NOx according to the injected flow rate of recirculation gas was the largest. In addition, considering that when 10% of CO2/N2 was injected at a flow rate of 1.5 and 3 L/min, the NOx emission was 6.5 and 5.5 g NOx/kg fuel, respectively, it was shown that there is little effect by steam.
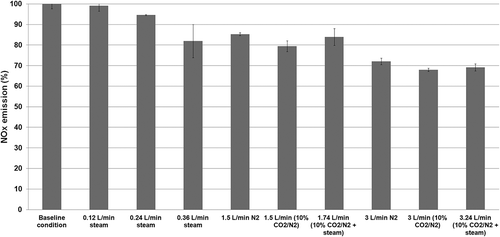
In order to effectively compare the changes in the NOx emission with regard to all recirculation gas conditions, we averaged the amounts of NOx emission for each condition and present them compared to the NOx emission at the baseline condition in . In this figure, the average amount of NOx emission in the baseline condition is presented as 100%, and its amounts in the other conditions are presented in percentages of the baseline condition amount. The conditions shown on the x-axis of the figure are indicated according to the order of flow rate increase, and the mean and standard deviation gained from the results of the experiment repeated twice are shown in the graph. As seen in the graph, as the flow rate of recirculation gas increases, the NOx emission decreases; the lowest emission rate of NOx appears under the condition of recirculation flow rate being at 3 and 3.24 L/min, which are 68–72% of the NOx emission under the baseline combustion condition. Therefore, the primary reason that the emission concentration of NOx decreases in MILD combustion of coal that uses high-temperature recirculation seems to be due to the restraint of the NOx production resulting from the effect of dilution in oxygen concentration and the reduction of the peak combustion temperature.
Conclusion
We conducted this study to find the factors that determine the NOx reduction in employing the MILD combustion technology, which applies high-temperature flue gas recirculation known for its effectiveness in reducing NOx emissions. In MILD combustion technology, the reduction efficiency of NOx is reported to depend on the flue gas recirculation ratio and the composition of recirculation gas; therefore, in this study we performed the combustion experiment by changing the flow rate and concentration of N2, CO2, and steam that simulate the recirculation gas using a lab-scale drop tube furnace. Under the condition of 6 L/min air established as the baseline combustion condition, the average NOx emission turned out to be 8.2 g NOx/kg fuel. While maintaining the excess air ratio of 1.2, which is the same as the baseline condition, we additionally injected simulated recirculation gas at a flow rate of 1.5 and 3 L/min, which indicate approximately 25 and 50% (v/v) flue gas recirculation, respectively. To begin with, when N2 of 1.5 and 3 L/min was injected, the NOx emission was emitted was 6.9 and 5.8 g NOx/kg fuel, respectively, which indicates a great reduction. On the other hand, we used 10% CO2 as N2 background gas and injected it additionally at a recirculation ratio of 0.25 and 0.5 and burned it. As a result, the NOx emission was 6.5 and 5.5 g NOx/kg fuel, respectively, which shows results similar to when only N2 was additionally injected. When steam was additionally injected at a flow rate of 0.12, 0.24, and 0.36 L/min, the reduction rate in the NOx concentration was minimal. Relatively unstable combustion was shown with an injection of 0.36 L/min steam, which increases the water vapor concentration in the flue gas by approximately 6 percentage points. Lastly, as the gas that simulates the composition of recirculation gas, steam at a flow rate of 0.24 L/min was additionally injected to 10% CO2/N2 at a recirculation ratio of 0.25 and 0.5. The results indicate that the NOx emission was 6.8 and 5.6 g NOx/kg fuel, respectively, which shows results similar to when 10% CO2/N2 was additionally injected. Therefore, among the injected gases that simulate the recirculation gas, it turned out that the influence of steam was little and that the influence of CO2 is also minimal. On the other hand, it turned out that the flow rate of recirculation gas, which means the flue gas recirculation ratio, influences the reduction of NOx emissions the most. Therefore, it is deemed that in MILD combustion of coal that applies high-temperature flue gas recirculation, the primary reason for the reduction of NOx emissions is due to the reductions of oxygen concentration and peak combustion temperature resulting from the injection of recirculation gas.
Funding
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (number 20153010102030) and by a research project from Korea Institute of Machinery and Materials (KIMM).
Additional information
Funding
Notes on contributors
Min Park
Min Park is a graduate student in the Department of Environmental Engineering at Chungbuk National University, Korea.
Sung Hoon Shim
Sung Hoon Shim and Sang Hyun Jeong are principal researchers in the Research Division for Environment and Energy Systems at Korea Institute of Machinery & Materials (KIMM), Korea.
Sang Hyun Jeong
Kwang-Joong Oh is a professor in the Department of Environmental Engineering at Pusan National University, Busan, Korea.
Sang-Sup Lee
Sang-Sup Lee is an associate professor in the Department of Environmental Engineering at Chungbuk National University, Korea.
References
- ASTM. 2008. ASTM D5373-08: Standard test methods for instrumental determination of carbon, hydrogen, and nitrogen in laboratory samples of coal. West Conshohocken, PA: ASTM International.
- ASTM. 2012. ASTM D4239-12: Standard test method for sulfur in the analysis sample of coal and coke using high-temperature tube furnace combustion methods. West Conshohocken, PA: ASTM International.
- Cooper, C.D., and F.C. Alley. 2011. Air Pollution Control: A Design Approach, 4th ed. Long Grove, IL: Waveland Press.
- Dally, B.B., S.H. Shim, R.A. Craig, P.J. Ashman, and G.G. Szegö. 2010. On the burning of sawdust in a MILD combustion furnace. Energy Fuels 24(6):3462–3470. doi: 10.1021/ef901583k
- Fenimore, C.P. 1981. Formation of nitric oxide in premixed hydrocarbon flames. Paper presented at the Thirteenth Symposium (International) on Combustion, The Combustion Institute, Pittsburgh, PA, pp. 373–380.
- Mosca, S., P. Benedetti, E. Guerriero, and M. Rotatori. 2014. Assessment of N2O emission from cement plants: real data measured with both FTIR and NDIR. J. Air Waste Manage. Assoc. 64:1270–78. doi: 10.1080/10962247.2014.936986
- Saha, M., B.B. Dally, P.R. Medwell, and E.M. Cleary. 2014. Moderate or intense low oxygen dilution combustion characteristics of pulverized coal in a self-recuperative furnace. Energy Fuels 28: 6046–57. doi: 10.1021/ef5006836
- Schaffel-Mancini, N., M. Mancini, A. Szlek, and R. Weber. 2010. Novel conceptual design of a supercritical pulverized coal boiler utilizing high temperature air combustion (HTAC) technology. Energy 35(7):2752–60. doi: 10.1016/j.energy.2010.02.014
- Shim, S.H. 2014. Combustion characteristics and analyzing results of exhaust gas of solid fuel with measuring position in a pilot scale MILD combustor using high temperature exhaust gas recirculation. J. Korea Soc. Waste Manage. 31:89–95. doi: 10.9786/kswm.2014.31.1.89
- Shim, S.H., S.H. Jeong, and S.-S. Lee. 2014. Reduction in nitrogen oxides emissions by MILD combustion of dried sludge. Renew. Energy 65(0): 29–35. doi: 10.1016/j.renene.2013.07.005
- Shim, S.H., S.H. Jeong, and S.-S. Lee. 2015. Low-nitrogen oxides combustion of dried sludge using a pilot-scale cyclone combustor with recirculation. J. Air Waste Manage. Assoc. 65:413–22. doi: 10.1080/10962247.2014.998353
- Stadler, H., D. Ristic, M. Forster, A. Schuster, R. Kneer, and G. Scheffknecht. 2009. NOx-emissions from flameless coal combustion in air, Ar/O2 and CO2/O2. Proc. Combust. Inst. 32:3131–38. doi: 10.1016/j.proci.2008.06.025
- Turns, S.R. 2012. An Introduction to Combustion: Concepts and Applications, 3rd ed. New York, NY: McGraw-Hill.
- Wang, Y.D., Y. Huang, D. McIlveen-Wright, J. McMullan, N. Hewitt, P. Eames, and S. Rezvani. 2006. A techno-economic analysis of the application of continuous staged-combustion and flameless oxidation to the combustor design in gas turbines. Fuel Process. Technol. 87(8):727–36. doi: 10.1016/j.fuproc.2006.02.003
- Weber, R.W., J.P. Smart, and W. Kamp. 2005. On the (MILD) combustion of gaseous, liquid, and solid fuels in high temperature preheated air. Proc. Combust. Inst. 30:2623≠29. doi: 10.1016/j.proci.2004.08.101
- Wunning, J.A. and Wunning J.G. 1997. Flameless oxidation to reduce thermal NO-formation. Prog. Energy Combust. 23:81–94. doi: 10.1016/S0360-1285(97)00006-3